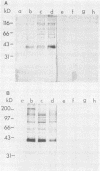
Abstract
Peripheral blood mononuclear cells from cattle experimentally infected with Babesia bovis were examined for parasite-specific cell-mediated immune responses. Unfractionated merozoites and soluble and membrane fractions derived from merozoites were all antigenic for immune cattle, although the membrane fraction was the most stimulatory. Cattle responded to different antigenic fractions in a differential manner, and only that animal immunized with autologous cultured parasites responded to parasitized erythrocyte culture supernatants. Plastic-adherent cells (presumably monocytes/macrophages) were required for a proliferative response to babesial antigens but not to the T-cell mitogen concanavalin A, suggesting that babesial proteins are not simply mitogenic for T cells. Lymphocyte responses directed against a different hemoparasite from Mexico, Babesia bigemina, indicate that this parasite shares cross-reactive T-cell epitopes with B. bovis. These studies define a system whereby T lymphocytes from babesia-immune cattle can be used in proliferation assays to identify babesial merozoite antigens which are immunogenic for T cells. Because identification of helper T-cell epitopes is important for the design of a babesial subunit vaccine which will evoke anamnestic responses, the studies described here provide a basis for such experiments.
Full text
PDF

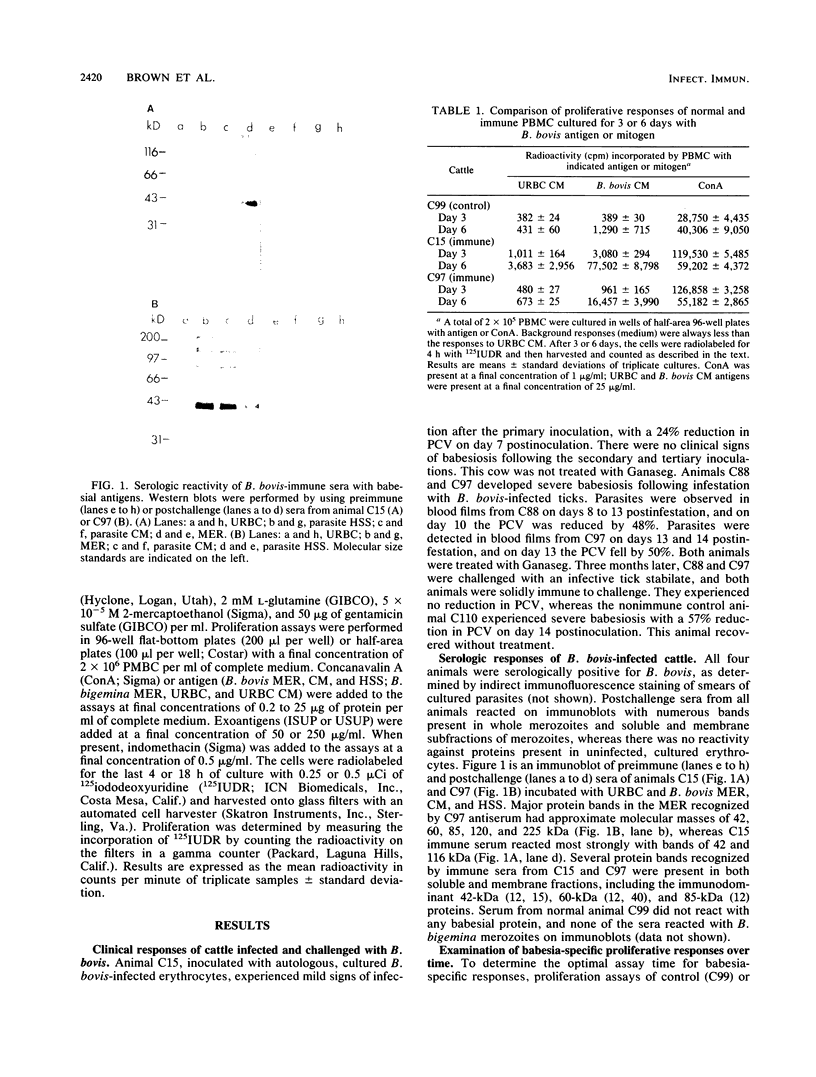
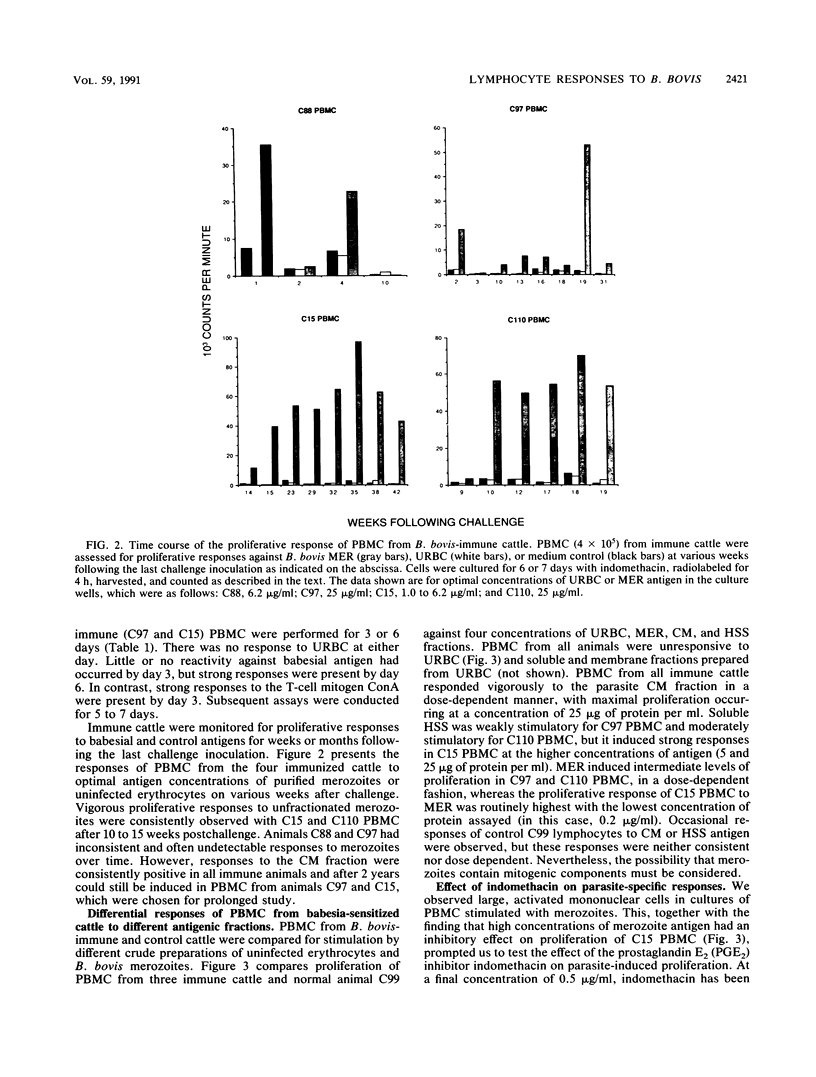
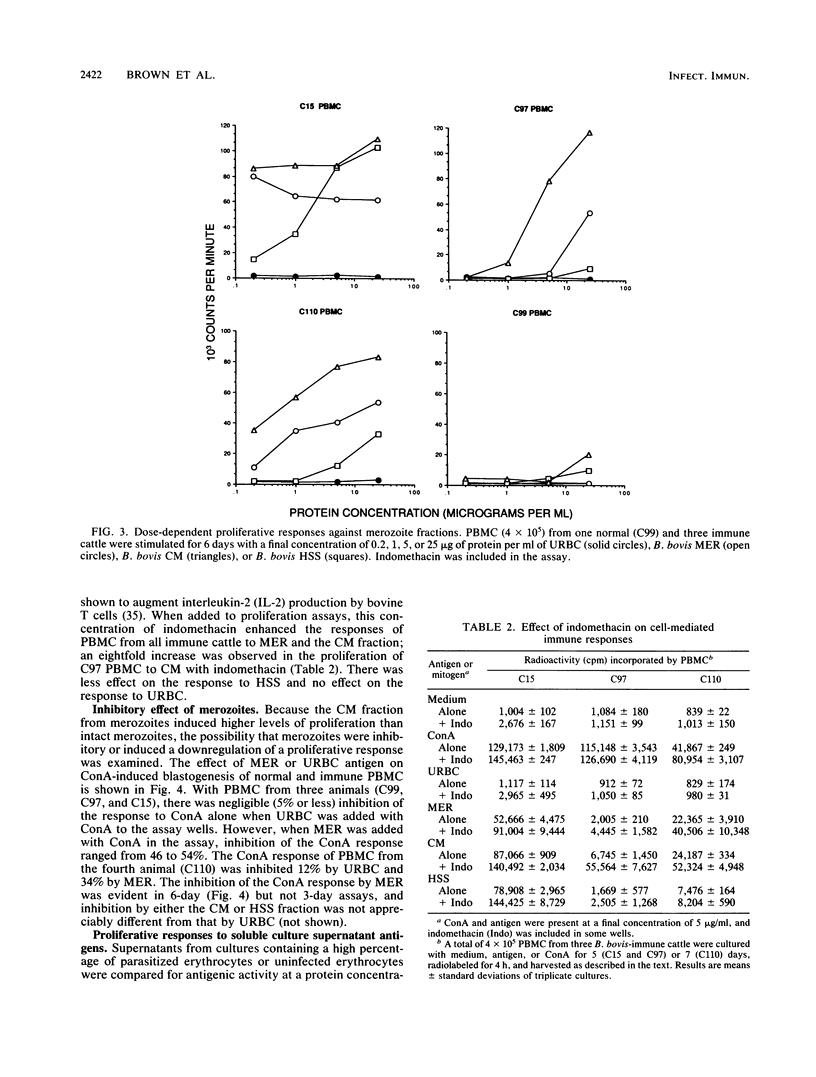



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. C., Logan K. S. Bovine T-cell clones infected with Theileria parva produce a factor with IL 2-like activity. Parasite Immunol. 1986 Mar;8(2):189–192. doi: 10.1111/j.1365-3024.1986.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Sugimoto C., Conrad P. A., Grab D. J. Differential response of bovine T-cell lines to membrane and soluble antigens of Theileria parva schizont-infected cells. Parasite Immunol. 1989 Nov;11(6):567–583. doi: 10.1111/j.1365-3024.1989.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Chatenoud L., Klatzmann D., Fradelizi D. The mechanisms of inhibition of human IL 2 production. II. PGE2 induction of suppressor T lymphocytes. J Immunol. 1984 Apr;132(4):1851–1857. [PubMed] [Google Scholar]
- Chulay J. D. Development of sporozoite vaccines for malaria. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):61–66. doi: 10.1016/0035-9203(89)90606-8. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Emery D. L. Adoptive transfer of immunity to infection with Theileria parva (East Coast fever) between cattle twins. Res Vet Sci. 1981 May;30(3):364–367. [PubMed] [Google Scholar]
- Goddeeris B. M., Baldwin C. L., ole-MoiYoi O., Morrison W. I. Improved methods for purification and depletion of monocytes from bovine peripheral blood mononuclear cells. Functional evaluation of monocytes in responses to lectins. J Immunol Methods. 1986 May 22;89(2):165–173. doi: 10.1016/0022-1759(86)90354-6. [DOI] [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Yunker C. E. Babesia bovis: increased percentage parasitized erythrocytes in cultures and assessment of growth by incorporation of [3H]hypoxanthine. Exp Parasitol. 1986 Oct;62(2):202–210. doi: 10.1016/0014-4894(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Hines S. A., McElwain T. F., Buening G. M., Palmer G. H. Molecular characterization of Babesia bovis merozoite surface proteins bearing epitopes immunodominant in protected cattle. Mol Biochem Parasitol. 1989 Nov;37(1):1–9. doi: 10.1016/0166-6851(89)90096-0. [DOI] [PubMed] [Google Scholar]
- Holman P. J., Waldrup K. A., Wagner G. G. In vitro cultivation of a Babesia isolated from a white-tailed deer (Odocoileus virginianus). J Parasitol. 1988 Feb;74(1):111–115. [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Kuttler K. L., Levy M. G., James M. A., Ristic M. Efficacy of a nonviable culture-derived Babesia bovis vaccine. Am J Vet Res. 1982 Feb;43(2):281–284. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy M. G., Ristic M. Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science. 1980 Mar 14;207(4436):1218–1220. doi: 10.1126/science.7355284. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Kerr J. D., Goodger B. V., Wright I. G. The immune response of cattle to Babesia bovis (syn. B. argentina). Studies on the nature and specificity of protection. Int J Parasitol. 1979 Aug;9(4):297–306. doi: 10.1016/0020-7519(79)90078-x. [DOI] [PubMed] [Google Scholar]
- Mahoney D. F., Wright I. G., Goodger B. V. Bovine babesiosis: the immunization of cattle with fractions of erythrocytes infected with Babesia bovis (syn B. argentina). Vet Immunol Immunopathol. 1981 Apr;2(2):145–156. doi: 10.1016/0165-2427(81)90046-5. [DOI] [PubMed] [Google Scholar]
- McElwain T. F., Palmer G. H., Goff W. L., McGuire T. C. Identification of Babesia bigemina and Babesia bovis merozoite proteins with isolate- and species-common epitopes recognized by antibodies in bovine immune sera. Infect Immun. 1988 Jun;56(6):1658–1660. doi: 10.1128/iai.56.6.1658-1660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. L., Silverman P. H., Kullgren B., Mahlmann L. J. Tumor necrosis factor alpha and the anemia associated with murine malaria. Infect Immun. 1989 May;57(5):1542–1546. doi: 10.1128/iai.57.5.1542-1546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro-James S., Ristic M., Toro Benitez M., Leon E., Lopez R. Heterologous strain immunity in bovine babesiosis using a culture-derived soluble Babesia bovis immunogen. Vet Parasitol. 1985 Dec;18(4):321–337. doi: 10.1016/0304-4017(85)90067-6. [DOI] [PubMed] [Google Scholar]
- Morrison W. I., Goddeeris B. M., Brown W. C., Baldwin C. L., Teale A. J. Theileria parva in cattle: characterization of infected lymphocytes and the immune responses they provoke. Vet Immunol Immunopathol. 1989 Feb;20(3):213–237. doi: 10.1016/0165-2427(89)90003-2. [DOI] [PubMed] [Google Scholar]
- Pearson T. W., Lundin L. B., Dolan T. T., Stagg D. A. Cell-mediated immunity to Theileria-transformed cell lines. Nature. 1979 Oct 25;281(5733):678–680. doi: 10.1038/281678a0. [DOI] [PubMed] [Google Scholar]
- Ruebush M. J., Hanson W. L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980 Jul;29(4):507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- Ruebush M. J., Steel L. K., Kennedy D. A. Prostaglandin-mediated suppression of delayed-type hypersensitivity to infected erythrocytes during Babesia microti infection in mice. Cell Immunol. 1986 Apr 1;98(2):300–310. doi: 10.1016/0008-8749(86)90290-x. [DOI] [PubMed] [Google Scholar]
- Sambhara S. R., Belden E. L. Bovine interleukin 2: production and characterization. Vet Immunol Immunopathol. 1988 Mar;18(2):165–172. doi: 10.1016/0165-2427(88)90058-x. [DOI] [PubMed] [Google Scholar]
- Scott P., Caspar P., Sher A. Protection against Leishmania major in BALB/c mice by adoptive transfer of a T cell clone recognizing a low molecular weight antigen released by promastigotes. J Immunol. 1990 Feb 1;144(3):1075–1079. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., Carpenter J., Cabrera A., Gravely S. M., Erp E. E., Osorno M., Ristic M. Bovine babesiosis: vaccination against tick-borne challenge exposure with culture-derived Babesia bovis immunogens. Am J Vet Res. 1979 Dec;40(12):1678–1682. [PubMed] [Google Scholar]
- Suarez C. E., Palmer G. H., Jasmer D. P., Hines S. A., Perryman L. E., McElwain T. F. Characterization of the gene encoding a 60-kilodalton Babesia bovis merozoite protein with conserved and surface exposed epitopes. Mol Biochem Parasitol. 1991 May;46(1):45–52. doi: 10.1016/0166-6851(91)90197-e. [DOI] [PubMed] [Google Scholar]
- Timms P. Development of babesial vaccines. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):73–79. doi: 10.1016/0035-9203(89)90608-1. [DOI] [PubMed] [Google Scholar]
- Timms P., Stewart N. P., Rodwell B. J., Barry D. N. Immune responses of cattle following vaccination with living and non-living Babesia bovis antigens. Vet Parasitol. 1984 Nov;16(3-4):243–251. doi: 10.1016/0304-4017(84)90042-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troye-Blomberg M., Perlmann H., Patarroyo M. E., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. II. Antigen specific proliferative responses in vitro. Clin Exp Immunol. 1983 Aug;53(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Winger C. M., Canning E. U., Culverhouse J. D. Induction of protective immunity to Babesia divergens in Mongolian gerbils, Meriones unguiculatus, using culture-derived immunogens. Vet Parasitol. 1987 Dec;26(1-2):43–53. doi: 10.1016/0304-4017(87)90075-6. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Buffington G. D., Clark I. A., Parrodi F., Waltisbuhl D. J. Immunopathophysiology of babesial infections. Trans R Soc Trop Med Hyg. 1989;83 (Suppl):11–13. doi: 10.1016/0035-9203(89)90596-8. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Goodger B. V., Clark I. A. Immunopathophysiology of Babesia bovis and Plasmodium falciparum infections. Parasitol Today. 1988 Aug;4(8):214–218. doi: 10.1016/0169-4758(88)90161-5. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Brown J. Malaria antigen-specific T-cell responsiveness during infection with Plasmodium falciparum. Clin Exp Immunol. 1977 Sep;29(3):401–407. [PMC free article] [PubMed] [Google Scholar]