Abstract
This review highlights the developments in dendrimer-based micelles for drug delivery. Dendrimers, the perfectly branched monodisperse macromolecules, have certain structural advantages that make them attractive candidates as drug carriers for controlled release or targeted delivery. As polymeric micelle-based approaches precede the work in dendrimers, these are also discussed briefly. The review concludes with a perspective on possible applications of biaryl-based dendrimeric micelles that exhibit environment-dependent conformations, in drug delivery.
Introduction
The discovery and development of new drugs is a time consuming and expensive process. Each new candidate is estimated to take around 12 to 15 years to bring it from conception to market, with the cost of more than $500 million.1 However with most of the drugs, only a small part of the administered active ingredient reaches the desired site due to loss in metabolism and excretion. Every drug molecule has a therapeutic window in terms of concentration above which the drug is toxic and below which it is ineffective. Conventional dosage of drugs results in frequent cycling between the toxic and ineffective levels depending on the frequency with which the drug is administered. An attractive solution to this issue involves the development of a controlled release system, where the drug molecule is slowly released over time thereby the concentration fluctuations are negligibly small.2 Another advantage of controlled release system involves patient compliance. Treatment of tuberculosis patients provides a glaring example of the need for patient compliance.3 Symptoms in tuberculosis are substantially reduced with the administration of appropriate drugs within a few weeks. As a result, the patients stop taking the dosage. However, it is necessary that the patients continue to take the drugs for a much longer time (6 months to a few years) since, a lapse in the drug administration results in drug-resistant forms of the disease. If one could achieve an effective controlled release system, where the patient needs to take the drugs once every few weeks or months, patient compliance is likely to be a lot better, thus reducing the incidence of drug-resistant forms of the disease.
Another drug delivery system that has attracted a lot of attention involves targeted delivery of drug molecules to a specific cell type. The need for such a system is obvious in chemotherapy and radiotherapy of cancer patients. For example, the painful side effects of chemotherapy are attributed to the drug’s ability to kill cells indiscriminately, i.e., both cancer and healthy cells. The efficacy of these drugs will be enhanced and toxicity may be reduced, if a vehicle is developed that selectively delivers the drug molecules to tumor cells.
It is well known that a pharmaceutical agent can be targeted to the desired site or its release can be controlled by encapsulation within a polymer, lipid, or surfactant thus significantly improving the efficacy of the drug. The growing field of innovative polymer chemistry and sophisticated analytical chemistry combined with biomacromolecules has led to a variety of polymeric drug delivery systems that are suitable for in vivo studies. The vast array of research on polymer therapeutics includes macromolecular drugs, polymer-drug conjugates, polymer-protein conjugates and polymeric micelles containing the covalently bound drug.4,5 More recently, dendrimers have gained attention in this area, because of certain structural advantages that they offer. Dendrimers can be achieved with a precise molecular weight that is necessary for reproducible pharmacokinetic data, high density of surface functionalities that may allow a higher payload and well-defined structure that provides a handle to tune toxicity and release properties. The primary focus of this review is to highlight the use of dendrimer-based micelles in drug delivery. However, since polymeric micelle-based approach for controlled and targeted drug delivery is well established, we first give a brief account of those systems. Note that the systems discussed here are representative examples of micellar drug delivery vehicles based on macromolecules. We conclude by giving a perspective on the possible applications of our own amphiphilic dendrimer design in drug delivery. The intention of this review is to highlight the concepts and therefore the references are neither exhaustive nor inclusive of non-micellar systems.
Polymeric micelles as drug carriers
Micellization is a general phenomenon, studied widely in the field of drug delivery to increase the bioavailability of lipophilic drugs and nutrients. Macromolecules are better suited than small molecules for this purpose due to their lower critical micelle concentration (CMC) and higher stability.6 Polymeric micelles can physically entrap the hydrophobic drugs at the core that can be transported at concentrations exceeding their intrinsic water solubility. The hydrophilic periphery of the micelle renders the polymer water-soluble and provides a tight shell around the drug-loaded core. This minimizes drug degradation and harmful side effects, but increases bioavailability. Thus overall, polymeric micelles can give a better therapeutic profile. These macromolecular micelles are also very attractive in targeted drug delivery to cancer cells, because of the following reasons6: 1) the accumulation of polymeric micelles in tumors can be increased due to leaky vasculature of cancer zone. Such a passive targeting approach is possible due to the enhanced permeation and retention (EPR) effect of polymers.7 2) Polymeric micelles can be made sensitive to temperature or pH changes, which is potentially useful for targeted drug delivery in cancer, since many pathological processes in cancer tissues are accompanied with increase in temperature and/or acidity. 3) Ligands that are complementary to the receptors specific to cancer cells can also be attached to the hydrophilic unit of the micelle. This approach is known as active targeting.
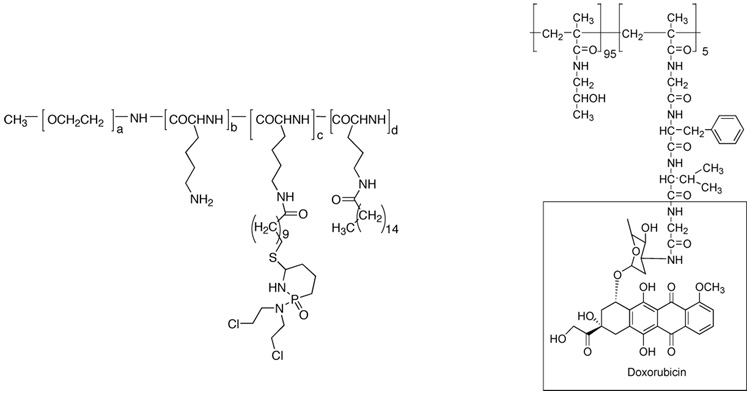
The drug can be encapsulated into a polymeric micelle in two ways – by covalent attachment (polymer-drug conjugate) or by physical encapsulation. In early 1980s Ringsdorf et al.8 and Kopecek et al.9 introduced the idea of using polymeric micelles for sustained drug release via polymer-drug conjugates. Here, poly(ethylene oxide)-poly(L-lysine) block copolymer based micelle was employed wherein sulfido derivatives of cyclophosphamide - an anticancer agent were attached to the poly(L-lysine) block unit8 as shown in Scheme 1. It was found that the rate of hydrolysis of cyclophosphamide to the active metabolite 4-hydroxy-cyclophosphamide varied with structure of the carrier. In another example, poly(ethylene oxide)-poly(aspartic acid) block copolymers were shown to form micelles after covalent attachment of hydrophobic drug adriamycin to poly (aspartic acid) block.12 These polymeric micelles showed enhanced accumulation on tumors, prolonged circulation time11 and reduced toxicity.12 The choice of linker in polymer-drug conjugates is critical, since it must be stable during circulation but should be allowed to be cleaved intratumorally. For example, HPMA copolymer-Gly-Phe-Leu-Gly-doxorubicin conjugates that have a peptide linker have been demonstrated for targeted delivery as shown in Scheme 1.13 This linker was found to be stable in the circulation, but was cleaved inside the tumor cells by an enzyme cathepsin B.
Scheme 1.
Structures of polymer-drug conjugates cyclophosphamide attached to poly(ethylene oxide)-poly(L-lysine) (left) and polymer-drug conjugate with a peptide linker
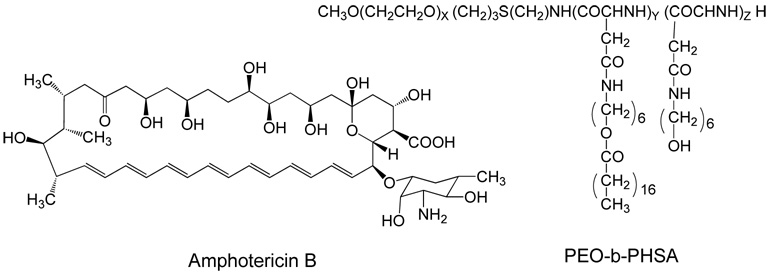
On the other hand, release of a physically encapsulated drug from the micelle would be easier and encapsulation can be achieved by simple mixing, avoiding synthetic procedures necessary for covalent attachment of the linker and drug. Amphotericin B (AmB) was physically encapsulated in poly(ethylene oxide)-poly(hydroxy stearyl L-aspartamide) block copolymer14 (Scheme 2). The encapsulation with this polymer was found to be better than with poly(ethylene oxide)-poly(β-benzyl L-aspartate),15 since the stearic acid component of the former polymer is more compatible with AmB. The toxicity was also found to be reduced. In another case, slow and prolonged release of doxorubicin hydrochloride was observed, when entrapped in oligo(methyl methacrylate)-poly(acrylic acid) block copolymer micelle.16
Scheme 2.
Structures of Amphotericin B (left) and the copolymer used for its physical encapsulation
Physical encapsulation is most suitable for controlled release systems since drug release is governed by diffusion and does not depend on cleavage of a linker that may be buried in core of the micelle. On the other hand, covalent attachment is useful for targeted delivery, since the drug molecule is less likely to be pre-maturely released. In order to eventually release the drug, a stimuli-responsive cleavable linker (by pH change or irradiation) can be used. Thus, it is clear that both these approaches offer complementary advantages to drug delivery.
Dendritic micelles as drug delivery vehicles
Dendrimers, the highly branched monodisperse macromolecules, have a large number of “tunable” surface groups and an interior that provides space as well as microenvironment suitable for host-guest chemistry.17,18 Dendritic micelles are generally unimolecular and do not suffer from even the low CMC that the linear polymer based micelles have. Also, these have been shown to be rapidly internalized into cells through endocytosis due to their nanometer-scale dimensions. By virtue of these unique features, dendrimers are being recently studied for applications in gene therapy and as drug carriers and as contrast agents in imaging.19,20
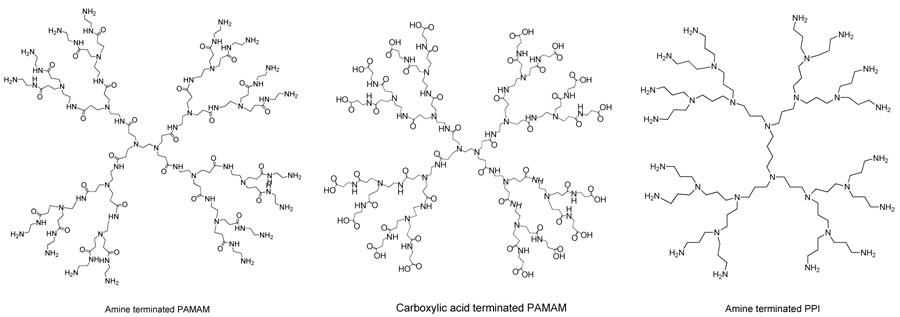
Poly(amidoamine) (PAMAM)21 and poly(propylene imine) (PPI),22 the commercially available dendrimers, have been widely studied for biological applications. As shown in Scheme 3, PAMAM dendrimers can have either amine or carboxylic groups and PPI dendrimers have amine groups on the periphery. These functional groups have a well-established chemistry and hence provide opportunity for attaching various functionalities through routine chemical transformations. These dendrimers can also be tailored to have a relatively hydrophobic interior for guest encapsulation23 and tertiary amines for non-covalent interaction. Despite these interesting features, their suitability to biological systems is not sufficiently established. In this direction, structure-biocompatibility relationship for PAMAM, poly (propylene imine) and poly(ethylene oxide) (PEO)- grafted carbosilane dendrimers (CSi-PEO) has been studied recently.24 The in vitro studies indicated that regardless of internal repeat unit structure, dendrimers with cationic end groups induced hemolysis and were cytotoxic above a concentration of 1mg/mL. However, anionic and CSi-PEO dendrimers were not hemolytic up to a concentration of 2 mg/mL, except for the higher generation carboxylate PAMAM dendrimers. Preliminary in vivo studies on 125I labeled PAMAM dendrimers showed that anionic dendrimers had much longer circulation time than their cationic counterparts. It was also observed that increased branching and a greater surface coverage of potentially toxic core by biocompatible end groups like PEO are necessary to make these dendrimers useful in biology. The ability of such poly(ethylene glycol) (PEG) functionalized dendrimers to sequester lipophilic guests has been reported, where PEG chains were attached to periphery of PAMAM dendrimers and effect of length of PEG arms on the solubility of pyrene in aqueous solutions of the dendrimers was studied.25 In another example, PEG-grafted PAMAM dendrimers were loaded with anticancer drugs like adriamycin and methotrexate with 6.5 and 26 molecules in fourth generation dendrimer, respectively. Release of these drugs was slow at low ionic strength; however, it was relatively rapid in isotonic solutions.26 Similarly, dendrimers can also be functionalized with biocompatible carbohydrate moieties. Mannose residues were attached to periphery of PAMAM dendrimers along with hydroxyl functional groups.27 The degree of mannose functionalization was controlled by varying the stoichiometry of the isothiocyanate derivative of the carbohydrate. These dendrimers were used as a model to study protein-carbohydrate interactions.
Scheme 3.
Structures of PAMAM and PPI dendrimers
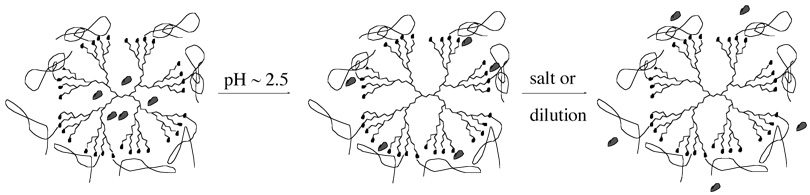
The periphery of PPI dendrimers has also been modified in different ways to develop pH sensitive controlled-release systems.28 The peripherally unmodified dendrimers also showed pyrene sequestering properties due to a hydrophobic interior and pyrene could be released by protonation of internal tertiary amines by lowering the pH.28 This however worked on a broad pH range. Introduction of hydrophilic quaternary ammonium groups on the periphery led to enhanced water solubility of the dendrimer as well as improved release properties.29 Release of pyrene in this case was observed within a narrow pH region. It was argued that quaternary ammonium groups seal the surface and delay the release of sequestered molecules. From NMR studies, it was observed that a significant release was due to protonation of tertiary amines in the intermediate layers. PEG units were attached to the periphery of PPI dendrimers to obtain a potentially biocompatible material with long circulation time in blood.30 These dendrimers were then used for solubilizing betamethasone valerate and betamethasone dipropionate as active ingredients. It was found that even at a low pH the guest molecules were not completely released; these molecules were apparently trapped in the peripheral PEG chains and complete release was obtained only upon dilution making them possible candidates for controlled drug release vehicles as illustrated in Figure 1. In another study, besides PEG chains guanidinium units were incorporated into the periphery to make them useful for targeted drug delivery.31 These doubly functionalized dendrimers were found to have higher loading capacity (11 wt%) for betamethasone valerate than that of simply PEGylated dendrimers (6 wt%) and unfunctionalized parent dendrimers (1.7 wt%). The drug could be completely released by addition of NaCl solution as a result of complexation between sodium ions and PEG chains. Even in isotonic solutions, a gradual release of drug was observed indicating the possible ‘uncontrolled leakage’ from PEGylated dendrimers when used in vivo.
Figure 1.
Schematic representation of stepwise probe release from PEG-modified PPI dendrimer
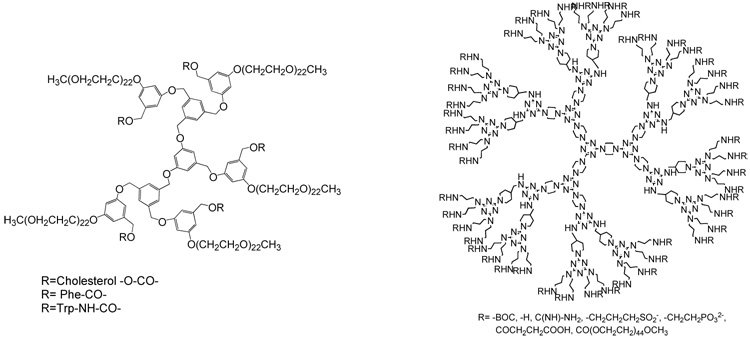
Attachment of PEG chains has been extended to benzyl ether and melamine-based dendrimers as well (Scheme 4). To the periphery of partially PEGylated benzyl ether dendrimers model drugs like cholesterol and amino acids were attached via hydrolyzable ester/carbonate/carbamate linkages for preliminary studies on water-soluble dendrimer-drug conjugates.32 The fully PEGylated benzyl ether dendrimers were used for physical encapsulation of a drug indomethacin. Encapsulation of the drug in the dendritic micelles was about 11 wt% and in vitro studies showed sustained release characteristics.33
Scheme 4.
Structures of PEGylated benzyl ether-model drug conjugate (left) and surface-modified melamine-based dendrimers
Dendrimers based on melamine have been modified on their periphery with different cationic and anionic groups and PEG units.34 Cationic dendrimers were found to be more cytotoxic and hemolytic than anionic and PEGylated species. The PEGylated dendrimers when evaluated in vivo showed no toxicity in doses up to 2.56 g/Kg i.p and 1.28 g/Kg i.v. Reduction in hepatotoxicity of methotrexate and 6-mercaptopurine, both anticancer drugs, was observed when encapsulated in melamine-based dendrimers besides improvement in their solubility.35
Designing controlled-release systems for hydrophilic drugs is an even greater challenge. In this direction, an attempt was made by synthesizing nanoparticles composed of multilayer deposition of poly(styrene sulphonate)(PSS)/PAMAM films on colloidal particles.36 Carboxyfluorescein, a hydrophilic dye, could be released from these systems to an extent of 90% in 4 h after an initial burst. The colloidal core could be removed by treatment with 0.1M HCl to afford hollow nanoparticles that further extended the potential of these materials.
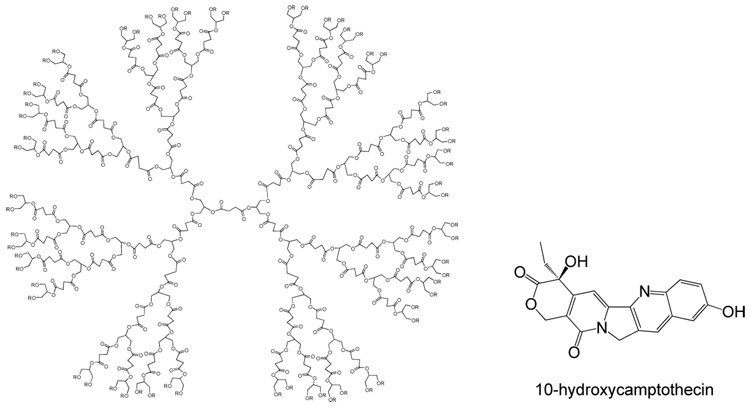
Another approach to obtain biocompatible dendrimers is to synthesize them from biocompatible monomers. In fact there are many reports on dendrimers and dendritic polymers made from monomers like glycerol37 aliphatic polyether-esters38,39 amino acids40 and carbohydrates.41 As an example, glycerol-lactic acid based42 and poly(glycerol succinic acid) (PGLSA) dendrimers43 have been synthesized for biomedical applications (Scheme 5). Hydroxyl terminated PGLSA dendrimers were first tested for their ability to encapsulate hydrophobic compounds by experiments with Reichardt’s dye. G4 dendrimer encapsulated a maximum of 2 dye molecules per dendrimer that was ~2000 fold improvement over solubility without the dendrimer. The carboxylated PGLSA dendrimer was further used to encapsulate a poorly water-soluble anticancer drug 10-hydroxycamptothecin (10-HCPT) and cytotoxicity assays on human breast cancer cells showed that the drug retained its activity upon encapsulation.
Scheme 5.
Structure of PGLSA dendrimer and hydrophobic anticancer drug 10-HCPT
It has been noted that it is difficult to control the release of entrapped molecules from a dendritic micelle as the drug molecules are either released rapidly or require harsh conditions for release.44 In an approach to overcome this problem, linear polymers and dendritic molecules have been combined to give a new class of polymeric micelle-based drug delivery vehicles. Linear-dendritic block copolymers have been synthesized that self-assemble to form micelles (Figure 2).45 Poly(ethylene oxide) was used as the hydrophilic linear block and polylysine/polyester dendrons with hydrophobic groups attached to their periphery via acid-labile (acetal) linkers as the dendritic block. The rate of hydrolysis of the acetal groups at pH 5 and that of release of the encapsulated dye was found to depend on structure and molecular weight of the copolymer and the dendritic backbone. A library of linear PEO-dendritic hybrids has also been prepared for possible drug-delivery applications.46
Figure 2.
Schematic representation of release of guest molecules from a pH sensitive micelle of linear-dendritic copolymer
Perspective
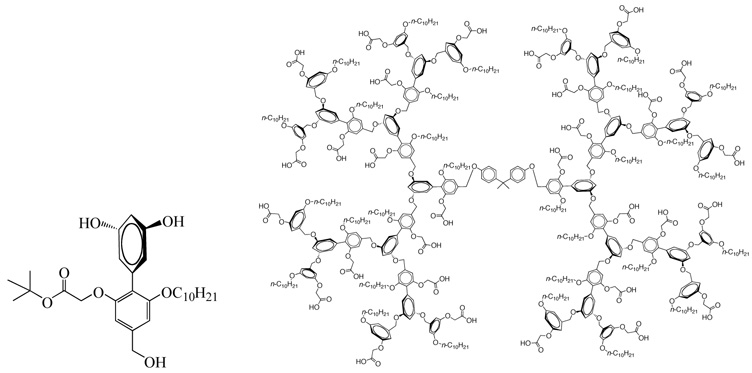
It is evident from the above discussion that dendritic micelles with anionic or PEG groups on the periphery are promising carriers for drug delivery. Further, functionalized biocompatible dendrimers will be attractive for multiple dendrimer-drug conjugates. It will be interesting not only to have highly functionalized dendrimers but also to direct the functionalities towards the concave interior of the dendrimer for better encapsulation of the drug. Recently, we introduced a new class of dendrimers that are facially amphiphilic.47 The biaryl monomer design, shown in Scheme 6, allows for the placement of hydrophilic and hydrophobic moieties at an orthogonal plane containing the AB2 functionalities. Thus, when the dendrimer is constructed, a solvophobic driving force helps direct the functional groups towards the dendritic interior that leads to a switch from micellar to reverse micellar conformation with change in solvent polarity, i.e. surrounding environment. Here, we use a carboxylic acid moiety as the hydrophilic group and a long carbon chain as the hydrophobic group. These dendrimers are soluble in aqueous KOH solutions as well as in toluene suggesting that these can form both normal and reverse micelles. Thus these molecules are capable of acting as hydrophobic and hydrophilic containers for a variety of guest molecules even at concentrations as low as 10−6 M of the dendrimer. We studied the sequestration of various hydrophobic and hydrophilic molecules like Reichardt’s dye, orange OT, and proflavin and found that the guest molecules experience highly apolar environment in the interior of micelles and polar environment in reverse micelles. From spectroscopic studies it was found that a G3 dendritic micelle could encapsulate ~8.5 dye molecules while the reverse micelle could sequester ~1.5 dye molecules per dendrimer.
Scheme 6.
The biaryl building block (left) and a G3 dendrimer synthesized from it.
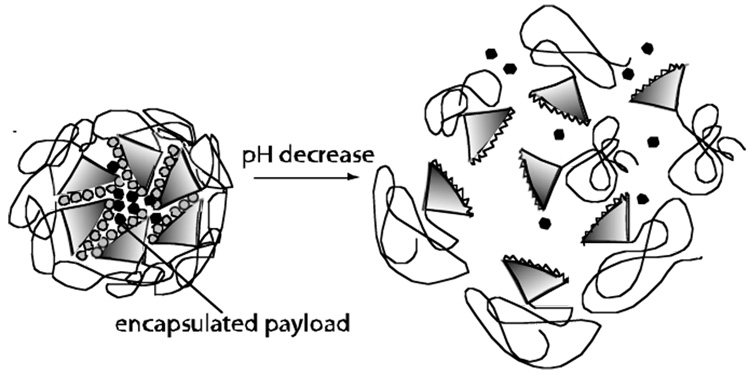
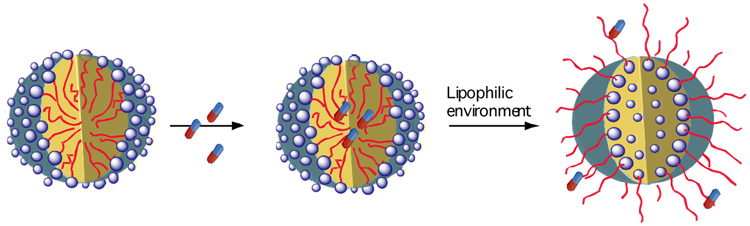
We have also prepared biaryl dendrimers where a triethylene glycol group replaces carboxylic group as the hydrophilic unit affording neutral amphiphilic dendritic micelles.48 These biaryl-based dendritic molecules with carboxylate or oligoethylene glycol moieties in every repeat unit are expected to be more biocompatible and less toxic. It is also supported by the fact that non-ionic surfactants like Tween 80 containing polyoxyethylene units have been approved by Food and Drug Administration. Also, using the methodology developed by us for sequencing dendrimers,49 we can control the number of carboxylates or oligoethyleneoxy units on periphery or in the intermediate layers of these dendrimers to tune the toxicity and release properties. Lipophilic drugs have been shown to be selectively cytotoxic towards cancer cells.50 Since the conformation of biaryl dendrimers can switch from a micellar structure to a reverse micellar structure with a change in environment (Figure 3), we expect that the encapsulated drug may be released upon reaching the targeted cells by adopting an inverted-micellar structure. The drug may be released slowly to enter the cell via diffusion leading to controlled delivery, whereas the built-in hydrophobicity and anionic groups can respond to the lipophilicity and acidity of malignant cells resulting in targeted delivery.
Figure 3.
Environment dependent conformational changes in biaryl-based amphiphilic dendrimers leading to probable targeted-drug release systems
It is interesting that a single dendrimer can act as both a micelle and a reverse micelle, since reverse micelles are also attractive from the point of view of mimicking biological systems and have applications in entrapping and stabilizing protein molecules in organic solvents.51 We have used a similar design to synthesize an amphiphilic homopolymer that exhibits solvent-dependent conformation changes.52 These amphiphilic polystyrenes have an advantage over the biaryl dendrimers in terms of ease of synthesis. It is our hope that a combination of dendritic and polymeric micelle-based drug carriers may augment the capabilities of the micellar drug delivery systems.
References
- 1.Bolten BM, DeGregorio T. Trends in development cycles. Nature Rev. Drug Discovery. 2002;1:335–336. doi: 10.1038/nrd805. [DOI] [PubMed] [Google Scholar]
- 2.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 3.Dutt M, Khuller GK. Chemotherapy of Mycobacterium tuberculosis infections in mice with a combination of isoniazid and rifampicin entrapped in poly (DL-lactide-co-glycolide) microparticles. J. Antimicrob. Chemother. 2001;47:829–835. doi: 10.1093/jac/47.6.829. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R. The dawning era of polymer therapeutics. Nature Rev. Drug Discovery. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 5.Duncan R. In: Handbook of Anticancer Drug Development. Budman DR, Calvert AH, Rowinski EK, editors. Baltimore: Lippincott Williams & Wilkins; 2003. pp. 239–260. [Google Scholar]
- 6.Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J. Controlled Release. 2001;73:137–172. doi: 10.1016/s0168-3659(01)00299-1. [DOI] [PubMed] [Google Scholar]
- 7.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and antitumor agent smancs. Cancer Res. 1986;6:6387–6392. [PubMed] [Google Scholar]
- 8.Bader H, Ringsdorf H, Schmidt B. Water soluble polymers in medicine. Angew. Makromol. Chem. 1984;123/124:457–485. [Google Scholar]
- 9.Kopecek J. Soluble Biomedical polymers. Polym. Med. 1977;7:191–221. [PubMed] [Google Scholar]
- 10.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjugate Chem. 1992;3:295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51:3229–3236. [PubMed] [Google Scholar]
- 12.Yokoyama M, Okano T, Sakurai Y, Naito M, Kataoka K. Influencing factors on in vitro micelle stability of adriamycin-block copolymer conjugates. J. Controlled Release. 1994;28:59–65. [Google Scholar]
- 13.Duncan R, Cable HC, Lloyd JB, Rejmanova P, Kopecek J. Polymers containing enzymatically degradable bonds. 7. Design of oligopeptide side-chains in poly[N-(2-hydroxypropyl)methacrylamide] copolymers to promote efficient degradation by lysosomal enzymes. Makromol. Chem. 1983;184:1997–2008. [Google Scholar]
- 14.Lavasanifar A, Samuel J, Kwon GS. Micelles of poly(ethylene oxide)-block-poly(hydroxy alkyl L-aspartamide): synthetic analogues of lipoproteins for drug delivery. J. Biomed. Mater. Res. 2000;52:831–835. doi: 10.1002/1097-4636(20001215)52:4<831::aid-jbm29>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Yu BG, Okano T, Kataoka K, Sardari S, Kwon GS. In vitro dissociation and antifungal efficacy and toxicity of amphotericin B-loaded poly(ethylene oxide)-block-poly(β-benzyl L-aspartate) micelles. J. Controlled Release. 1998;56:285–291. doi: 10.1016/s0168-3659(98)00095-9. [DOI] [PubMed] [Google Scholar]
- 16.Inoue T, Chen G, Nakamae K, Hoffman AS. An AB block copolymer of oligo(methyl methacrylate) and poly(acrylic acid) for micellar delivery of hydrophobic drugs. J. Controlled Release. 1998;51:221–229. doi: 10.1016/s0168-3659(97)00172-7. [DOI] [PubMed] [Google Scholar]
- 17.Zheng F, Zimmerman SC. Dendrimers in supramolecular chemistry: from molecular recognition to self-assembly. Chem. Rev. 1997;97:1681–1712. doi: 10.1021/cr9603892. [DOI] [PubMed] [Google Scholar]
- 18.Basu S, Sandanaraj BS, Thayumanavan S. Molecular recognition in dendrimers. In: Mark HF, editor. Encyclopedia of Polymer Science and Technology. 4th ed. John Wiley & Sons, Inc.; 2004. [Google Scholar]
- 19.Patri AK, Majoros IJ, Baker JR. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002;6:466–471. doi: 10.1016/s1367-5931(02)00347-2. [DOI] [PubMed] [Google Scholar]
- 20.Bosman AW, Janssen HM, Meijer EW. About dendrimers: Structure, physical properties, and applications. Chem. Rev. 1999;99:1665–1688. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 21.Tomalia DA, Naylor M, Goddard WA., III Starburst dendrimers: Molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem., Int. Ed. Engl. 1990;29:138–175. [Google Scholar]
- 22.Baars MWPL, Kleppinger R, Koch MHJ, Yeu S-L, Meijer EW. The localization of guests in water-soluble oligoethyleneoxy-modified poly(propylene imine) dendrimers. Angew. Chem. Int. Ed. 2000;39:1285–1288. doi: 10.1002/(sici)1521-3773(20000403)39:7<1285::aid-anie1285>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Watkins DM, Sayed-Sweet Y, Klimash JW, Turro NJ, Tomalia D. Dendrimers with hydrophobic cores and the formation of supramolecular dendrimer-surfactant assemblies. Langmuir. 1997;13:3136–3141. [Google Scholar]
- 24.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: Relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Controlled Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Morris JJ, Lopina ST. Polyethylene glycol–polyamidoamine dendritic micelle as solubility enhancer and the effect of the length of polyethylene glycol arms on the solubility of pyrene in water. J. Colloid Interface Sci. 2004;273:148–154. doi: 10.1016/j.jcis.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 26.Kojima C, Kono K, Maruyama K, Takagishi T. Synthesis of polyamidoamine dendrimers with poly(ethylene glycol) grafts and their ability to encapsulate anticancer drugs. Bioconjugate Chem. 2000;11:910–917. doi: 10.1021/bc0000583. [DOI] [PubMed] [Google Scholar]
- 27.Woller EK, Walter ED, Morgan JR, Singel DJ, Cloninger MJ. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl-functionalized dendrimers. J. Am. Chem. Soc. 2003;125:8820–8826. doi: 10.1021/ja0352496. [DOI] [PubMed] [Google Scholar]
- 28.Pistolis G, Malliaris A, Tsiourvas D, Paleos CM. Polypropylenimine dendrimers as pH-sensitive controlled-release systems. Chem. Eur. J. 1999;5:1440–1444. [Google Scholar]
- 29.Sideratou Z, Tsiourvas D, Paleos CM. Quaternized poly(propylene imine) dendrimers as novel pH-sensitive controlled-release systems. Langmuir. 1999;16:1766–1769. [Google Scholar]
- 30.Sideratou Z, Tsiourvas D, Paleos CM. Solubilization and release properties of PEGylated diaminobutane poly(propylene imine) dendrimers. J. Colloid Interface Sci. 2001;242:272–276. [Google Scholar]
- 31.Paleos CM, Tsiourvas D, Sideratou Z, Tziveleka L. Acid- and salt-triggered multifunctional poly(propylene imine) dendrimer as a prospective drug delivery system. Biomacromolecules. 2004;5:524–529. doi: 10.1021/bm030068h. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Kono K, Frechet JMJ. Water-soluble dendrimer-poly(ethylene glycol) starlike conjugates as potential drug carriers. J. Polym. Sci. Part A: Polym. Chem. 1999;37:3492–3503. [Google Scholar]
- 33.Liu M, Kono K, Frechet JMJ. Water-soluble dendritic unimolecular micelles: Their potential as drug delivery agents. J. Controlled Release. 2000;65:121–131. doi: 10.1016/s0168-3659(99)00245-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen H-T, Neerman MF, Parrish AR, Simanek EE. Cytotoxicity, hemolysis, and acute in vivo toxicity of dendrimers based on melamine, candidate vehicles for drug delivery. J. Am. Chem. Soc. 2004;126:10044–10048. doi: 10.1021/ja048548j. [DOI] [PubMed] [Google Scholar]
- 35.Neerman MF, Chen H-T, Parrish AR, Simanek EE. Reduction of drug toxicity using dendrimers based on melamine. Mol. Pharm. 2004;1:390–393. doi: 10.1021/mp049957p. [DOI] [PubMed] [Google Scholar]
- 36.Khopade AJ, Caruso F. Electrostatically assembled polyelectrolyte/dendrimer multilayer films as ultrathin nanoreservoirs. Nano Lett. 2002;2:415–418. [Google Scholar]
- 37.Frey H, Haag R. Dendritic polyglycerol: a new versatile biocompatible material. Rev. Mol. Biotech. 2002;90:257–267. doi: 10.1016/s1389-0352(01)00063-0. [DOI] [PubMed] [Google Scholar]
- 38.Seebach D, Herrmann GF, Lengweiler UD, Bachmann BM, Amrein W. Synthesis and enzymic degradation of dendrimers from (R)-3-hydroxybutanoic acid and trimesic acid. Angew. Chem. Int. Ed. 1996;35:2795–2797. [Google Scholar]
- 39.Ihre H, Padilla De Jesus OL, Frechet JMJ. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J. Am. Chem. Soc. 2001;123:5908–5917. doi: 10.1021/ja010524e. [DOI] [PubMed] [Google Scholar]
- 40.Sadler K, Tam JP. Peptide dendrimers: applications and synthesis. Rev. Mol. Biotech. 2002;90:195–229. doi: 10.1016/s1389-0352(01)00061-7. [DOI] [PubMed] [Google Scholar]
- 41.Turnbull WB, Pease AR, Stoddart JF. Synthetic carbohydrate dendrimers, Part 8. Toward the synthesis of large oligosaccharide-based dendrimers. ChemBioChem. 2000;1:70–74. doi: 10.1002/1439-7633(20000703)1:1<70::AID-CBIC70>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 42.Carnahan MA, Grinstaff MW. Synthesis and characterization of polyether-ester dendrimers from glycerol and lactic acid. J. Am. Chem. Soc. 2001;123:2905–2906. doi: 10.1021/ja005726+. [DOI] [PubMed] [Google Scholar]
- 43.Morgan MT, Carnahan MA, Immoos CE, Ribeiro AA, Finkelstein S, Lee SJ, Grinstaff MW. Dendritic molecular capsules for hydrophobic compounds. J. Am. Chem. Soc. 2003;125:15485–15489. doi: 10.1021/ja0347383. [DOI] [PubMed] [Google Scholar]
- 44.Gillies ER, Frechet JMJ. Dendrimers and dendritic polymers in drug delivery. Drug Discovery Today. 2005;10:35–43. doi: 10.1016/S1359-6446(04)03276-3. [DOI] [PubMed] [Google Scholar]
- 45.Gillies ER, Jonsson TB, Frechet JMJ. Stimuli-responsive supramolecular assemblies of linear-dendritic copolymers. J. Am. Chem. Soc. 2004;126:11936–11943. doi: 10.1021/ja0463738. [DOI] [PubMed] [Google Scholar]
- 46.Gillies ER, Frechet JMJ. Designing macromolecules for therapeutic applications: polyester dendrimer-poly(ethylene oxide) "bow-tie" hybrids with tunable molecular weight and architecture. J. Am. Chem. Soc. 2002;124:14137–14146. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 47.Vutukuri D, Basu S, Thayumanavan S. Dendrimers with both polar and apolar nanocontainer characteristics. J. Am. Chem. Soc. 2004;126:15636–15637. doi: 10.1021/ja0449628. [DOI] [PubMed] [Google Scholar]
- 48.Bharathi P, Zhao H, Thayumanavan S. Toward globular macromolecules with functionalized interiors: design and synthesis of dendrons with an interesting twist. Org. Lett. 2001;3:1961–1964. doi: 10.1021/ol016064b. [DOI] [PubMed] [Google Scholar]
- 49.Sivanandan K, Sandanaraj B, Thayumanavan S. Sequences in dendrons and dendrimers. J. Org. Chem. 2004;69:2937–2944. doi: 10.1021/jo049878p. [DOI] [PubMed] [Google Scholar]
- 50.Christman JE, Miller DS, Coward P, Smith LH, Teng NN. Study of selective cytotoxic properties of cationic, lipophilic mitochondrial-specific compounds in gynecologic malignancies. Gynec. Oncology. 1990;39:72–79. doi: 10.1016/0090-8258(90)90402-7. [DOI] [PubMed] [Google Scholar]
- 51.Chang G-G, Huang T-M, Hung H-C. Reverse micelles as life-mimicking systems. Proc. Natl. Sci. Counc. ROC(B) 2000;24:89–100. [PubMed] [Google Scholar]
- 52.Basu S, Vutukuri D, Shyamroy S, Sandanaraj B, Thayumanavan S. Invertible amphiphilic homopolymers. J. Am. Chem. Soc. 2004;126:9890–9891. doi: 10.1021/ja047816a. [DOI] [PubMed] [Google Scholar]