Abstract
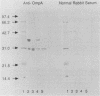
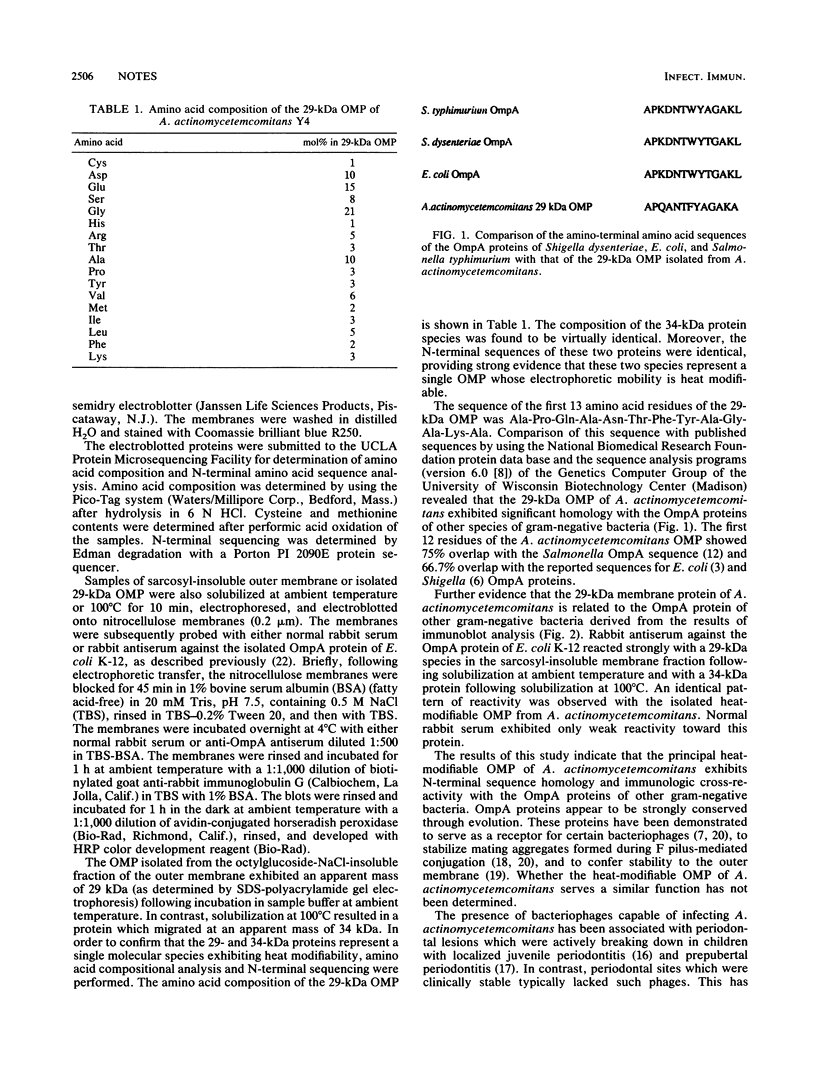
The outer membrane of Actinobacillus actinomycetemcomitans contains a 29-kDa protein which exhibits heat modifiability on sodium dodecyl sulfate-polyacrylamide gels and represents a major target for immunoglobulin G antibody in sera of periodontitis patients colonized by this organism. In the present study, the N-terminal amino acid sequence of the 29-kDa outer membrane protein was determined and compared with reported sequences for other known proteins. The heat-modifiable outer membrane protein of A. actinomycetemcomitans was found to exhibit significant N-terminal homology with the OmpA proteins of other gram-negative bacteria. Moreover, this protein reacted with antiserum raised against the purified OmpA protein of Escherichia coli K-12. Whether the heat-modifiable OMP of A. actinomycetemcomitans also shares functional properties of OmpA proteins, particularly with respect to bacteriophage receptor activity, is presently under investigation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Baker P. J., Wilson M. E. Opsonic IgG antibody against Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Oral Microbiol Immunol. 1989 Jun;4(2):98–105. doi: 10.1111/j.1399-302x.1989.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad A. I., Kristoffersen T., Olsen I., Preus H. R., Jesen H. B., Vasstrand E. N., Bakken V. Outer membrane proteins of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus studied by SDS-PAGE and immunoblotting. Oral Microbiol Immunol. 1990 Jun;5(3):155–161. doi: 10.1111/j.1399-302x.1990.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. The nucleotide sequence coding for major outer membrane protein OmpA of Shigella dysenteriae. Nucleic Acids Res. 1982 Apr 10;10(7):2367–2378. doi: 10.1093/nar/10.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo J. M., Spieler E. L. Identification and characterization of the major cell envelope proteins of oral strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1983 Jan;39(1):253–261. doi: 10.1128/iai.39.1.253-261.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farida R., Wilson M., Ivanyi L. Serum IgG antibodies to lipopolysaccharides in various forms of periodontal disease in man. Arch Oral Biol. 1986;31(11):711–715. doi: 10.1016/0003-9969(86)90001-4. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Cole S. T. Cloning and molecular characterization of the ompA gene from Salmonella typhimurium. Eur J Biochem. 1983 Aug 15;134(3):497–502. doi: 10.1111/j.1432-1033.1983.tb07594.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preus H. R., Olsen I., Namork E. Association between bacteriophage-infected Actinobacillus actinomycetemcomitans and rapid periodontal destruction. J Clin Periodontol. 1987 Apr;14(4):245–247. doi: 10.1111/j.1600-051x.1987.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Preus H. R., Olsen I., Namork E. The presence of phage-infected Actinobacillus actinomycetemcomitans in localized juvenile periodontitis patients. J Clin Periodontol. 1987 Nov;14(10):605–609. doi: 10.1111/j.1600-051x.1987.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Henning U. Action of a major outer cell envelope membrane protein in conjugation of Escherichia coli K-12. J Bacteriol. 1977 Mar;129(3):1651–1652. doi: 10.1128/jb.129.3.1651-1652.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag I., Schwarz H., Hirota Y., Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978 Oct;136(1):280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alphen L., Havekes L., Lugtenberg B. Major outer membrane protein d of Escherichia coli K12. Purification and in vitro activity of bacteriophages k3 and f-pilus mediated conjugation. FEBS Lett. 1977 Mar 15;75(1):285–290. doi: 10.1016/0014-5793(77)80104-x. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Marsh P. D., Ivanyi L. Antigens of Actinobacillus actinomycetemcomitans identified by immunoblotting with sera from patients with localized human juvenile periodontitis and generalized severe periodontitis. Arch Oral Biol. 1989;34(8):649–656. doi: 10.1016/0003-9969(89)90020-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. E. IgG antibody response of localized juvenile periodontitis patients to the 29 kilodalton outer membrane protein of Actinobacillus actinomycetemcomitans. J Periodontol. 1991 Mar;62(3):211–218. doi: 10.1902/jop.1991.62.3.211. [DOI] [PubMed] [Google Scholar]
- Zambon J. J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985 Jan;12(1):1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]