Abstract
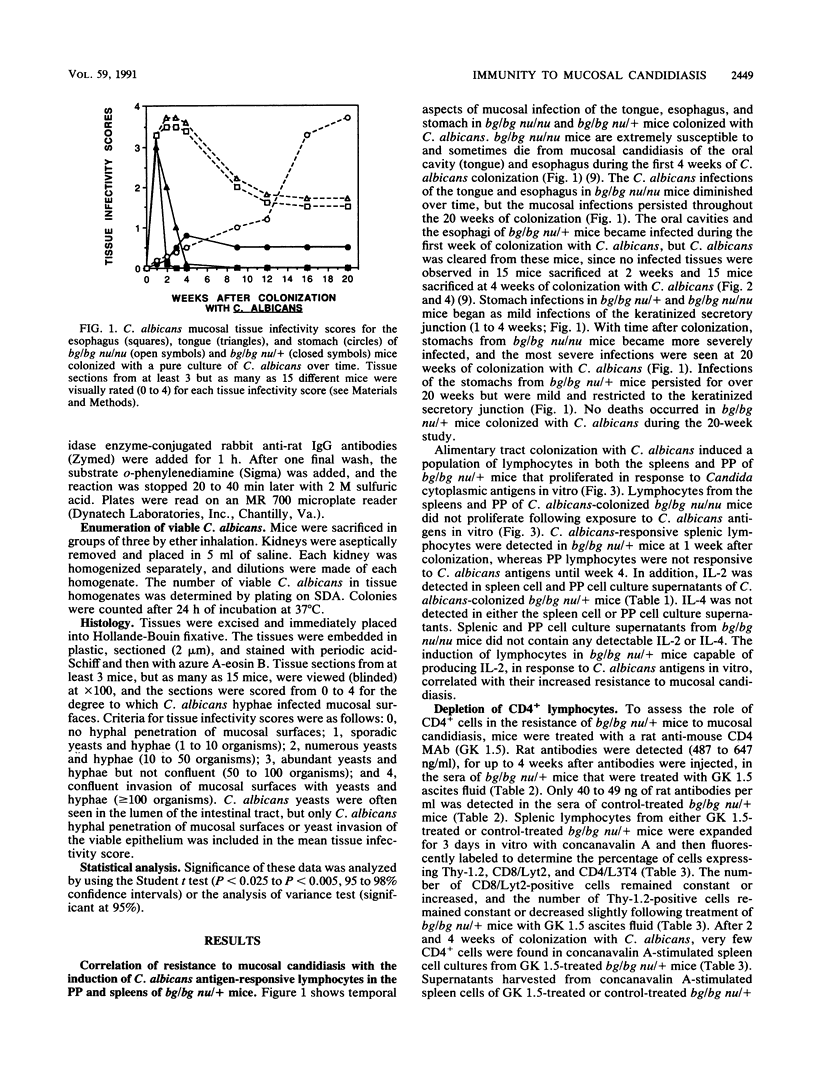
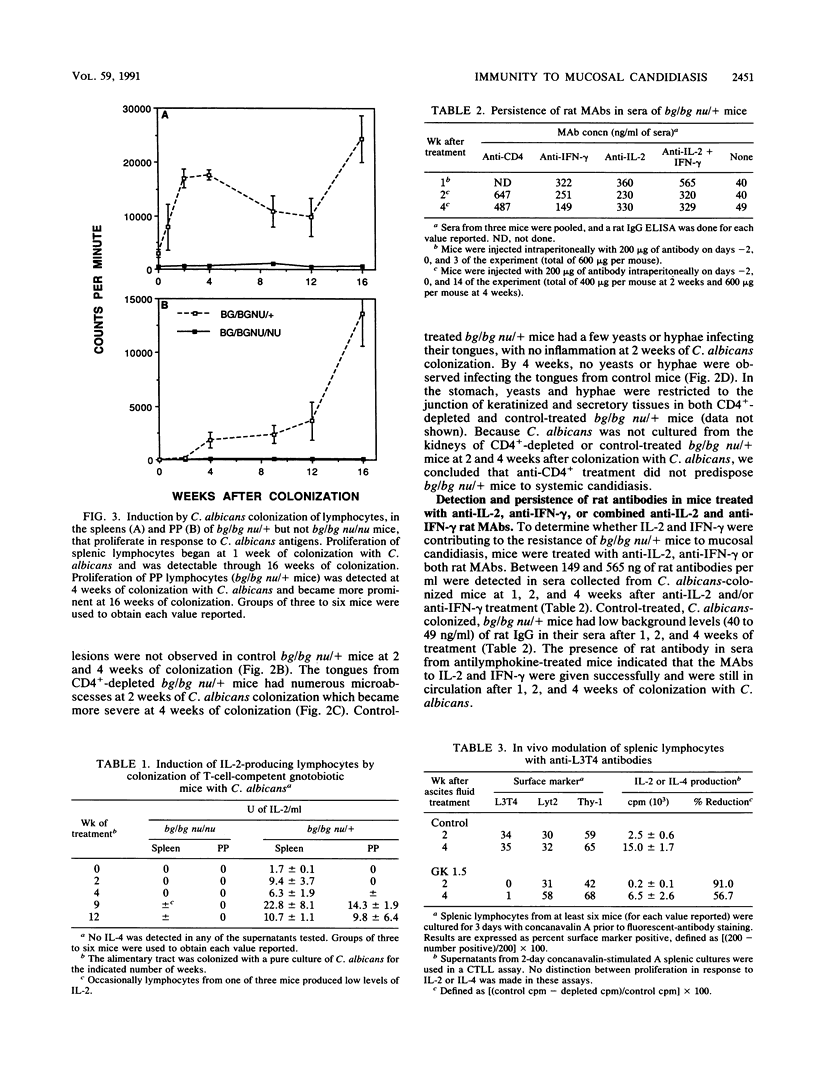
The role of CD4+ lymphocytes in resistance of N:NIH(S) III bg/bg nu/+ mice to mucosal candidiasis was evaluated. Alimentary tract colonization with a pure culture of Candida albicans induced a population of lymphocytes in both the Peyer's patches and spleens of bg/bg nu/+ mice, but not bg/bg nu/nu mice, that proliferated and produced interleukin-2 (IL-2) in response to C. albicans antigens. The induction of candida-specific lymphocytes correlated with the clearance of C. albicans from the esophagus and tongue of resistant bg/bg nu/+ mice. Isogenic bg/bg nu/nu mice which do not develop candida-reactive lymphocytes were unable to clear C. albicans from their tongues and esophagi. Treatment of bg/bg nu/+ mice with anti-CD4+ monoclonal antibodies depleted their CD4+ lymphocytes and increased their susceptibility to mucosal candidiasis of the tongue and esophagus. In vivo treatment of bg/bg nu/+ mice with anti-IL-2, anti-gamma interferon (IFN-gamma), or both anti-IL-2 and anti-IFN-gamma monoclonal antibodies did not abrogate their resistance to mucosal candidiasis. Furthermore, treatment of C. albicans-susceptible bg/bg nu/nu mice with IFN-gamma and IL-2 did not protect them from mucosal candidiasis. Thus, CD4+ cells apparently play a critical role in resistance to mucosal candidiasis; however, we were unable to demonstrate a role for IL-2 and IFN-gamma in mediating resistance to mucosal candidiasis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendorf T. M., Walker D. M. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol. 1980;25(1):1–10. doi: 10.1016/0003-9969(80)90147-8. [DOI] [PubMed] [Google Scholar]
- Balish E., Balish M. J., Salkowski C. A., Lee K. W., Bartizal K. F. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl Environ Microbiol. 1984 Apr;47(4):647–652. doi: 10.1128/aem.47.4.647-652.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Filutowicz H., Oberley T. D. Correlates of cell-mediated immunity in Candida albicans-colonized gnotobiotic mice. Infect Immun. 1990 Jan;58(1):107–113. doi: 10.1128/iai.58.1.107-113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balish E., Phillips A. W. Growth and virulence of Candida albicans after oral inoculation in the chick with a monoflora of either Escherichia coli or Streptococcus faecalis. J Bacteriol. 1966 May;91(5):1744–1749. doi: 10.1128/jb.91.5.1744-1749.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Finbloom D. S., Van Der Meide P. H., Slayter M. V., Nacy C. A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989 Jul 1;143(1):266–274. [PubMed] [Google Scholar]
- Blasi E., Farinelli S., Varesio L., Bistoni F. Augmentation of GG2EE macrophage cell line-mediated anti-Candida activity by gamma interferon, tumor necrosis factor, and interleukin-1. Infect Immun. 1990 Apr;58(4):1073–1077. doi: 10.1128/iai.58.4.1073-1077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Anaissie E. J. Chronic systemic candidiasis. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):855–857. doi: 10.1007/BF01963770. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990 Apr;58(4):1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. T cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J Immunol. 1990 Jun 1;144(11):4333–4339. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F., Young K. M., Cooley A. J. Administration of purified anti-L3T4 monoclonal antibody impairs the resistance of mice to Listeria monocytogenes infection. Infect Immun. 1989 Jan;57(1):100–109. doi: 10.1128/iai.57.1.100-109.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps M. M., Pape J. W., Verdier R. I., DeHovitz J., Thomas F., Johnson W. D., Jr Treatment of candida esophagitis in AIDS patients. Am J Gastroenterol. 1988 Jan;83(1):20–21. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Fujihashi K., Kiyono H., Aicher W. K., Green D. R., Singh B., Eldridge J. H., McGhee J. R. Immunoregulatory function of CD3+, CD4-, and CD8- T cells. Gamma delta T cell receptor-positive T cells from nude mice abrogate oral tolerance. J Immunol. 1989 Dec 1;143(11):3415–3422. [PubMed] [Google Scholar]
- Galli M., Lazzarin A., Saracco A., Balotta C., Castagna A., Negri C., Ridolfo A. L., Uberti-Foppa C., Corbellino M., Moroni M. Clinical and immunological aspects of HIV infection in drug addicts. Clin Immunol Immunopathol. 1989 Jan;50(1 Pt 2):S166–S176. doi: 10.1016/0090-1229(89)90124-4. [DOI] [PubMed] [Google Scholar]
- Garner R. E., Kuruganti U., Czarniecki C. W., Chiu H. H., Domer J. E. In vivo immune responses to Candida albicans modified by treatment with recombinant murine gamma interferon. Infect Immun. 1989 Jun;57(6):1800–1808. doi: 10.1128/iai.57.6.1800-1808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez A. M., Bullock W. E., Taylor C. L., Deepe G. S., Jr Role of L3T4+ T cells in host defense against Histoplasma capsulatum. Infect Immun. 1988 Jul;56(7):1685–1691. doi: 10.1128/iai.56.7.1685-1691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizawa Y., Nishi T., Kondo M., Tsuchiya K., Imada A. Effect of recombinant human interleukin-2 on the course of experimental chronic respiratory tract infection caused by Klebsiella pneumoniae in mice. Infect Immun. 1988 Jan;56(1):45–50. doi: 10.1128/iai.56.1.45-50.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevan A., Asherson G. L. Recombinant interleukin-2 limits the replication of Mycobacterium lepraemurium and Mycobacterium bovis BCG in mice. Infect Immun. 1988 Mar;56(3):660–664. doi: 10.1128/iai.56.3.660-664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Li H., Jerrells T. R., Spitalny G. L., Walker D. H. Gamma interferon as a crucial host defense against Rickettsia conorii in vivo. Infect Immun. 1987 May;55(5):1252–1255. doi: 10.1128/iai.55.5.1252-1255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H., Onozuka K., Terada Y., Nakano Y., Nakano M. Effect of murine recombinant interferon-gamma in the protection of mice against Salmonella. Int J Immunopharmacol. 1990;12(1):49–56. doi: 10.1016/0192-0561(90)90067-w. [DOI] [PubMed] [Google Scholar]
- Mody C. H., Lipscomb M. F., Street N. E., Toews G. B. Depletion of CD4+ (L3T4+) lymphocytes in vivo impairs murine host defense to Cryptococcus neoformans. J Immunol. 1990 Feb 15;144(4):1472–1477. [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Munk M. E., Gatrill A. J., Kaufmann S. H. Target cell lysis and IL-2 secretion by gamma/delta T lymphocytes after activation with bacteria. J Immunol. 1990 Oct 15;145(8):2434–2439. [PubMed] [Google Scholar]
- Old L. J. Tumour necrosis factor. Polypeptide mediator network. 1987 Mar 26-Apr 1Nature. 326(6111):330–331. doi: 10.1038/326330a0. [DOI] [PubMed] [Google Scholar]
- Pankhurst C., Peakman M. Reduced CD4 + T cells and severe oral candidiasis in absence of HIV infection. Lancet. 1989 Mar 25;1(8639):672–672. doi: 10.1016/s0140-6736(89)92176-4. [DOI] [PubMed] [Google Scholar]
- Phillips A. W., Balish E. Growth and invasiveness of Candida albicans in the germ-free and conventional mouse after oral challenge. Appl Microbiol. 1966 Sep;14(5):737–741. doi: 10.1128/am.14.5.737-741.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkowski C. A., Balish E. A monoclonal antibody to gamma interferon blocks augmentation of natural killer cell activity induced during systemic cryptococcosis. Infect Immun. 1991 Feb;59(2):486–493. doi: 10.1128/iai.59.2.486-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. D., Hofflin J. M., Remington J. S. In vivo recombinant interleukin 2 administration enhances survival against a lethal challenge with Toxoplasma gondii. J Immunol. 1985 Dec;135(6):4160–4163. [PubMed] [Google Scholar]
- Wang M., Friedman H., Djeu J. Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [PubMed] [Google Scholar]
- Williams D. M., Byrne G. I., Grubbs B., Marshal T. J., Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988 Nov;56(11):3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]





