Abstract
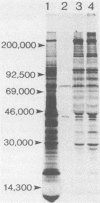
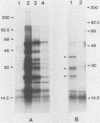
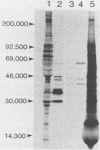
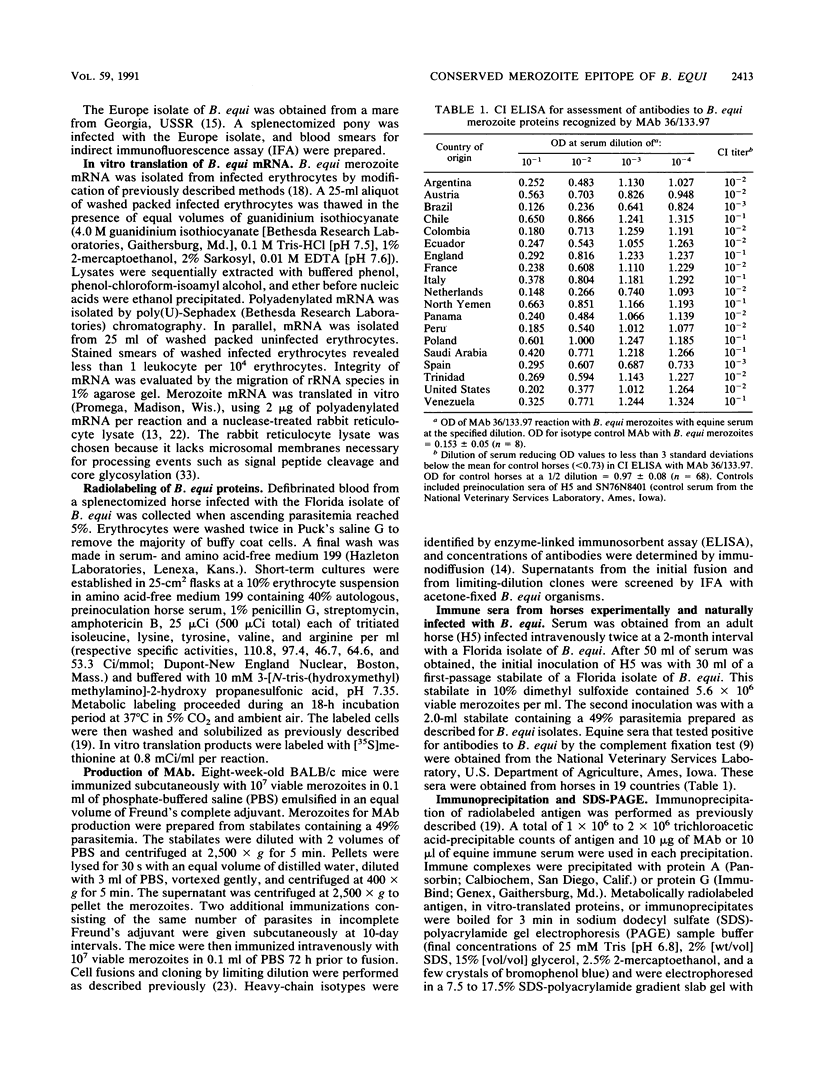
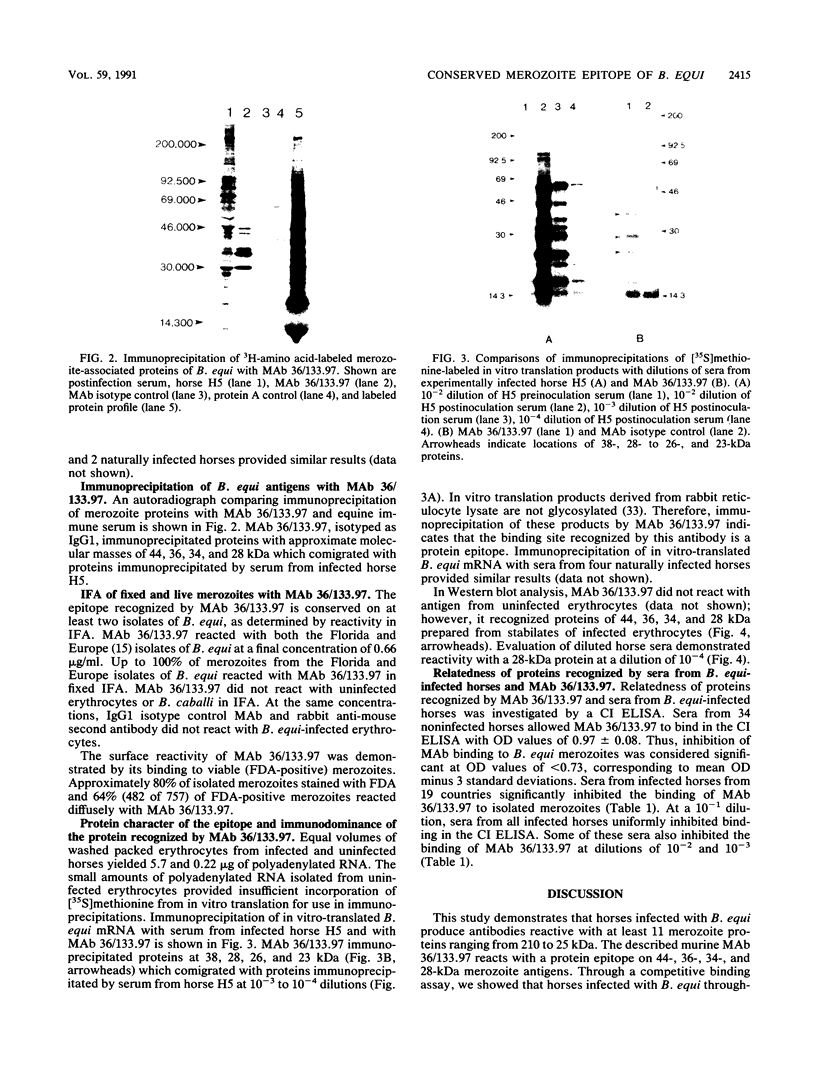
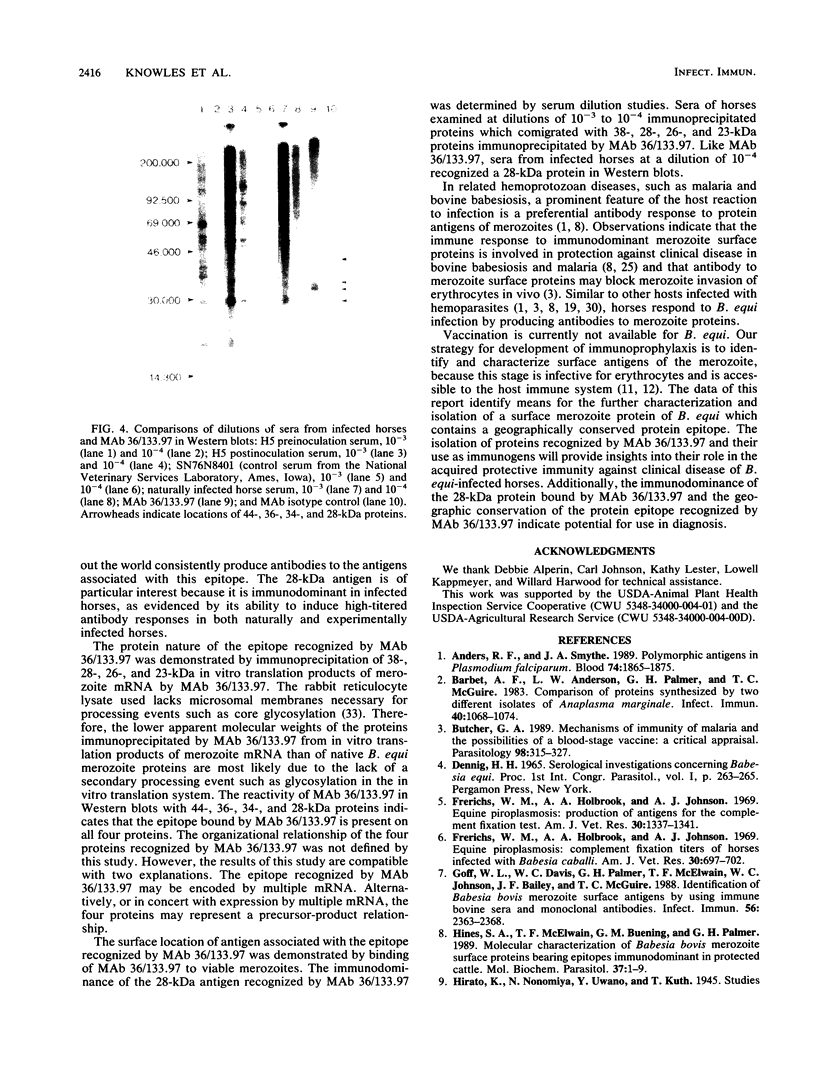
Babesiosis is a tick-borne hemoparasitic disease affecting horses worldwide. To investigate mechanisms of immunity to this parasite, the antibody response of infected horses to Babesia equi merozoite proteins was evaluated. Immunoprecipitation of B. equi merozoite antigens with sera from infected horses revealed 11 major proteins of 210, 144, 108, 88, 70, 56, 44, 36, 34, 28, and 25 kDa. Monoclonal antibody (MAb) 36/133.97, which binds to live merozoites, immunoprecipitated proteins of 44, 36, 34, and 28 kDa. When immunoprecipitations were performed with in vitro translation products of merozoite mRNA, MAb 36/133.97 immunoprecipitated proteins of 38, 28, 26, and 23 kDa which comigrated with proteins immunoprecipitated by sera from infected horses at 10(-3) to 10(-4) dilutions. In Western blot analysis, MAb 36/133.97 recognized proteins of 44, 36, 34, and 28 kDa, and a 28-kDa protein was identified by sera from infected horses at a dilution of 10(-4). MAb 36/133.97 bound to B. equi isolates from Florida and Europe. Furthermore, the binding of MAb 36/133.97 to merozoite proteins was inhibited by sera of infected horses from 19 countries. Collectively, these data indicate MAb 36/133.97 binds to a geographically conserved peptide epitope on multiple B. equi merozoite proteins, including a merozoite surface protein, and MAb 36/133.97 reacts with a B. equi protein immunodominant in infected horses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Smythe J. A. Polymorphic antigens in Plasmodium falciparum. Blood. 1989 Nov 1;74(6):1865–1875. [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher G. A. Mechanisms of immunity to malaria and the possibilities of a blood-stage vaccine: a critical appraisal. Parasitology. 1989 Apr;98(Pt 2):315–327. doi: 10.1017/s0031182000062247. [DOI] [PubMed] [Google Scholar]
- Frerichs W. M., Holbrook A. A., Johnson A. J. Equine piroplasmosis: complement-fixation titers of horses infected with Babesia caballi. Am J Vet Res. 1969 May;30(5):697–702. [PubMed] [Google Scholar]
- Frerichs W. M., Holbrook A. A., Johnson A. J. Equine piroplasmosis: production of antigens for the complement-fixation test. Am J Vet Res. 1969 Aug;30(8):1337–1341. [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Holbrook A. A. Biology of equine piroplasmosis. J Am Vet Med Assoc. 1969 Jul 15;155(2):453–454. [PubMed] [Google Scholar]
- Howard R. J. Vaccination against malaria: recent advances and the problems of antigenic diversity and other parasite evasion mechanisms. Int J Parasitol. 1987 Feb;17(1):17–29. doi: 10.1016/0020-7519(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Kuttler K. L., Gipson C. A., Goff W. L., Johnson L. W. Experimental Babesia equi infection in mature horses. Am J Vet Res. 1986 Aug;47(8):1668–1670. [PubMed] [Google Scholar]
- Madden P. A., Holbrook A. A. Equine piroplasmosis: indirect fluorescent antibody test for Babesia caballi. Am J Vet Res. 1968 Jan;29(1):117–123. [PubMed] [Google Scholar]
- McElwain T. F., Perryman L. E., Davis W. C., McGuire T. C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987 Apr 1;138(7):2298–2304. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. A., Buening G. M., Carson C. A. Cryopreservation of Babesia bovis for in vitro cultivation. Parasitology. 1982 Jun;84(Pt 3):567–572. doi: 10.1017/s0031182000052835. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Riggs M. W., McGuire T. C., Mason P. H., Perryman L. E. Neutralization-sensitive epitopes are exposed on the surface of infectious Cryptosporidium parvum sporozoites. J Immunol. 1989 Aug 15;143(4):1340–1345. [PubMed] [Google Scholar]
- Rotman B., Papermaster B. W. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci U S A. 1966 Jan;55(1):134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIBINOVIC K. G., RISTIC M., SIBINOVIC S., PHILLIPS T. N. EQUINE BABESIOSIS: ISOLATION AND SEROLOGIC CHARACTERIZATION OF A BLOOD SERUM ANTIGEN FROM ACUTELY INFECTED HORSES. Am J Vet Res. 1965 Jan;26:147–153. [PubMed] [Google Scholar]
- SIPPEL W. L., COOPERRIDER D. E., GAINER J. H., ALLEN R. W., MOUW J. E., TEIGLAND M. B. Equine piroplasmosis in the United States. J Am Vet Med Assoc. 1962 Sep 15;141:694–698. [PubMed] [Google Scholar]
- Saul A. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 1987 Jan;9(1):1–9. doi: 10.1111/j.1365-3024.1987.tb00483.x. [DOI] [PubMed] [Google Scholar]
- Sibinovic K. H., Milar R., Ristic M., Cox H. W. In vivo and in vitro effects of serum antigens of babesial infection and their antibodies on parasitized and normal erythrocytes. Ann Trop Med Parasitol. 1969 Sep;63(3):327–336. doi: 10.1080/00034983.1969.11686633. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]