Abstract
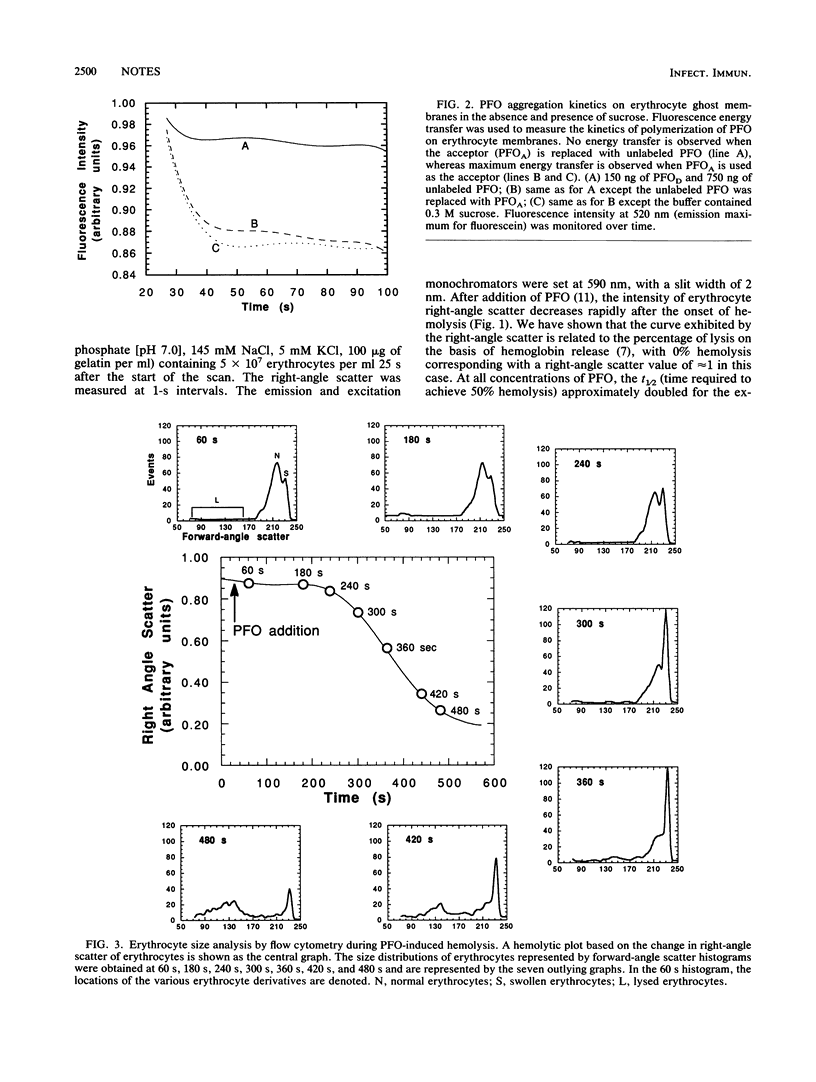
Clostridium perfringens theta-toxin was shown to lyse target erythrocytes by a colloid-osmotic mechanism. Analysis showed the onset of lysis of erythrocytes by theta-toxin could be temporarily stabilized with 0.3 M sucrose. Flow cytometry analysis of the size distribution of theta-toxin-treated erythrocytes showed swelling of the erythrocytes prior to lysis.
Full text
PDF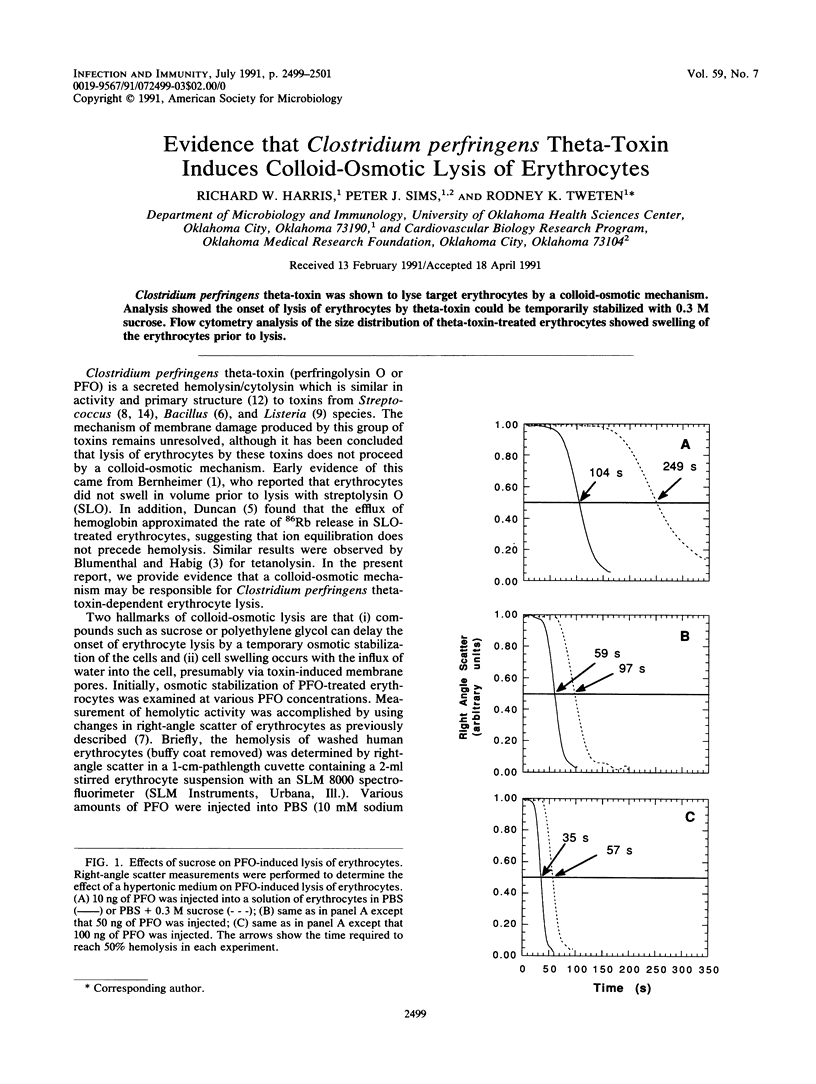

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W., Rudy B. Interactions between membranes and cytolytic peptides. Biochim Biophys Acta. 1986 Jun 12;864(1):123–141. doi: 10.1016/0304-4157(86)90018-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal R., Habig W. H. Mechanism of tetanolysin-induced membrane damage: studies with black lipid membranes. J Bacteriol. 1984 Jan;157(1):321–323. doi: 10.1128/jb.157.1.321-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER L. Z., MADOFF M. A., WEINSTEIN L. HEMOLYSIS OF RABBIT ERYTHROCYTES BY PURIFIED STAPHYLOCOCCAL ALPHA-TOXIN. II. EFFECTS OF INHIBITORS ON THE HEMOLYTIC SEQUENCE. J Bacteriol. 1964 Jan;87:136–144. doi: 10.1128/jb.87.1.136-144.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. L. Characteristics of streptolysin O hemolysis: kinetics of hemoglobin and 86rubidium release. Infect Immun. 1974 Jun;9(6):1022–1027. doi: 10.1128/iai.9.6.1022-1027.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy C., Mengaud J., Alouf J. E., Cossart P. Alveolysin, the thiol-activated toxin of Bacillus alvei, is homologous to listeriolysin O, perfringolysin O, pneumolysin, and streptolysin O and contains a single cysteine. J Bacteriol. 1990 Dec;172(12):7301–7305. doi: 10.1128/jb.172.12.7301-7305.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. W., Sims P. J., Tweten R. K. Kinetic aspects of the aggregation of Clostridium perfringens theta-toxin on erythrocyte membranes. A fluorescence energy transfer study. J Biol Chem. 1991 Apr 15;266(11):6936–6941. [PubMed] [Google Scholar]
- Kehoe M. A., Miller L., Walker J. A., Boulnois G. J. Nucleotide sequence of the streptolysin O (SLO) gene: structural homologies between SLO and other membrane-damaging, thiol-activated toxins. Infect Immun. 1987 Dec;55(12):3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengaud J., Vicente M. F., Chenevert J., Pereira J. M., Geoffroy C., Gicquel-Sanzey B., Baquero F., Perez-Diaz J. C., Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988 Apr;56(4):766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. Ultrastructure of membrane lesions in immune lysis, osmotic lysis and drug-induced lysis. Fed Proc. 1974 Oct;33(10):2116–2124. [PubMed] [Google Scholar]
- Tweten R. K. Cloning and expression in Escherichia coli of the perfringolysin O (theta-toxin) gene from Clostridium perfringens and characterization of the gene product. Infect Immun. 1988 Dec;56(12):3228–3234. doi: 10.1128/iai.56.12.3228-3234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten R. K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988 Dec;56(12):3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J. W., van den Engh G. J., van Bekkum D. W. Light scattering properties of murine hemopoietic cells. Blood Cells. 1980;6(3):391–407. [PubMed] [Google Scholar]
- Walker J. A., Allen R. L., Falmagne P., Johnson M. K., Boulnois G. J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987 May;55(5):1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


