Abstract
We examined the mechanisms of kainate (KA) induced modulation of GABA release in rat prefrontal cortex. Pharmacologically isolated IPSCs were recorded from visually identified layer II/III pyramidal cells using whole cell patch clamp techniques. KA produced an increase in evoked IPSC amplitude at low nanomolar concentrations (100–500 nM). The frequency but not the amplitude of miniature (m) IPSCs was also increased. The GluR5 subunit selective agonist (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA) caused an increase in mIPSC frequency whereas (3S,4aR,6S,8aR)-6-(4-carboxyphenyl)methyl-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid (LY382884), a selective GluR5 subunit antagonist, inhibited this facilitation. Philanthotoxin-433 (PhTx) blocked the effect of KA, indicating involvement of Ca2+-permeable GluR5 receptors. No IPSC facilitation was seen when Ca2+ was omitted from the bathing solution. Facilitation was observed when slices were preincubated in ruthenium red or high concentrations of ryanodine, but was inhibited with application of thapsigargin. The IP3 receptor (IP3R) antagonists diphenylboric acid 2-amino-ethyl ester (2-APB) (15 µM) and Xestospongin C (XeC) blocked IPSC facilitation. These results show that activation of KA receptors (KARs) on GABAergic nerve terminals results is linked to intracellular Ca2+ release via activation of IP3, but not ryanodine, receptors. This represents a new mechanism of presynaptic modulation whereby Ca2+ entry thru Ca2+-permeable GluR5 subunit containing KARs activates IP3Rs receptors leading to an increase in GABA release.
Keywords: neocortex, kainate receptors, IP3, IPSCs, modulation, GluR5
1. Introduction
Prefrontal cortex, like other parts of neocortex, contains extensively interconnected networks of pyramidal cells and interneurons (Bartho et al., 2004; Douglas and Martin, 2004). Feedback inhibition via GABAergic interneurons provides a stabilizing influence and prevents runaway excitation (Galarreta and Hestrin, 1998; Varela et al., 1999). It is well established that cortical networks are modulated via dopaminergic (Bjorklund and Lindvall, 1984), noradrenergic (Kawaguchi and Shindou, 1998) and cholinergic (Mesulam et al., 1983) inputs. Control over local cortical circuits is also exerted by metabotropic glutamate (Bandrowski et al., 2003) and GABAB receptors (Sutor and Luhmann, 1998). It is becoming clear that presynaptic KARs also modulate cortical inhibitory synaptic transmission (Ali et al., 2001; Ren et al., 2007).
KARs are widely expressed in the neocortex where five different KAR subunits are found, glutamate receptors 5, 6, 7 (GluR5–7) and kainate receptors 1 and 2 (KA1 and KA2) (Wisden and Seeburg, 1993). GluR5–7 are known to form functional homomeric glutamate receptors (Hollmann and Heinemann, 1994; Bettler and Mulle, 1995; Schiffer et al., 1997). Functional KA1 and KA2 homomeric receptors are not found (Werner et al., 1991; Herb et al., 1992) but these subunits can co-assemble with GluR5–7 to make channels with unique properties (Hollmann and Heinemann, 1994; Chittajallu et al., 1999). KARs are found both pre- (Kidd et al., 2002; Ali et al., 2001) and postsynaptically (Ali, 2003; Eder et al., 2003) in the neocortex. Presynaptic receptors can facilitate both glutamatergic (Campbell et al., 2007) and inhibitory GABAergic transmission (Xu et al., 2006; Ren et al., 2007). The precise mechanism whereby presynaptic KARs modulate GABA release in the neocortex is not clear and the role of specific subunits has not been established.
Neurotransmitter release can be altered by calcium release from intracellular stores (Meldolesi, 2001; Rose and Konnerth, 2001; Lauri et al., 2003; Simkus and Stricker, 2002). Such liberation from the endoplasmic reticulum (ER) is mediated through RyRs and IP3Rs (Berridge, 1998). Both function as calcium-permeable channels in the ER membrane, the opening of which is promoted by cytosolic calcium, leading to the phenomenon of Ca2+-induced Ca2+-release (CICR) (Friel and Tsien, 1992; Nakamura et al., 1999). In addition to Ca2+, the gating of IP3Rs requires IP3, an intracellular messenger that is generated through a Gq-coupled signal transduction pathway. Ca2+ and IP3 can thus function as chemical computational signals, mediating complex, bidirectional interactions between the plasma membrane and ER that underlie diverse processes, including excitability, neurotransmitter release, plasticity, and gene transcription (Berridge, 1998).
Presynaptic activation of CICR is known to regulate neurotransmitter release (Narita et al., 1998, 2000) and has been implicated in some (Lauri et al., 2005) but not all (Breustedt and Schmitz 2004) studies of KAR induced facilitation. We demonstrate the presence of functional presynaptic KARs in the prefrontal cortex that are involved in the modulation of GABAergic transmission. In contrast to previous studies showing IPSC depression (Clarke et al., 1997; Rodriguez-Moreno et al., 1997; Frerking et al., 1998; Min et al., 1999; Ali et al., 2001; Cossart et al., 2001), we observe facilitatory effects of KA on IPSCs. This facilitation involves the GluR5 subunit and CICR from intracellular stores. Our results indicate a novel mechanism whereby KAR activation leads to an IP3 receptor mediated increase in Ca2+ release from intracellular stores producing IPSC facilitation in layer II/III pyramidal cells.
2. Materials and Methods
2.1 Slice preparation
Slices from 18–25 day-old rat neocortex were prepared using procedures similar to those described previously (Campbell et al., 2007). Animals were handled and housed according to the NIH Committee on Laboratory Animal Resources guidelines. All experimental protocols were approved by the University of Alabama Institutional Animal Care and Use Committee. Every effort was made to minimize pain and discomfort. In brief, rats of either sex were anesthetized with ketamine (100 mg/kg) and decapitated. The brain was quickly removed and placed in ice-cold oxygenated saline containing (in mM): 125 NaCl, 3.5 KCl, 0.5 CaCl2, 3.5 MgCl2, 26 NaHCO3 and 10 D-glucose. A block of frontal association cortex (Paxinos and Watson, 1997) was prepared and cut in 300 µm thick slices using an Oxford Series 1000 Vibratome (Ted Pella Inc., Redding CA). Slices were initially stored at 37 °C for one hour. After an additional recovery period of at least 1 hr at room temperature, individual slices were transferred to a recording chamber mounted on the stage of a Leica DM LFSA (Leica Microsystems Wetzlar GMBH, Wetzlar, Germany) microscope equipped with infrared Nomarski differential interference contrast optics. The chamber was continuously perfused with oxygenated saline at a rate of 2–4 ml/min at 32 °C. For whole-cell patch clamp recordings, individual cells were visualized using a Leica 40X, .75 NA long-working distance water immersion objective. Images were detected using an infrared-sensitive video camera (Newvicon C2400-07-C, Hamamatsu, Japan) and displayed on a video monitor. Pyramidal neurons in layers II/III were identified during recording by their depth below the pial surface, their regular spiking properties, pyramidal shape and prominent apical dendrites.
2.2 Solutions and chemicals
The extracellular recording saline had the following composition (in mM): 125 NaCl, 3.5 KCl, 1.25 NaH2PO4, 2.5 CaCl2, 1.3 MgSO4, 26 NaHCO3, and 10 D-glucose. The patch pipette solution had the following composition (in mM): 134 KCl, 0.5 ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N',N',-tetraacetic acid (EGTA), 2 Mg-ATP, 0.2 NaGTP and 10 HEPES. The pH was adjusted to 7.3 with 1M KOH and osmolarity was adjusted to 290–300 mOsm with sucrose. Cadmium, 2-APB, PhTx, ryanodine and thapsigargin were obtained from Sigma-Aldrich (St. Louis, MO). D-2-amino-5-phosphonovalerate (D-APV), 4-(8-Methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepine-5-yl)-benzenamine hydrochloride (GYKI52466), (S)-4-carboxyphenylglycine ((S)-4-CPG), KA and (2S)-(+)-5,5-Dimethyl-2-morpholineacetic acid (SCH50911) were purchased from Tocris Cookson Inc. (Ellsville, MO). XeC was purchased from Calbiochem (San Diego, CA, USA). LY382884 was a gift from Eli Lilly (Indianapolis, IN, USA).
2.3 Stimulation
Synaptic responses were evoked with a bipolar stimulating electrode (twisted pair of 25 µm Formvar insulated nichrome wires) positioned 150–200 µm below the recording pipette. Stimulation pulses were 50–200 µA in amplitude and 80–100 µs in duration. A series of increasing stimulations were applied until stable IPSCs were evoked. This stimulation level was termed the threshold value. A stimulation frequency of 0.05 Hz was used to establish control responses. Averages of 10 consecutive responses were routinely collected and displayed. IPSC amplitudes were measured as the difference between baseline and peak.
2.4 Recording and analysis
Recordings were obtained at 32°C using whole-cell patch-clamp techniques. Patch pipettes were pulled from borosilicate glass capillaries on a Narishige Model PP-83 puller and had resistances of 3–4 MΩ. Whole-cell recordings were made in voltage-clamp mode using an Axopatch 1B amplifier (Molecular Devices, Sunnyvale, CA), controlled by Clampex 8.0 software via a Digidata 1322A interface (Molecular Devices). Evoked responses were filtered at 2 kHz, digitized at 10 kHz and analyzed using Clampfit 8.0 software (Molecular Devices). Spontaneous and miniature IPSCs were analyzed with Mini Analysis Program (Synaptosoft, Inc., Leonia, NJ). Continuous records, five minutes in duration, of IPSCs were obtained before, during and after kainate application. The root mean square (RMS) value of the baseline noise was determined. An amplitude threshold of 2–3 times the RMS noise level was used. Events exceeding this amplitude threshold and having rise times of less than 1.2 ms and decay time less than 20 ms were automatically detected. Subsequent visual analysis was used to verify each event as an IPSC. Cumulative probability plots were used using routines available in the Mini Analysis program. Data are expressed as mean ± SEM. Statistical analysis of response amplitudes before, during, and after drug applications was carried out using a two-tailed Student’s t-test with Statmost software (Data-Most). p < 0.05 was considered significant.
3. Results
3.1 Kainate facilitation of evoked IPSCs
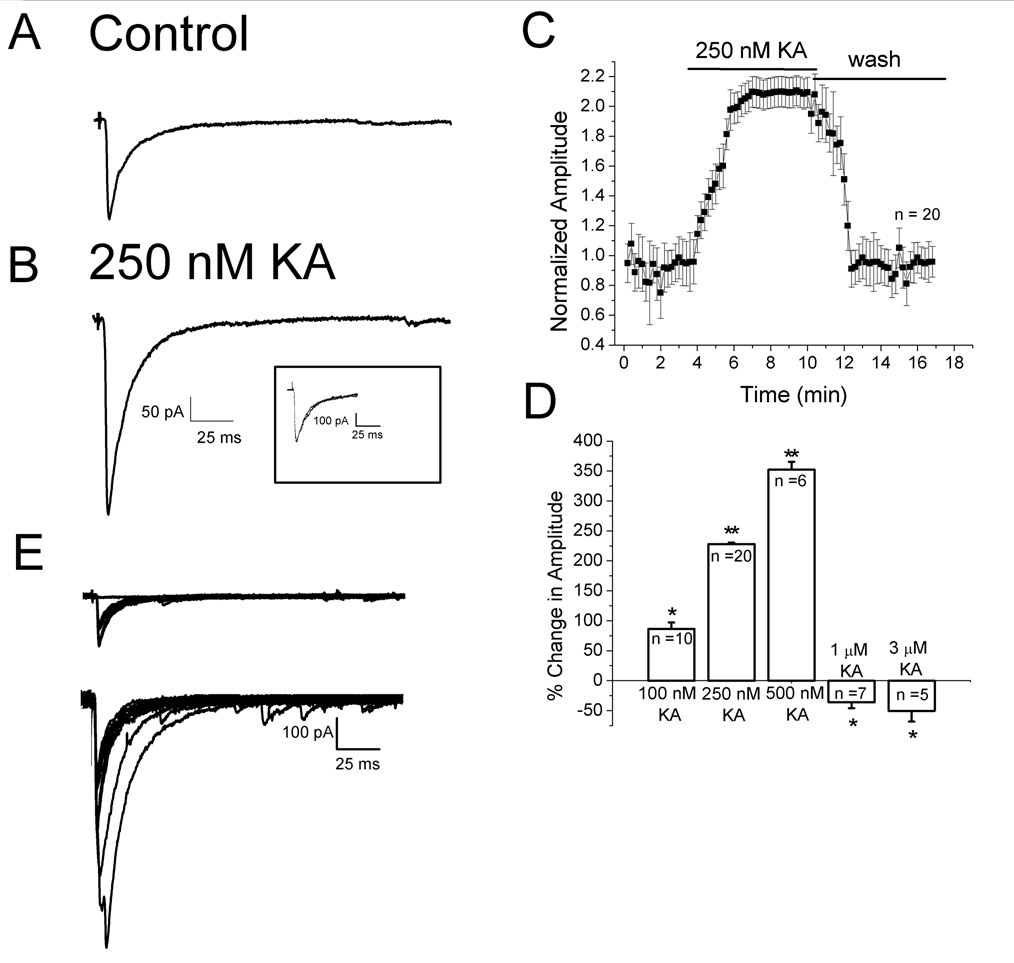
Under direct visualization, layer II–III pyramidal neurons in prefrontal cortex were studied under voltage clamp conditions. In order to test the effect of KA on pharmacologically isolated evoked IPSCs, NMDA- and AMPA-mediated EPSCs were blocked with 50 µM D-APV and 50 µM GYKI52466, respectively. GABAB receptors were blocked with 2 µM SCH50911. Synaptic responses were elicited by intracortical stimulation near the layer III/IV border. An example of a typical evoked IPSC is shown in Fig. 1A. Responses began after a short synaptic delay, had a rapid rising phase and decayed exponentially. Evoked currents were blocked by 10 µM bicuculline, a GABAA receptor antagonist, suggesting that the observed currents were mediated through activation of GABAA receptors (data not shown). An increase in amplitude of the evoked response was seen after bath application of 250 nM KA (Fig. 1B). The inset shows the records scaled to the same peak amplitude and superimposed. No change in the decay kinetics upon application of KA was observed. A summary plot of the effects of 250 nM KA, as a function of time, is shown in Fig. 1C. IPSC amplitude increased during KA application and recovered upon washing. IPSC facilitation was seen with KA concentrations from 100–500 nM whereas depression of IPSCs was observed after bath application of 1–10 µM KA (Fig. 1D). High concentrations of kainate also produced an increase in holding current, as described previously (Campbell et al., 2007). At 1 µM, KA produced a change in holding current of −90.32 ± 8.34 pA (n = 7). When applied in the presence of 20 µM CNQX, the change in holding current observed with 1 µM KA was −10.1 ± 2.5 pA (n = 4). An inward current of −175.24 ± 15.46 pA was seen with application of 3 µM KA. When 3 µM KA was applied in the presence of CNQX, an inward current of −8.6 ± 3.1 pA (n = 3) was observed. The results with CNQX suggest that the changes in holding current induced by 1–3 µM KA are due to activation of postsynaptic kainate receptors.
Figure 1. Concentration dependent effects of KA on evoked IPSC amplitudes.
A: specimen record of IPSCs evoked under control conditions. B: response to same stimulation after bath application of 250 nM KA. Scale bar applies to A and B. Each trace is an average of 10 traces. Insert shows responses scaled to the same peak amplitude. No change in kinetics was observed. Cells were held at −70 mV. C: plot of IPSP amplitude as a function of time before during and after 250 nM KA application (n = 20). D: histogram showing effects of different concentrations of KA on IPSCs. Low nM concentrations of KA facilitated IPSC amplitudes whereas higher µM concentrations depressed evoked IPSCs. E: KAR activation decreased the failure rate. Top panel, evoked IPSCs are shown superimposed. 35% of responses were synaptic failures in control conditions. Lower panel, in the presence of 250 nM KA synaptic failures were not observed. * indicates p<0.05; ** indicates p<0.01.
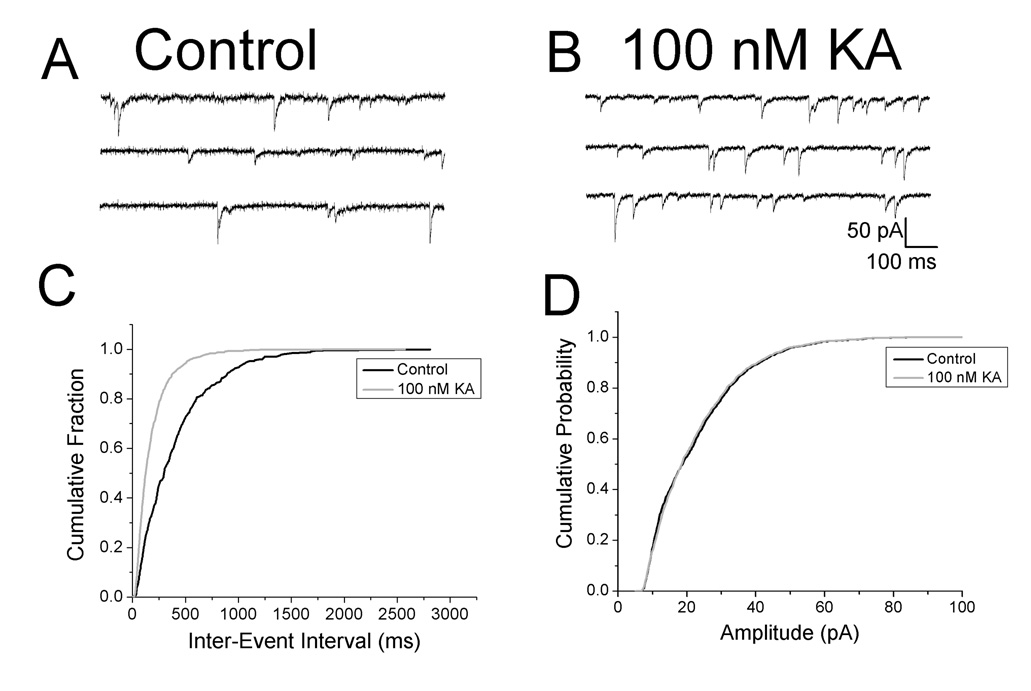
If low concentrations of KA were acting presynaptically to enhance transmitter release, a decrease in the number of failures would be expected, as reported at inhibitory synapses in the hippocampus (Jiang et al., 2001). In control conditions, synaptic failures occurred 35% of the time (Fig. 1E, upper panel). Failures were not observed after application of 250 nM KA (Fig. 1E, lower panel). Overall, the failure rate was 35 ± 0.4% under control conditions and decreased to 1.4 ± 0.2% (n = 20) in the presence of KA. We also reasoned that if facilitatory KARs were present on the nerve terminals of GABAergic interneurons, KA should increase the frequency of mIPSCs without affecting their amplitude. To block Na+ dependent action potentials, 1 µM TTX was bath applied. As shown in Fig. 2A and B, mIPSC frequency increased in the presence of 100 nM KA. Cumulative probability plots demonstrated that mIPSC frequency was significantly increased (Fig. 2C, 1.38 ± 0.5 Hz control vs. 2.34 ± 0.4 Hz in KA, p < 0.05, n = 8) whereas amplitudes were not affected (Fig. 2D, 34.84 ± 4.1 pA control vs. 39.20 ± 7.1 pA in KA, p > 0.05, n = 8). The results of the experiments with number of failures and mIPSC frequency suggest that KA was acting presynaptically to facilitate GABA release.
Figure 2. KA increases mIPSC frequency.
A: specimen records showing mIPSCs under control conditions. B: mIPSCs recorded in same cell in presence of 100 nM KA. C: cumulative probability plots of mIPSC inter-event intervals before and during bath application of KA. A leftward shift was observed in the presence of KA, indicating an increase in mIPSC frequency. D: cumulative probability plot of mIPSC amplitudes. There was no change in the amplitude of mIPSCs with KA application. Similar results were seen in all cells tested (n = 8).
3.2 Role of extracellular Ca2+ in kainate mIPSC facilitation
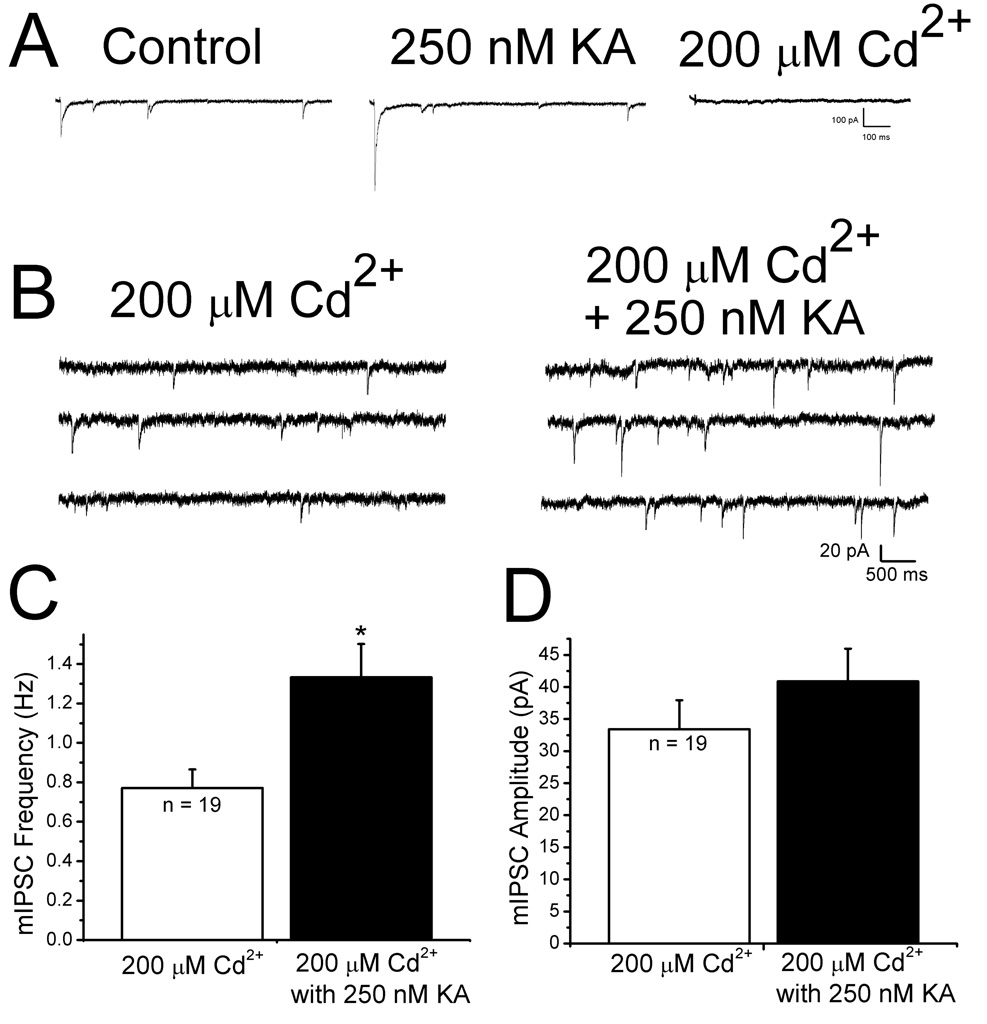
KARs could depolarize GABAergic terminals and facilitate IPSCs via activation of voltagedependent Ca2+ channels (Ren et al., 2007). To test this, 200 µM Cd2+ was applied. As a positive control for the efficacy of Cd2+ in blocking presynaptic Ca2+ channels, we examined its effect on evoked IPSCs. Evoked IPSCs that had been facilitated by 250 nM KA were completely blocked upon application of 200 µM Cd2+ (Fig. 3A), indicating that presynaptic Ca2+ channels were blocked. In the presence of 200 µM Cd2+, Ca2+-independent mIPSCs (Nicola and Malenka, 1997) were observed to occur at a low rate. Under these conditions, 250 nM KA significantly increased the frequency of mIPSCs (control: 0.8 ± 0.1 Hz, KA: 1.5 ± 0.3 Hz, p < 0.05, n = 19, Fig. 3C) without a change in the mean amplitude (Fig. 3D) or decay time constant. Since Cd2+ did not block KA facilitation of mIPSC frequency, the enhanced rate of mIPSCs was not due to Ca2+ influx through voltage-dependent Ca2+ channels.
Figure 3. KA increases mIPSC frequency in the presence of cadmium.
A: representative traces of evoked IPSCs under control conditions (leftmost trace), in the presence of 250 nM KA (middle trace) and after application of 200 µM Cd2+ (rightmost trace). Complete blockade of evoked IPSCs in the presence of 200 µM cadmium was used as a positive control for the efficacy of cadmium in blocking calcium dependent release. Each trace is an average of ten responses. B: specimen records of mIPSCs recorded in the presence of cadmium before (left) and after bath application of 250 nM KA (right). mIPSC frequency was increased by KA. C: summary bar graphs showing mIPSC frequency in the presence of 200 µM Cd2+ before (white column) and after addition of 250 nM KA (black column). There was a significant increase in the frequency of mIPSCs in the presence of 200 µM Cd2+ and 250 nM KA. D: summary bar graphs showing that KA did not affect mIPSC amplitude in the presence of Cd2+. These results show that activation of voltage-dependent calcium channels is not required for the KA-induced mIPSC frequency facilitation. * indicates p<0.05
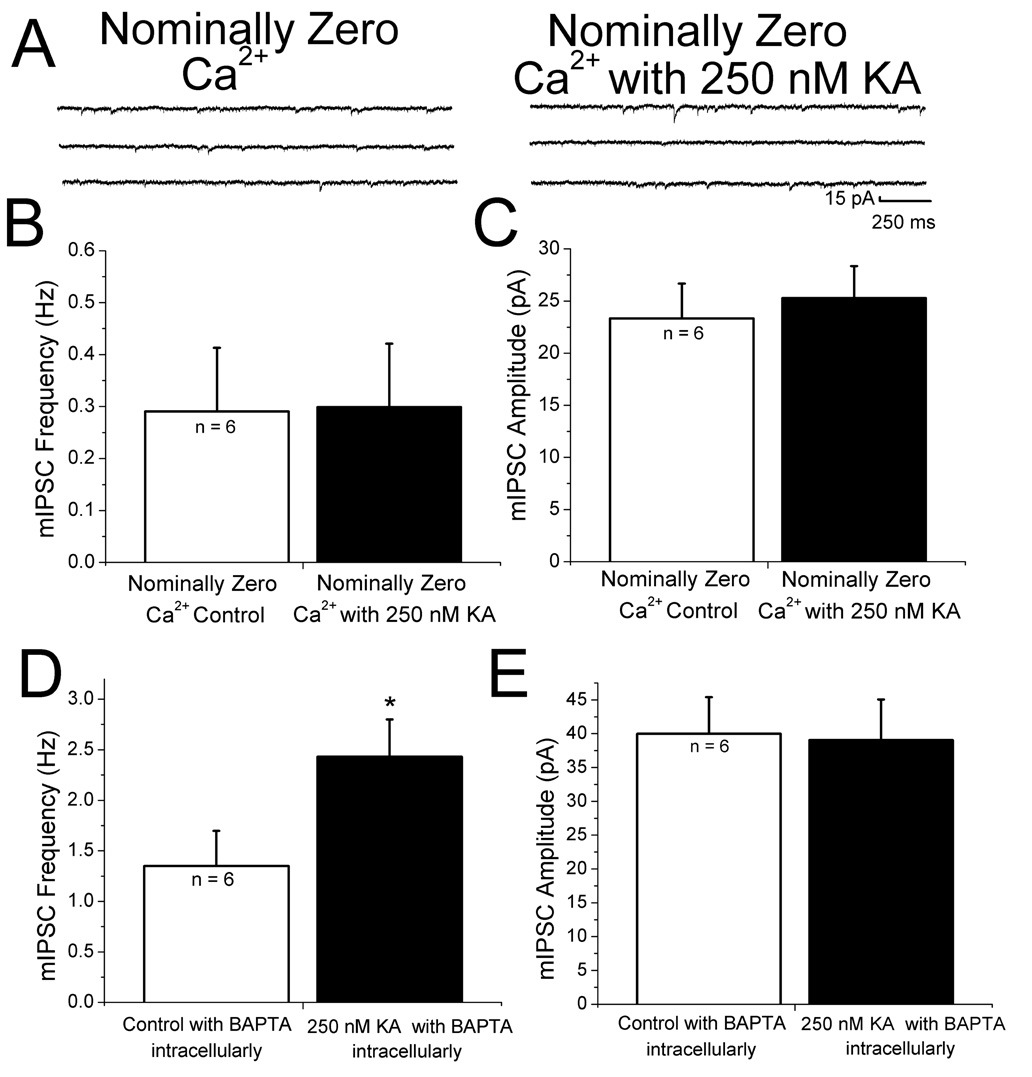
Transmitter release is highly dependent on Ca2+. Both extra- and intracellular Ca2+ stores contribute to regulation of mEPSCs in neocortex (Simkus and Stricker 2002). We therefore examined the roles of both Ca2+ sources in KAR-mediated facilitation. To test if extracellular Ca2+ was necessary for KA-induced increases in mIPSC frequency, slices were bathed in a solution containing no added Ca2+ and 4.8 mM MgCl2 (to maintain the total divalent cation concentration constant) for 25 minutes prior to recording. Under these conditions, no increase in miniature IPSC frequency was observed with bath application of 250 nM KA (Fig. 4A and B). There also was no significant change in amplitude (Fig. 4C). These results show that Ca2+ entry from the extracellular space is necessary for KA-induced changes in IPSC frequency. We next addressed the question of whether postsynaptic Ca2+ influx was involved in KA-induced facilitation. The Ca2+ chelator BAPTA (10 mM) was included in the recording pipette and allowed to diffuse into the cell for 20 minutes after obtaining a stable whole-cell recording. KA-induced increases in miniature IPSC frequency without a significant change in amplitude were still observed in cells recorded with BAPTA in the recording pipette (Fig. 4D and E) and were comparable to those observed without BAPTA (Fig. 2C and D). These results suggest that the increase in IPSC amplitude induced by KA does not require Ca2+ elevation in the postsynaptic cell.
Figure 4. Effects of changes in extracellular and intracellular Ca2+ on KA-induced changes in IPSCs.
A: in saline containing nominally zero extracellular Ca2+ and 4.8 mM Mg+, mIPSC frequency was not changed by 250 nM KA. B: summary graph of mIPSC frequency data showing that no significant change in the mIPSC frequency. C: summary of results of KA on mIPSC amplitude in nominally zero Ca2+. No effect of KA was observed under these conditions. D,E: summary plots showing that chelating postsynaptic calcium with 10 mM BAPTA did not affect the ability 250 nM KA to facilitate mIPSC frequency without changing amplitudes. * indicates p < 0.05.
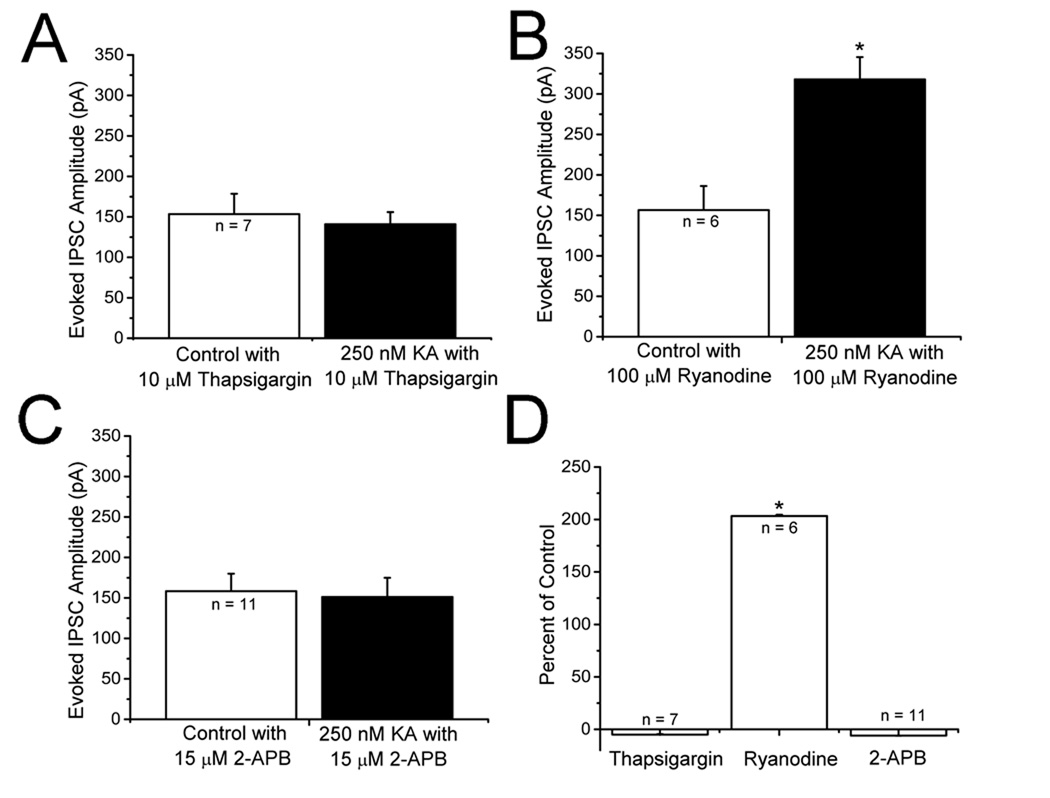
Knowing that extracellular Ca2+ is necessary for the KAR mediated facilitation of IPSCs and postsynaptic calcium is not, we tested for the involvement of intracellular Ca2+ stores. Slices were incubated in 10 µM thapsigargin, an inhibitor of the endosomal Ca2+-ATPase that prevents store refilling (Thastrup et al., 1990), for 30 minutes before experiments were conducted. In the presence of 10 µM thapsigargin, 250 nM KA did not produce an increase in evoked IPSC amplitudes (Fig. 5A). Under these conditions, application of 250 nM KA did not facilitate mIPSC frequency (1.52 ± 0.02 Hz control vs 1.49 ± 0.03 Hz in thapsigargin plus KA, p > 0.05, n = 9) and did not change amplitudes (44.65 ± 4.26 pA control versus 45.42 ± 6.12 pA in thapsigargin plus 250 nM KA, n = 9). These results suggest that intracellular Ca2+ stores contribute to KA facilitation of evoked IPSCs. Release of Ca2+ from internal stores is mediated both by RyRs and IP3Rs. Ryanodine sensitive stores have been previously reported to be involved in KA facilitation in some studies (Lauri et al., 2003) but not others (Breustedt and Schmitz, 2004). Bath application of 100 µM ryanodine, a concentration that blocks RyRs (Ehrlich et al., 1994; Liu et al., 2005), did not affect facilitation of evoked IPSC amplitudes (Fig. 5B). Ryanodine did not block KA induced mIPSC frequency facilitation (1.53 ± 0.08 Hz control vs 3.65 ± 0.11 Hz in ryanodine plus 250 nM KA, n = 7, p <0.05). Amplitudes were not affected by KA (42.16 ± 2.21 pA control vs 41.62 ± 2.31 pA in ryanodine plus 250 nM KA, n = 7, p >0.05) We also tested the effect of another ryanodine receptor antagonist, ruthenium red, and found that it did not block KA-induced facilitation of evoked IPSC amplitudes (134.65 ± 56.6 pA control vs 263.70 ± 90 pA in ruthenium red plus KA, n = 5, p < 0.05,). This suggests that RyRs are not involved in the observed IPSC facilitation.
Figure 5. Involvement of intracellular Ca2+ stores in KAR-induced changes in evoked IPSC amplitude.
A: bath application of 250 nM KA did not increase evoked IPSC amplitudes in the presence of thapsigargin. Slices were incubated with 10 µM thapsigargin for 30 minutes before recordings were conducted. B: application of KA in the presence of 100 µM ryanodine produced a significant increase in evoked IPSC amplitude, suggesting that ryanodine receptors are not involved. C: in the presence of 15 µM 2-APB, to block the IP3 receptors, 250 nM KA did not change eIPSC amplitude. D: summary graph showing that intracellular Ca2+ stores and IP3 receptors are involved in the KAR-mediated facilitation of IPSCs in neocortex. * indicates p < 0.05.
Release of Ca2+ from intracellular stores by IP3Rs represents an additional potential modulatory mechanism. To test this, we applied diphenylboric acid 2-amino-ethyl ester (2-APB), which blocks IP3Rs (Maruyama et al., 1997). We found that, in the presence of 15 µM 2-APB, evoked IPSC amplitudes were not changed by application of 250 nM KA (Fig. 5C). 2-APB also blocked facilitation of mIPSC frequency (1.42 ± 0.23 Hz control vs 1.46 ± 0.24 Hz in 2-APB plus 250 nM KA, n = 7, p >0.05) Amplitudes were not changed (47.86 ± 4.31 pA control vs 48.65 ± 5.32 pA in 2-APB plus 250 nM KA, n = 7, p>0.05). In other preparations, 2-APB has been suggested to act at additional, non IP3R, sites (Bootman et al., 2002). We therefore examined the effects of another IP3R antagonist, XeC (Gafni et al., 1997; Ta et al., 2006). KA did not facilitate mIPSC frequency in the presence of XeC (1.34 ± 0.27 Hz control vs 1.41 ± 0.4 Hz in XeC plus KA, n = 7, p > 0.05). The results with thapsigargin, ryanodine and 2-APB are summarized in Fig. 5D.
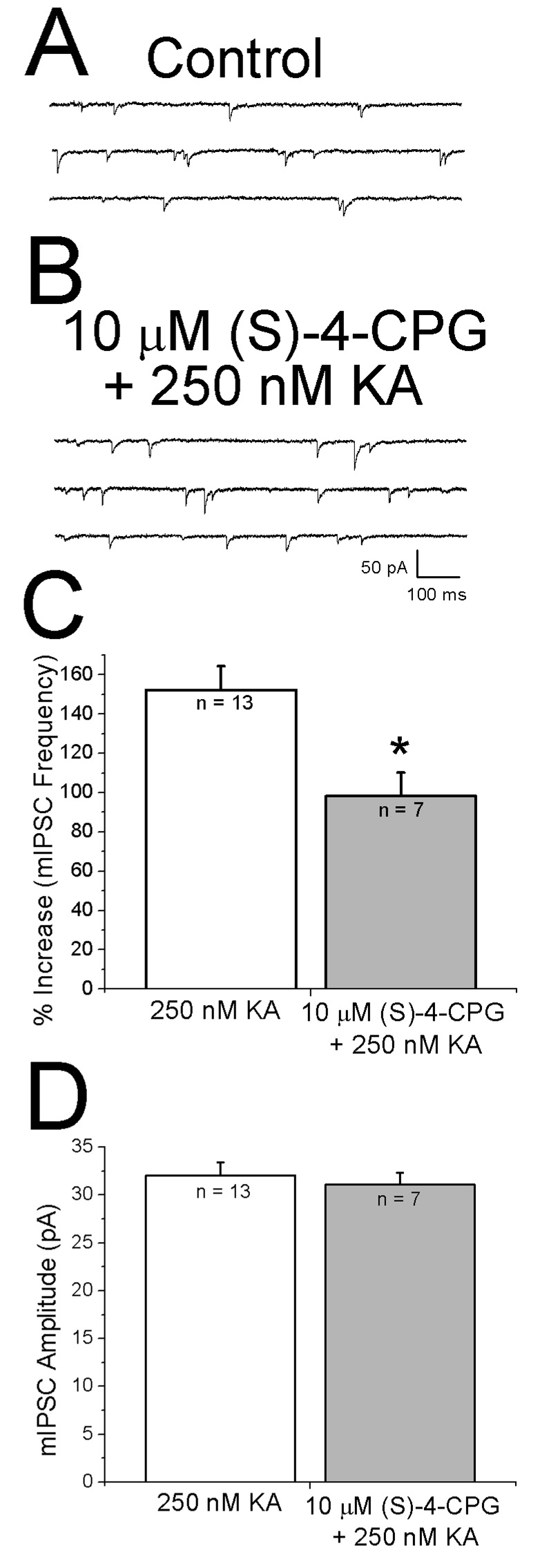
Activation of IP3Rs requires binding of both Ca2+ and IP3 (Berridge, 1998). Group I mGluRs are known to increase IP3 formation (Schoepp, 2001) and group I selective agonists increase mEPSC frequency in layer II pyramidal cells (Simkus and Sticker, 2002). Tonic activation of mGluRs by ambient glutamate could result in IP3 production. We therefore tested whether bath application of the group 1 mGluR antagonist (S)-4-CPG (10 µM) affected the ability of KA to facilitate mIPSCs. When (S)-4-CPG alone was bath applied, mIPSC frequency decreased (1.76 ± 0.08 Hz control: vs 1.0 ± 0.05 Hz after (S)-4-CPG, n = 8, p< 0.05). As shown in the specimen records in Fig. 6 A and B, when 250 nM KA was applied in the presence of (S)-4-CPG, mIPSC frequency increased. As shown in Fig. 6C, KA alone increased mIPSC frequency by 152 ± 12%. When 10 µM (S)-4-CPG was present KA increased mIPSC frequency by 98 ± 12%. The increase in mIPSC frequency in the presence of (S)-4-CPG was significantly less than that observed with KA alone (Fig. 6C). No effect on amplitude was observed in either group (Fig. 6D).
Figure 6. Kainate facilitation of IPSCs involves activation of mGluRs.
A: specimen records of mIPSCs recorded in the presence of the group I mGluR antagonist (S)-4-CPG. B: specimen records obtained after addition of 250 nM KA in continued presence of (S)-4-CPG. C: histograms showing effect of (S)-4-CPG on KA-induced facilitation. Increase in mIPSC frequency was significantly lower when KA was applied in the presence of (S)-4-CPG. D: mIPSC amplitudes were similar under control conditions and in presence of the antagonists. A did not affect amplitude. * indicates p < 0.05.
3.3 Role of GluR5 subunits
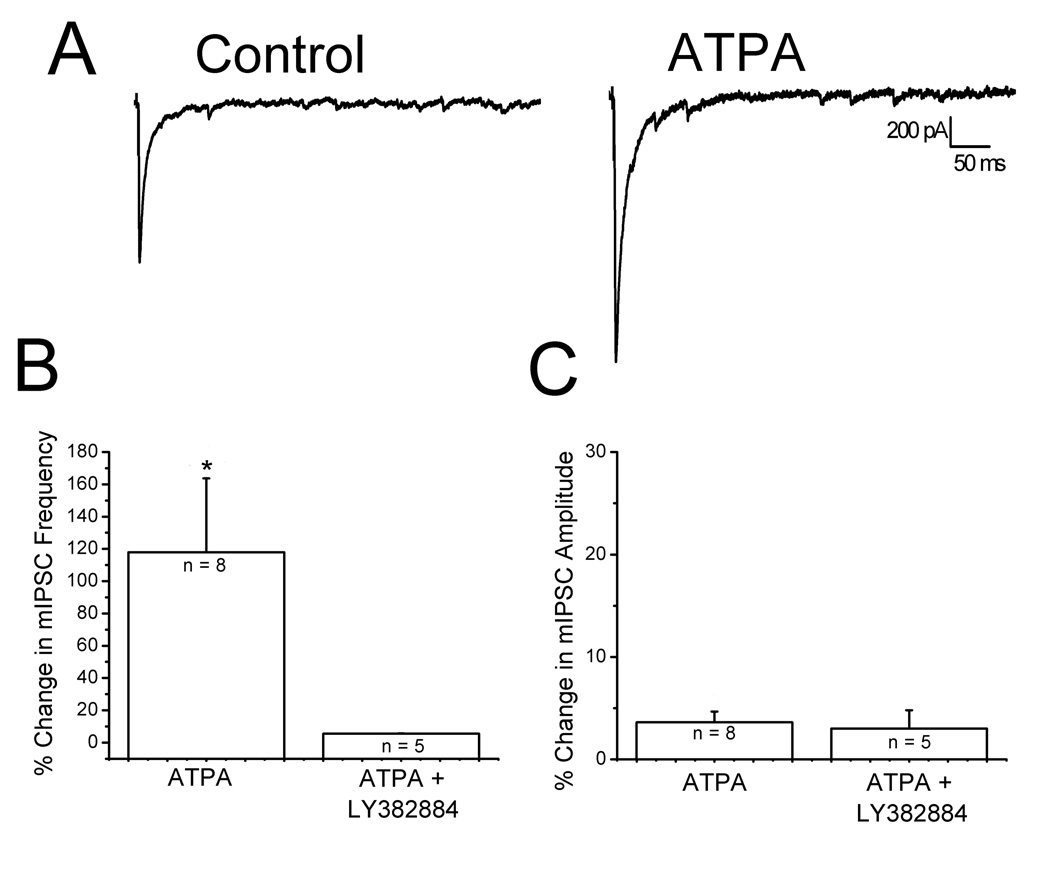
Homomeric, unedited GluR5 receptors display a significantly higher Ca2+ permeability than the edited forms (Egebjerg and Heinemann, 1993; Burnashev et al., 1995). It is possible that the extracellular Ca2+ needed for KA facilitation was entering through Ca2+ permeable KARs. The role of GluR5-containing KARs was determined using selective agonists and antagonists. ATPA is a selective GluR5 agonist (Clarke et al., 1997) and the GluR5 subunit is selectively blocked by LY382884 (Bleakman et al., 1996; Bortolotto et al., 1999). Fig. 7A shows that bath application of 1 µM ATPA increased evoked IPSC amplitudes, mimicking the effects of low concentrations of KA. Moreover, 1 µM ATPA caused a significant, reversible increase in the frequency of mIPSCs (Fig. 7B, left bar: 1.45 ± 0.02 Hz control vs 1.75 ± 0.05 Hz in ATPA, n = 8, p<0.05) without affecting amplitudes (Fig. 7C, left bar: 35 ± 1.2 pA control vs 36 ± 1.6 pA in ATPA, n = 8, p>0.05). These findings suggest that ATPA acts at presynaptic KARs containing GluR5 subunits. In further support of this, when 10 µM LY382884 was present in the bath, 250 nM KA caused no significant change in the frequency (Figure 7B, right bar: 0.81 ± 0.29 Hz LY vs 0.86 ± 0.29 Hz in LY plus KA, n = 5, p>0.05) or amplitude of mIPSCs (Figure 7C, right bar: 24.6 ± 4.3 pA LY vs 29.5 ± 3.1 pA in LY plus KA, n = 5, p>0.05).
Figure 7. Activation of GluR5 containing KARs mediate IPSC facilitation.
A: representative traces of evoked IPSCs under control conditions (left) and during application of 1 µM ATPA, a GluR5 agonist (right trace). A significant increase in IPSC amplitude was observed. B: summary histograms showing that 1 µM ATPA increases the frequency of mIPSCs (right). When ATPA was applied in the presence of the GluR5 antagonist LY382884, no change in frequency was observed. C: summary histograms showing ATPA had no effect on mIPSC amplitudes *p < 0.05
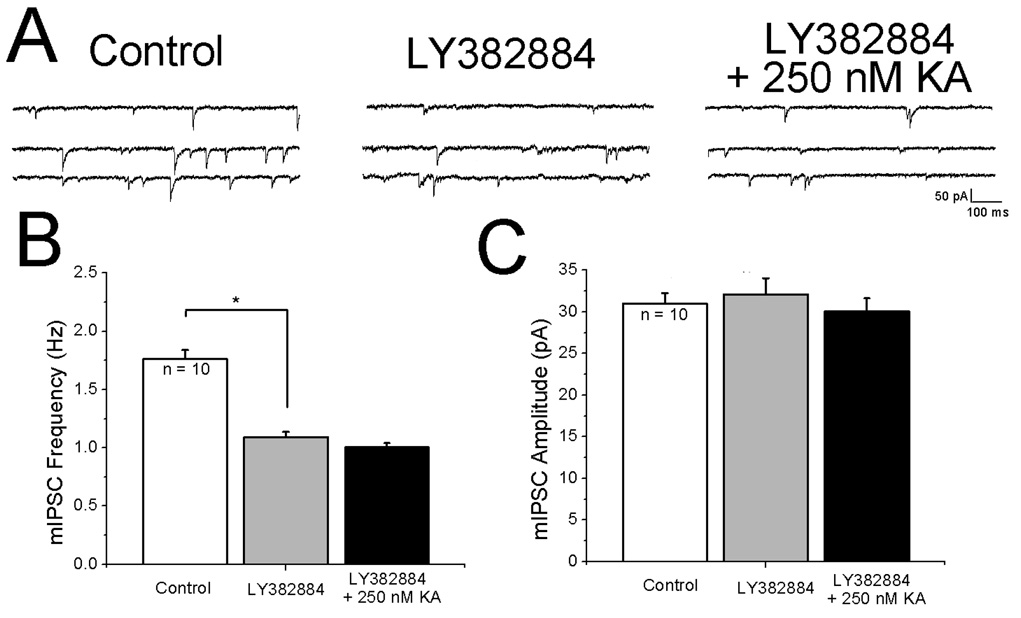
The results with (S)-4-CPG described above indicate that ambient levels of glutamate were activating mGluRs. It was reasoned that KARs should also be tonically activated by extracellular glutamate. In this situation, application of LY382884 would be expected to decrease mIPSC frequency and addition of KA in the presence of LY382884 should not facilitate mIPSC frequency. The specimen records shown in Fig. 8A indicate that mIPSC frequency was decreased by the antagonist and that 250 nM KA did not increase mIPSC rate. Quantitative analysis of results from ten cells showed that the effect of LY382884 on basal rate was significant (Fig. 8B) whereas KA was ineffective. Miniature IPSC amplitudes remained constant across all conditions (Fig. 8C).
Figure 8. Evidence that ambient glutamate levels tonically activate presynaptic receptors.
A: (left) specimen records of mIPSCs under control conditions, (middle) bath application of the GluR5 antagonist LY382884 decreases the frequency of mIPSCs, (right) in continued presence of LY382664, 250 nM KA does not increase mIPSC frequency. C, D: summary histograms showing effects of LY382664 and KA on mIPSC frequency and amplitude, respectively. *p < 0.05.
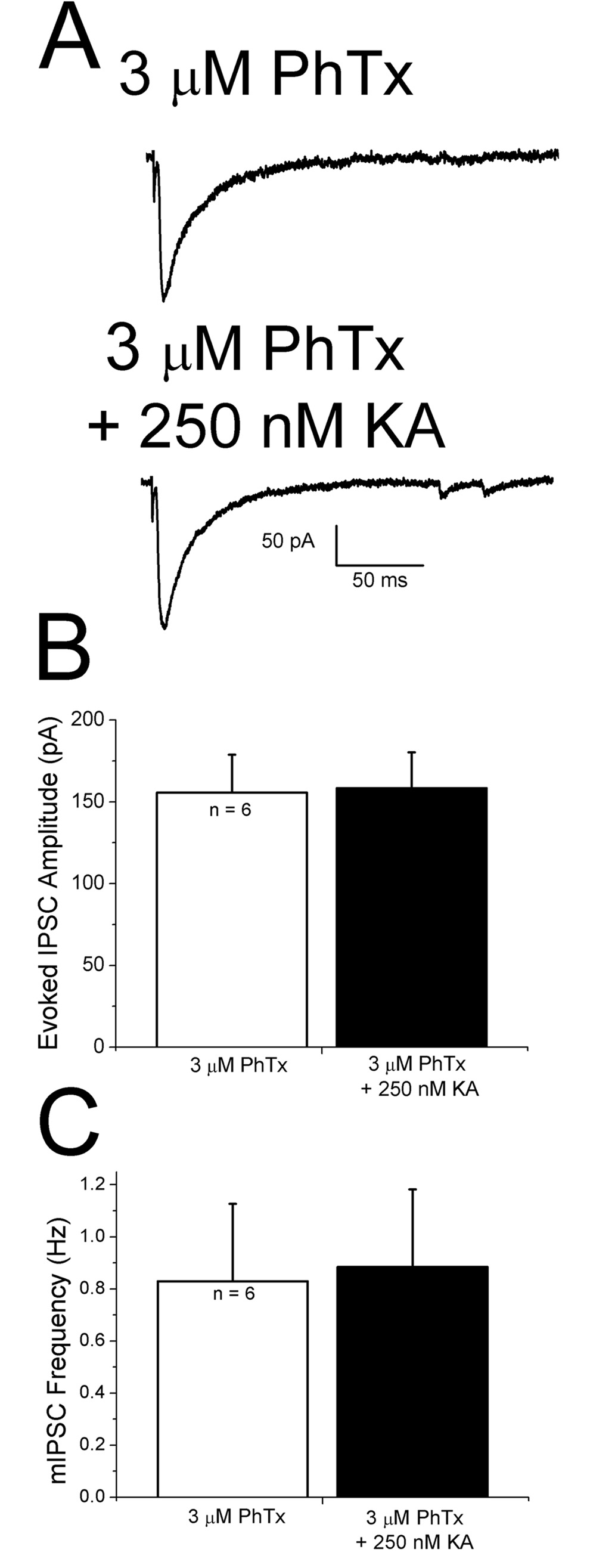
Taken together these results suggest that KA modulation of GABA release onto pyramidal cells involves GluR5 subunits. Unedited, Ca2+-permeable GluR5 containing receptors are blocked by philanthotoxin-433 (PhTx) (Washburn and Dingledine, 1996). PhTx (3 µM) was bath applied for at least 45 minutes prior to recording. Control IPSCs recorded in the presence of PhTx were not affected by 250 nM KA applied in presence of PhTx (Fig. 9A and B). Miniature IPSC frequency was not increased by KA in the presence of PhTx (Figure 9C). It thus appears that Ca2+-permeable, GluR5-containing KARs are present and contribute to release of Ca2+ from IP3 sensitive stores.
Figure 9. Philanthotoxin-433 (PhTx), a blocker of Ca2+-permeable glutamate receptors, blocks KA-induced effects on IPSCs.
A: after pretreatment with PhTx, IPSCs evoked under control conditions (upper trace) and during KA (lower trace) were not significantly different. B,C: summary plot showing that evoked IPSC amplitude and mIPSC frequency, respectively, were unaffected when KA was applied in presence of PhTx.
4. Discussion
This study investigated mechanisms underlying KA-induced modulation of IPSCs in neocortical pyramidal cells. At concentrations of 1–3 µM, KA produced a decrease in evoked IPSC amplitudes, attributable to postsynaptic changes. Lower doses of KA facilitated IPSCs via a presynaptic mechanism that involved, at least in part, release of Ca2+ from intracellular stores. This required GluR5 subunit containing KARs and activation of IP3Rs. We propose a model whereby Ca2+ entry through Ca2+ permeable, GluR5 containing, KARs causes activation of IP3Rs. This IP3R activation causes a release of Ca2+ from intracellular stores resulting in an increase in neurotransmitter release and a facilitation of inhibitory synaptic transmission. This is a novel mechanism involving both ionotropic, via GluR5 subunits, and metabotropic, via IP3R, effects.
4.1 High KA concentrations decrease IPSCs
At high concentrations, KA decreases inhibitory synaptic transmission in hippocampus (Rodriguez-Moreno et al., 1997; Rodriguez-Moreno and Lerma, 1998; Maingret et al., 2005), amygdala (Braga et al., 2003) and spinal cord (Kerchner et al., 2001). This decrease was associated with an increase in synaptic failures, indicating a presynaptic action possibly involving direct coupling between KARs and G proteins (Rodriguez-Moreno et al., 1997; Rodriguez-Moreno and Lerma, 1998; Lerma et al., 2001). An indirect mechanism of KA inhibition, involving an increase in action potential firing which produces enhanced GABA release, has also been postulated (Frerking et al., 1999). This increase leads to activation of presynaptic GABAB receptors with depression of GABA release and activation of postsynaptic GABAA receptors producing passive shunting. Another alternative explanation for presynaptic KAR inhibition involves depolarization of the nerve terminal. This inactivates the voltage dependent Ca2+ channels that are required for neurotransmitter release (Mayer, 1997). KAR-induced inhibition is typically observed when high kainate concentrations are employed, as observed here. It is not clear whether these inhibitory mechanisms are engaged by endogenously released glutamate.
In the present experiments, higher concentrations of KA decreased evoked IPSC amplitudes and produced a significant increase in holding current. We have shown previously that such changes in holding current are sensitive to CNQX, but not D-APV and GYKI52466, indicating activation of postsynaptic kainate receptors (Campbell et al., 2007). The precise mechanism underlying evoked IPSCs depression is not clear but activation of postsynaptic KA receptors and generation of a large inward current could lead to decreased responsiveness due to shunting or altered dendritic-somatic coupling. It is unlikely that mechanisms involving GABAB receptors are involved since GABAB antagonists were always present in our studies.
4.2 Presynaptic KA receptors mediate IPSC facilitation
The frequency of spontaneous IPSCs in CA1 pyramidal neurons is increased by low nanomolar concentrations of KA (Frerking et al., 1999; Cossart et al., 2001) and the number of failures in response to stimulation is reduced (Cossart et al., 2001; Jiang et al., 2001). Activation of presynaptic KARs on GABAergic nerve terminals projecting to substantia nigra neurons also increased the frequency of mIPSCs in those cells (Nakamura et al., 2003). Facilitation of GABA release has been observed in basolateral amygdala neurons following GluR5 receptor activation with low concentrations of ATPA (Braga et al., 2003). Although presynaptic KARs located on terminals of fast-spiking GABAergic interneurons have been reported to decrease inhibitory transmitter release onto layer V neocortical pyramidal cells (Ali et al., 2001), our present results, and those of others (Ren et al., 2007) indicate that evoked and spontaneous IPSCs in superficial layers of neocortex are potently facilitated by activation of presynaptic KARs.
4.3 Involvement of Ca2+ and IP3Rs
Several different Ca2+ dependent mechanisms were examined in the present study, leading to the conclusion that IP3R mediated Ca2+ release from intracellular stores was involved in KA modulation. The KA mediated facilitation of GABA release was not dependent on voltage-dependent Ca2+ channels since it persisted in the presence of a concentration of cadmium which blocked synaptic transmission. Exposure to solutions containing no added extracellular Ca2+ inhibited the KAR mediated facilitation, suggesting that extracellular Ca2+ was necessary. KA was unable to induce facilitation of inhibitory synaptic transmission in the presence of PhTx, an inhibitor of Ca2+ permeable AMPA/KARs. These results suggest that Ca2+ entry via Ca2+-permeable KARs is involved. The facilitation of GABAergic transmission upon KAR activation was blocked after inhibition of endosomal Ca2+-ATPase- mediated Ca2+ uptake with thapsigargin, suggesting a role for intracellular stores in the KAR mediated facilitation of GABA release, as reported for hippocampal neurons (Lauri et al., 2003). The inability of high concentrations of ryanodine, which has an antagonist action by completely closing the release channels (Meissner, 1994; Masumiya et al., 2001), to block KAR-induced changes indicated that RyRs are not involved. The concentrations used here are comparable to those used previously (Ehrlich et al., 1994; Liu et al., 2005) to completely block opening of RyRs. Additionally, low doses of ruthenium red, which also blocks Ca2+ release from ryanodine-sensitive Ca2+ stores (Taylor and Broad, 1998) was unable to inhibit the KAR-induced facilitation.
In our studies, we used two different drugs to inhibit IP3R induced calcium release, XeC (Gafni et al., 1997) and 2-APB (Maruyama et al., 1997). Although these drugs can have multiple targets (Collin et al., 2005), 2-APB has proven to be an effective inhibitor of IP3-mediated calcium release in intact cells of vertebrates and invertebrates (Maruyama et al., 1997; Ma et al., 2000; Koganezawa and Shimada, 2002) and has not been found to alter either agonist-mediated IP3 production or IP3 binding to its receptor (Maruyama et al., 1997; Ma et al., 2000). Both compounds effectively blocked KAR-induced changes in IPSCs. Experiments with the IP3R antagonists were performed without blockage of RyRs, so calcium signals could have been amplified by subsequent CICR through RyRs. However, the consistent effects with the various IP3R antagonists and lack of effect with ryanodine indicate a principal role for IP3Rs.
Activation of IP3Rs requires binding of both Ca2+ and IP3 (Berridge, 1998). We have shown previously that extracellular glutamate levels are high enough to activate KARs on excitatory nerve terminals (Campbell et al. 2007). Previous studies have also shown that baseline glutamate levels affect mGluRs in layer V pyramidal neurons (Bandrowski et al., 2003) and that mGluRs tonically activate IP3 production (Simkus and Sticker, 2002). Increases in intracellular Ca2+ evoked by single action potentials in neocortical pyramidal cells are augmented by muscarinic acetycholine receptor stimulation (Yamamoto et al., 2000). This augmentation was shown to involve IP3R mediated Ca2+ release from intracellular stores via an IP3R dependent mechanism. In the present studies, KARs were the source of Ca2+ activating IP3Rs. The results with the mGluR antagonist (S)-4-CPG suggest that activity in mGluR modulatory systems, perhaps activated by ambient glutamate, provide levels of IP3 sufficient to open intracellular stores. Tonic activation of KARs has been reported previously (Lauri et al., 2006) and is also seen with presynaptic NMDA receptors (Beretta and Jones, 1996; Corlew et al., 2007)
In summary, it is suggested that following KAR activation, Ca2+ enters through Ca2+ permeable presynaptic GluR5 subunit containing KARs. This results in Ca2+ release from intracellular stores through IP3Rs which produces an increase in inhibitory synaptic transmission. KAR-induced increases in GABAergic transmission in upper layers of the PFC could provide a feedback mechanism for maintenance of stability in cortical circuits.
Acknowledgements
We would like to thank Dr. R.A.J. Lester for critical reading of the manuscript and K. Alison Margolies for excellent technical assistance. This work was supported by National Institutes of Health grants NS22373, P30-NS47466 and P30-NS57098.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali AB, Rossier J, Staiger JF, Audinat E. Kainate receptors regulate unitary IPSCs elicited in pyramidal cells by fast-spiking interneurons in the neocortex. Journal of Neuroscience. 2001;21:2992–2999. doi: 10.1523/JNEUROSCI.21-09-02992.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB. Involvement of post-synaptic kainate receptors during synaptic transmission between unitary connections in rat neocortex. European Journal of Neuroscience. 2003;17:2344–2350. doi: 10.1046/j.1460-9568.2003.02677.x. [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Huguenard JR, Prince DA. Baseline glutamate levels affect group I and II mGluRs in layer V pyramidal neurons of rat sensorimotor cortex. Journal of Neurophysiology. 2003;89:1308–1316. doi: 10.1152/jn.00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. Journal of Neurophysiology. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Berretta N, Jones RSG. Tonic facilitation of glutamate release by presynaptic Nmethyl-D-aspartate autoreceptors in the enthorinal cortex. Neuroscience. 1996;75:339–344. doi: 10.1016/0306-4522(96)00301-6. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bettler B, Mulle C. Review: Neurotransmitter receptors II: AMPA and kainite receptors. Neuropharmacology. 1995;34:123–139. doi: 10.1016/0028-3908(94)00141-e. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Dopamine-containing systems in the CNS. In: Bjorklund A, Hokfelt T, editors. Handbook of Chemical Neuroanatomy:Classical Transmitter in the Rat. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woody ML, Bufton HR, Kamboj RK, Tarnawa I, Lodge D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB Journal. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VRJ, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Braga MFM, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. Journal of Neuroscience. 2003;23:442–452. doi: 10.1523/JNEUROSCI.23-02-00442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breustedt J, Schmitz D. Assessing the role of GLUK5 and GLUK6 at hippocampal mossy fiber synapses. Journal of Neuroscience. 2004;24:10093–10098. doi: 10.1523/JNEUROSCI.3078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. Journal of Physiology (London) 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SL, Mathew SS, Hablitz JJ. Pre- and postsynaptic effects of kainate on layer II/III pyramidal cells in rat neocortex. Neuropharmacology. 2007;53:37–47. doi: 10.1016/j.neuropharm.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VRJ, Henley JM. Kainate receptors: Subunits, synaptic localization and function. Trends in Pharmacological Sciences. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Clarke VRJ, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas JP, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Current Opinion in Neurobiology. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot B. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. Journal of Neuroscience. 2007;27:9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29:497–508. doi: 10.1016/s0896-6273(01)00221-5. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KAC. Neuronal circuits of the neocortex. Annual Review of Neuroscience. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Eder M, Becker K, Rammes G, Schierloh A, Azad SC, Zieglgansberger W, Dodt HU. Distribution and properties of functional postsynaptic kainate receptors on neocortical layer V pyramidal neurons. Journal of Neuroscience. 2003;23:6660–6670. doi: 10.1523/JNEUROSCI.23-16-06660.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egebjerg J, Heinemann SF. Ca2+ permeability of unedited and edited versions of the kainate selective glutamate receptor GluR6. Proceedings of the National Academy of Sciences USA. 1993;90:755–759. doi: 10.1073/pnas.90.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I. The pharmacology of intracellular Ca2+-release channels. Trends in Pharmacological Sciences. 1994;15:145–149. doi: 10.1016/0165-6147(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neuroscience. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Petersen CCH, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proceedings of the National Academy of Sciences USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. Phase-dependent contributions from Ca2+ entry and Ca2+ release to caffeine-induced [Ca2+]i oscillations in bullfrog sympathetic neurons. Neuron. 1992;8:1109–1125. doi: 10.1016/0896-6273(92)90132-w. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nature Neuroscience. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg P. The KA-2 subunit of excitatory amino acid receptors show widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annual Review of Neuroscience. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30:503–513. doi: 10.1016/s0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Shindou T. Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. Journal of Neuroscience. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Li P, Zhuo M, Huettner JE. Presynaptic kainite receptors regulate spinal sensory transmission. Journal of Neuroscience. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd FL, Coumis U, Collingridge GL, Crabtree JW, Isaac JTR. A presynaptic kainate receptor is involved in regulating the dynamic properties of thalamocortical synapses during development. Neuron. 2002;34:635–646. doi: 10.1016/s0896-6273(02)00699-2. [DOI] [PubMed] [Google Scholar]
- Koganezawa M, Shimada I. Inositol 1,4,5-trisphosphate transduction cascade in taste reception of the fleshfly, Boettcherisca peregrina. Journal of Neurobiology. 2002;51:66–83. doi: 10.1002/neu.10047. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JTR, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Segerstrale M, Vesikansa A, Maingret F, Mulle C, Collingridge GL, Isaac JTR, Taira T. Endogenous activation of kainate receptors regulates glutamate release and network activity in the developing hippocampus. Journal of Neuroscience. 2005;25:4473–4484. doi: 10.1523/JNEUROSCI.4050-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Vesikansa, Segerstrale M, Collingridge GL, Usaac JTR, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainite receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–429. doi: 10.1016/j.neuron.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Rodriguez-Moreno A, Lopez-Garcia JC. Molecular physiology of kainate receptors. Physiology Reviews. 2001;81:971–998. doi: 10.1152/physrev.2001.81.3.971. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen B, Yankova M, Morest DK, Maryon E, Hand AR, Nonet ML, Wang ZW. Presynaptic ryanodine receptors are required for normal quantal size at the caenorhabditis elegans neuromuscular junction. Journal of Neuroscience. 2005;25:6745–6754. doi: 10.1523/JNEUROSCI.1730-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma HT, Patterson RL, Van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauri SE, Taira T, Isaac JTR. Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. Journal of Physiology (London) 2005;567:131–142. doi: 10.1113/jphysiol.2005.089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-Aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. Journal of Biochemistry. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Masumiya H, Li P, Zhang L, Chen SRW. Ryanodine sensitizes the Ca2+ release channel (ryanodine receptor) to Ca2+ activation. Journal of Biological Chemistry. 2001;276:39727–39735. doi: 10.1074/jbc.M106557200. [DOI] [PubMed] [Google Scholar]
- Mayer M. Kainate receptors: Finding homes at synapses. Nature. 1997;389:542–543. doi: 10.1038/39177. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annual Review of Physiology. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Meldolesi J. Rapidly exchanging Ca2+ stores in neurons: molecular, structural and functional properties. Progress in Neurobiology. 2001;65:309–338. doi: 10.1016/s0301-0082(01)00004-1. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Min MY, Melyan Z, Kullmann DM. Synaptically released glutamate reduces gamma -aminobutyric acid (GABA)ergic inhibition in the hippocampus via kainate receptors. Proceedings of the National Academy of Sciences USA. 1999;96:9932–9937. doi: 10.1073/pnas.96.17.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Jang IS, Ishibashi H, Watanabe S, Akaike N. Possible roles of kainate receptors on GABAergic nerve terminals projecting to rat substantia nigra dopaminergic neurons. Journal of Neurophysiology. 2003;90:1662–1670. doi: 10.1152/jn.01165.2002. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Barbara J-G, Nakamura K, Ross WN. Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials. Neuron. 1999;24:727–737. doi: 10.1016/s0896-6273(00)81125-3. [DOI] [PubMed] [Google Scholar]
- Narita K, Akita T, Hachisuka J, Huang SM, Ochi K, Kuba K. Functional coupling of Ca2+ channels to ryanodine receptors at presynaptic terminals: Amplification of exocytosis and plasticity. Journal of General Physiology. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita K, Akita T, Osanai M, Shirasaki T, Kijima H, Kuba K. A Ca2+-induced Ca2+ release mechanism involved in asynchronous exocytosis at frog motor nerve terminals. Journal of General Physiology. 1998;112:593–609. doi: 10.1085/jgp.112.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. Journal of Neuroscience. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Third Edition. San Diego: Academic Press; 1997. [Google Scholar]
- Ren M, Yoshimura Y, Takada N, Horibe S, Komatsu Y. Specialized inhibitory synaptic actions between nearby neocortical pyramidal neurons. Science. 2007;316:758–761. doi: 10.1126/science.1135468. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. Journal of Neuroscience. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2001;299:12–20. [PubMed] [Google Scholar]
- Simkus CRL, Stricker C. The contribution of intracellular calcium stores to mEPSCs recorded in layer II neurones of rat barrel cortex. Journal of Physiology (London) 2002;545:521–535. doi: 10.1113/jphysiol.2002.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Luhmann HJ. Involvement of GABAB receptors in convulsant-induced epileptiform activity in rat neocortex in vitro. European Journal of Neuroscience. 1998;10:3417–3427. doi: 10.1046/j.1460-9568.1998.00351.x. [DOI] [PubMed] [Google Scholar]
- Ta TA, Feng W, Molinski TF, Pessah IN. Hydroxylated xestospongins block inositol-1,4,5-trisphosphate-induced Ca2+ release and sensitize Ca2+ release mediated by ryanodine receptors. Molecular Pharmacology. 2006;69:532–538. doi: 10.1124/mol.105.019125. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends in Pharmacological Sciences. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proceedings of the National Academy of Sciences USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapse in visual cortex. Journal of Neuroscience. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. Journal of Pharmacology and Experimental Therapeutics. 1996;278:669–678. [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high affinity kainate receptor expressed predominately in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. Journal Neuroscience. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhao M-G, Toyoda H, Vadakkan KI, Jia Y, Pinaud R, Zhuo M. Presynaptic regulation of the inhibitory transmission by GluR5-containing kainate receptors in spinal substantia gelatinosa. Molecular Pain. 2006;2:29. doi: 10.1186/1744-8069-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Hashimoto K, Isomura Y, Shimohama S, Kato N. An IP3-assisted form of Ca2+-induced Ca2+ release in neocortical neurons. NeuroReport. 2000;11:535–539. doi: 10.1097/00001756-200002280-00022. [DOI] [PubMed] [Google Scholar]