Abstract
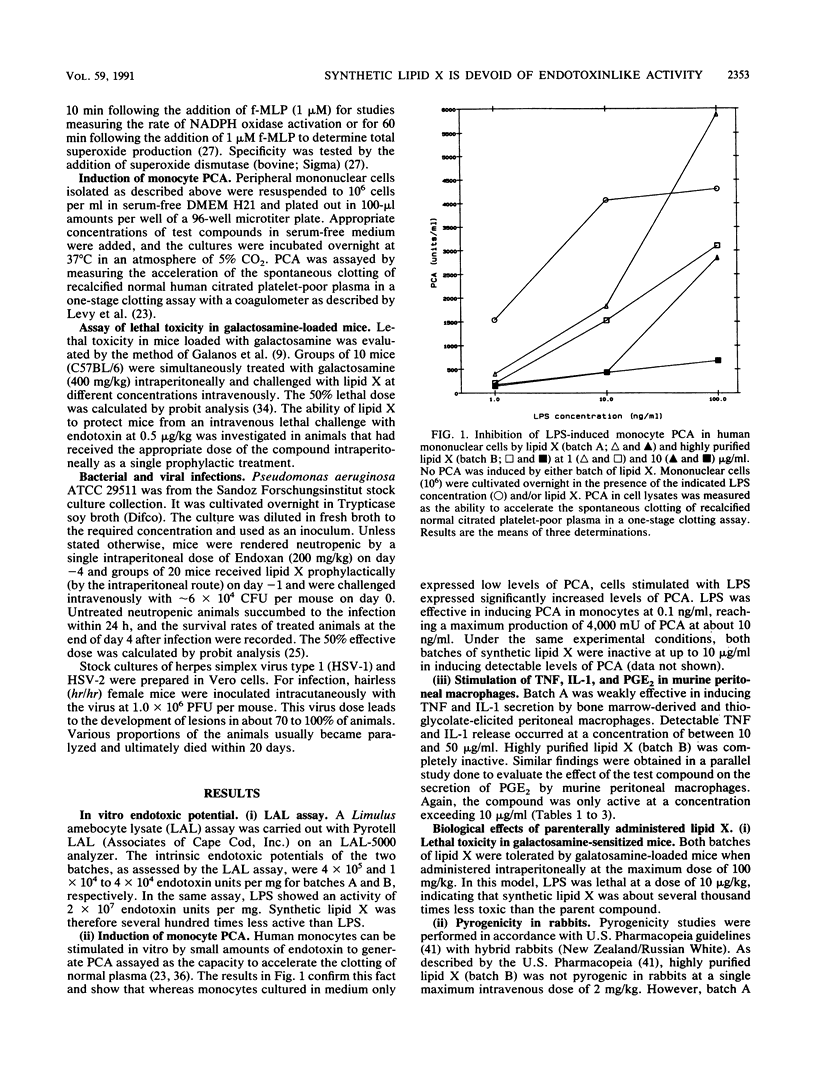
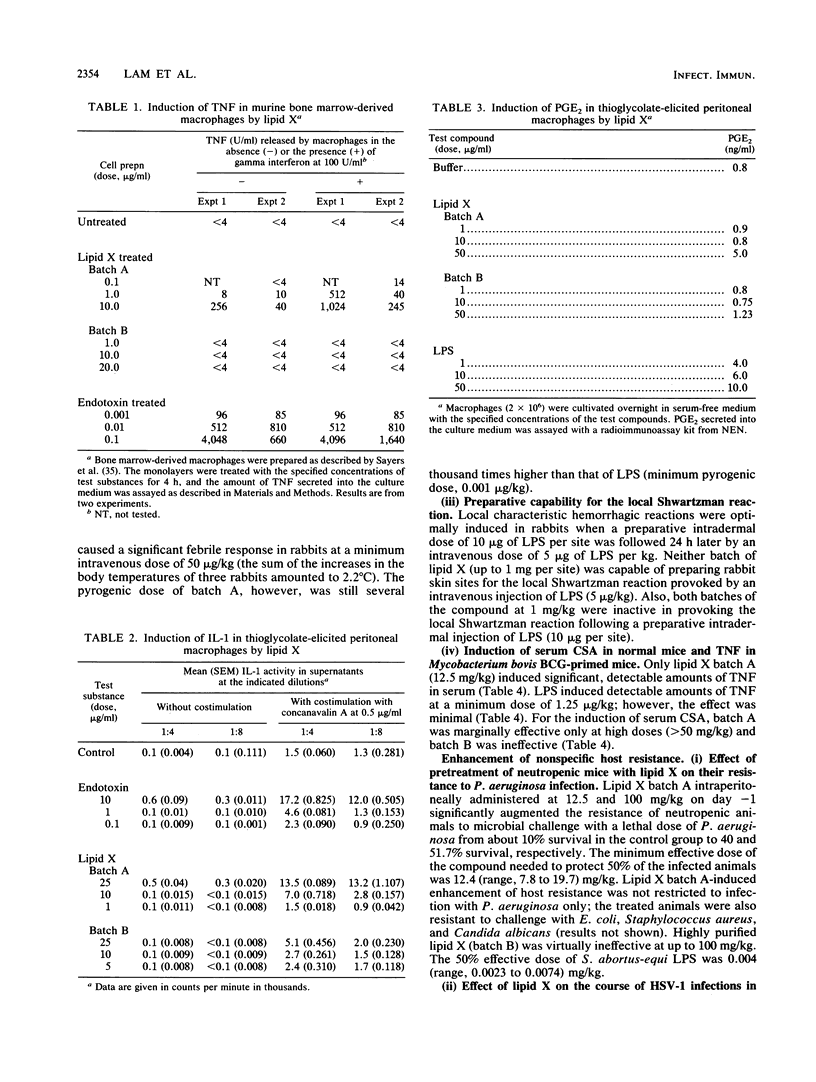
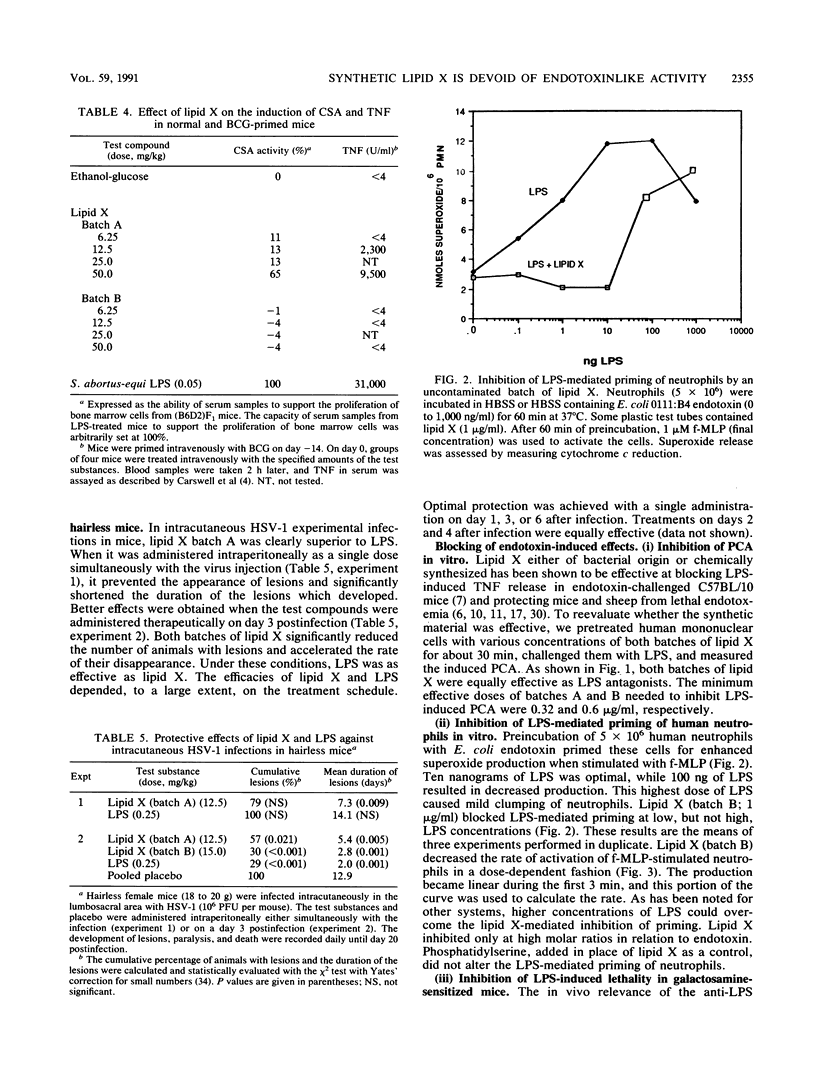
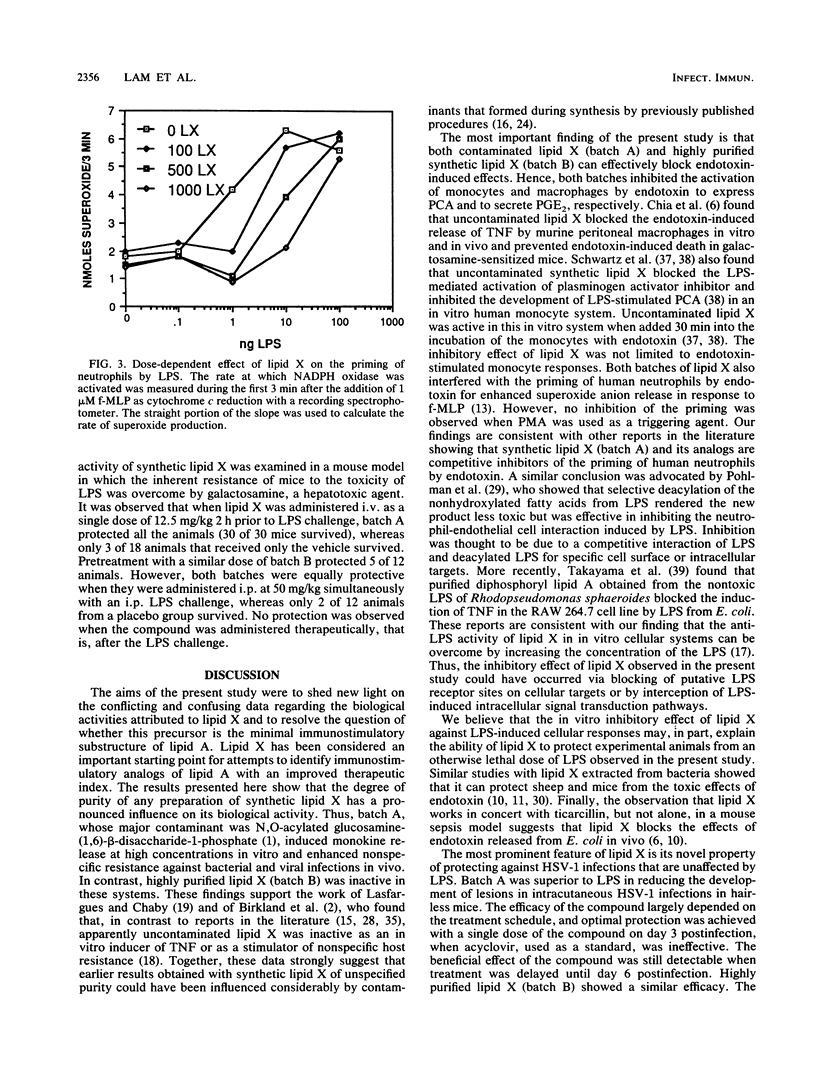
Lipid X, a precursor in the biosynthesis of lipid A, has been claimed to possess most of the immunostimulatory activity but none of the toxicity of endotoxin. However, recent work shows that synthetic lipid X can be contaminated with small amounts of N,O-acylated disaccharide-1-phosphate (H. Aschauer, A. Grob, J. Hildebrandt, E. Schuetze, and P. Steutz, J. Biol. Chem. 265:9159-9164, 1990). Because the impurities themselves exhibit immunostimulatory properties, it was necessary to establish whether chemically pure synthetic lipid X exhibits any of the endotoxinlike biological activities previously attributed to the native compound extracted from the Escherichia coli MN7 mutant. In the present study, two batches of synthetic lipid X were used: batch A contained the contaminating disaccharide, and batch B was pure lipid X. Batch A, previously believed to be pure on the basis of spectroscopic and microanalysis data, induced murine peritoneal macrophages to secrete tumor necrosis factor, interleukin-1, and prostaglandin E2 at a minimal dose of 10 micrograms/ml in vitro. Batch B, further purified by Sephadex LH 20 chromatography, was found virtually inactive in these in vitro assays. Furthermore, batch A was pyrogenic in rabbits at a dose of 0.05 mg/kg, whereas batch B was not pyrogenic at doses of up to greater than or equal to 2 mg/kg. However, both batches were equally tolerated by galactosamine-loaded mice at doses of up to 100 mg/kg. Surprisingly, while only batch A protected neutropenic mice against lethal infection with Pseudomonas aeruginosa (50% effective dose, 12.4 mg/kg), both batches were equally protective against infection with herpes simplex virus type 1 in mice and guinea pigs, even when lipid X was administered therapeutically. Interestingly, both batches of lipid X blocked endotoxin-induced expression of monocyte procoagulant activity and priming of human neutrophils for superoxide anion release. Similarly, both batches protected galactosamine-sensitized mice from otherwise lethal endotoxemia when administered prophylactically or simultaneously with the lipopolysaccharide challenge. Thus, our findings suggest that chemically pure lipid X (batch B) is devoid of the immunostimulatory properties of lipid A or endotoxin. Rather, it behaves as a competitive inhibitor of lipopolysaccharide. We conclude, therefore, that previous studies which attributed immunostimulatory activities to any batch of synthetic lipid X should be interpreted with caution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschauer H., Grob A., Hildebrandt J., Schuetze E., Stuetz P. Highly purified lipid X is devoid of immunostimulatory activity. Isolation and characterization of immunostimulating contaminants in a batch of synthetic lipid X. J Biol Chem. 1990 Jun 5;265(16):9159–9164. [PubMed] [Google Scholar]
- Birkland T. P., Cornwell R. D., Golenbock D. T., Proctor R. A. Comparative study of lipopolysaccharide-, lipid IVa-, and lipid X-induced tumor necrosis factor production in murine macrophage-like cell lines. Adv Exp Med Biol. 1990;256:399–402. doi: 10.1007/978-1-4757-5140-6_35. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. Y., Bright S. W., Pace J. L., Russell S. W., Morrison D. C. Induction of macrophage-mediated tumor cytotoxicity by a hamster monoclonal antibody with specificity for lipopolysaccharide receptor. J Immunol. 1990 Jul 1;145(1):8–12. [PubMed] [Google Scholar]
- Danner R. L., Joiner K. A., Parrillo J. E. Inhibition of endotoxin-induced priming of human neutrophils by lipid X and 3-Aza-lipid X. J Clin Invest. 1987 Sep;80(3):605–612. doi: 10.1172/JCI113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Leggett J. E., Rasmussen P., Craig W. A., Raetz C. R., Proctor R. A. Lipid X protects mice against fatal Escherichia coli infection. Infect Immun. 1988 Apr;56(4):779–784. doi: 10.1128/iai.56.4.779-784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenbock D. T., Will J. A., Raetz C. R., Proctor R. A. Lipid X ameliorates pulmonary hypertension and protects sheep from death due to endotoxin. Infect Immun. 1987 Oct;55(10):2471–2476. doi: 10.1128/iai.55.10.2471-2476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarek J., Timmons S., Hawiger J. Modulation of human platelet protein kinase C by endotoxic lipid A. J Clin Invest. 1988 Sep;82(3):964–971. doi: 10.1172/JCI113705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Raetz C. R. Lipid A binding sites in membranes of macrophage tumor cells. J Biol Chem. 1988 Oct 15;263(29):14802–14807. [PubMed] [Google Scholar]
- Ikeda K., Takahashi T., Shimizu C., Nakamoto S., Achiwa K. Lipid A and related compounds. XI. New, efficient synthesis of lipid X. Chem Pharm Bull (Tokyo) 1987 Apr;35(4):1383–1387. doi: 10.1248/cpb.35.1383. [DOI] [PubMed] [Google Scholar]
- Lam C., Schütze E., Walzl H., Basalka E. Protection of mice against lethal endotoxemia by lipid X is mediated through inhibition of neutrophil function. Circ Shock. 1987;22(4):311–321. [PubMed] [Google Scholar]
- Lasfargues A., Chaby R. Endotoxin-induced tumor necrosis factor (TNF): selective triggering of TNF and interleukin-1 production by distinct glucosamine-derived lipids. Cell Immunol. 1988 Aug;115(1):165–178. doi: 10.1016/0008-8749(88)90171-2. [DOI] [PubMed] [Google Scholar]
- Lasfargues A., Ledur A., Charon D., Szabo L., Chaby R. Induction by lipopolysaccharide of intracellular and extracellular interleukin 1 production: analysis with synthetic models. J Immunol. 1987 Jul 15;139(2):429–436. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. I. Detection of lipopolysaccharide-binding sites on splenocytes and splenocyte subpopulations. J Immunol. 1988 Aug 1;141(3):996–1005. [PubMed] [Google Scholar]
- Lei M. G., Morrison D. C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988 Aug 1;141(3):1006–1011. [PubMed] [Google Scholar]
- Levy G. A., Schwartz B. S., Edgington T. S. The kinetics and metabolic requirements for direct lymphocyte induction of human procoagulant monokines by bacterial lipopolysaccharide. J Immunol. 1981 Jul;127(1):357–363. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Cohen H. J. Activity and activation of the granulocyte superoxide-generating system. Blood. 1980 Jan;55(1):85–92. [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman T. H., Munford R. S., Harlan J. M. Deacylated lipopolysaccharide inhibits neutrophil adherence to endothelium induced by lipopolysaccharide in vitro. J Exp Med. 1987 May 1;165(5):1393–1402. doi: 10.1084/jem.165.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., Will J. A., Burhop K. E., Raetz C. R. Protection of mice against lethal endotoxemia by a lipid A precursor. Infect Immun. 1986 Jun;52(3):905–907. doi: 10.1128/iai.52.3.905-907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Purcell S., Takayama K. Molecular requirements for B-lymphocyte activation by Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4624–4628. doi: 10.1073/pnas.80.15.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B. L., Painter G., Raetz C. R. The biosynthesis of gram-negative endotoxin. Formation of lipid A disaccharides from monosaccharide precursors in extracts of Escherichia coli. J Biol Chem. 1984 Apr 25;259(8):4852–4859. [PubMed] [Google Scholar]
- Romano M., Hawiger J. Interaction of endotoxic lipid A and lipid X with purified human platelet protein kinase C. J Biol Chem. 1990 Jan 25;265(3):1765–1770. [PubMed] [Google Scholar]
- Sayers T. J., Macher I., Chung J., Kugler E. The production of tumor necrosis factor by mouse bone marrow-derived macrophages in response to bacterial lipopolysaccharide and a chemically synthesized monosaccharide precursor. J Immunol. 1987 May 1;138(9):2935–2940. [PubMed] [Google Scholar]
- Schwartz B. S., Monroe M. C., Bradshaw J. D. Endotoxin-induced production of plasminogen activator inhibitor by human monocytes is autonomous and can be inhibited by lipid X. Blood. 1989 Jun;73(8):2188–2195. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Beutler B., Kirkland T. N. Diphosphoryl lipid A from Rhodopseudomonas sphaeroides ATCC 17023 blocks induction of cachectin in macrophages by lipopolysaccharide. Infect Immun. 1989 Apr;57(4):1336–1338. doi: 10.1128/iai.57.4.1336-1338.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Mascagni P., Nashed M. A., Anderson L., Raetz C. R. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A. Complete structure of A diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983 Jun 25;258(12):7379–7385. [PubMed] [Google Scholar]
- van der Meer J. W., van de Gevel J. S., Elzenga-Claassen I., van Furth R. Suspension cultures of mononuclear phagocytes in the teflon culture bag. Cell Immunol. 1979 Jan;42(1):208–212. doi: 10.1016/0008-8749(79)90236-3. [DOI] [PubMed] [Google Scholar]



