Abstract
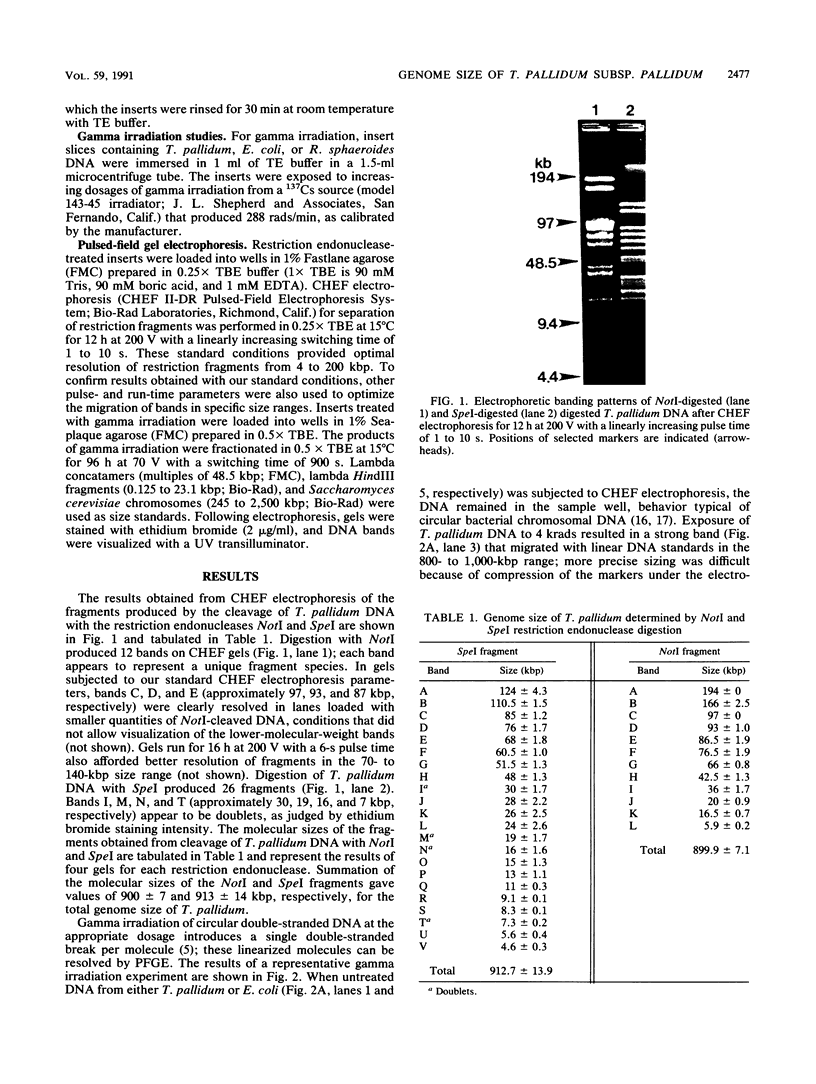
The genome size and chromosome conformation of Treponema pallidum subsp. pallidum, Nichols strain, were determined by contour-clamped homogeneous electric field electrophoresis, a pulsed-field gel electrophoresis technique. Digestion of T. pallidum subsp. pallidum DNA with the restriction endonucleases NotI and SpeI produced 12 and 26 fragments, respectively. Summation of the physical lengths of the fragments produced by NotI and SpeI cleavage yielded average sizes of 900 and 913 kbp, respectively, for the genome of T. pallidum subsp. pallidum. Contour-clamped homogeneous electric field electrophoresis of T. pallidum subsp. pallidum DNA exposed to 4 krads of gamma irradiation resolved a single band of 800 to 1,000 kbp; treatment of the DNA with 16 krads of gamma irradiation resulted in the production of smaller fragments, whereas untreated DNA did not migrate into the gels. The gamma irradiation results indicate that T. pallidum subsp. pallidum has a single, circular chromosome that was linearized at a dosage of 4 krads of gamma irradiation. The size estimate provided by restriction endonuclease digestion with NotI and SpeI shows that the genome of T. pallidum subsp. pallidum, at approximately 900 kbp, is considerably smaller than the 13,700-kbp genome size calculated from renaturation kinetics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Beverley S. M. Characterization of the 'unusual' mobility of large circular DNAs in pulsed field-gradient electrophoresis. Nucleic Acids Res. 1988 Feb 11;16(3):925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M. Estimation of circular DNA size using gamma-irradiation and pulsed-field gel electrophoresis. Anal Biochem. 1989 Feb 15;177(1):110–114. doi: 10.1016/0003-2697(89)90023-7. [DOI] [PubMed] [Google Scholar]
- Chang N., Taylor D. E. Use of pulsed-field agarose gel electrophoresis to size genomes of Campylobacter species and to construct a SalI map of Campylobacter jejuni UA580. J Bacteriol. 1990 Sep;172(9):5211–5217. doi: 10.1128/jb.172.9.5211-5217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff P. A., Norris S. J., Lovett M. A., Miller J. N. Purification of Treponema pallidum, Nichols strain, by Percoll density gradient centrifugation. Sex Transm Dis. 1984 Oct-Dec;11(4):275–286. doi: 10.1097/00007435-198410000-00003. [DOI] [PubMed] [Google Scholar]
- Hightower R. C., Metge D. W., Santi D. V. Plasmid migration using orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1987 Oct 26;15(20):8387–8398. doi: 10.1093/nar/15.20.8387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawiec S., Riley M. Organization of the bacterial chromosome. Microbiol Rev. 1990 Dec;54(4):502–539. doi: 10.1128/mr.54.4.502-539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Anomalous values of Mycoplasma genomes sizes determined by pulse-field gel electrophoresis. Nucleic Acids Res. 1989 Feb 11;17(3):1268–1268. doi: 10.1093/nar/17.3.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Miller J. N. Plasmid DNA in Treponema pallidum (Nichols): potential for antibiotic resistance by syphilis bacteria. Science. 1981 Jul 31;213(4507):553–555. doi: 10.1126/science.6264606. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Condemine G. New approaches for physical mapping of small genomes. J Bacteriol. 1990 Mar;172(3):1167–1172. doi: 10.1128/jb.172.3.1167-1172.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: genome size, fragment identification, and gene localization. J Bacteriol. 1989 Nov;171(11):5840–5849. doi: 10.1128/jb.171.11.5840-5849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]