Abstract
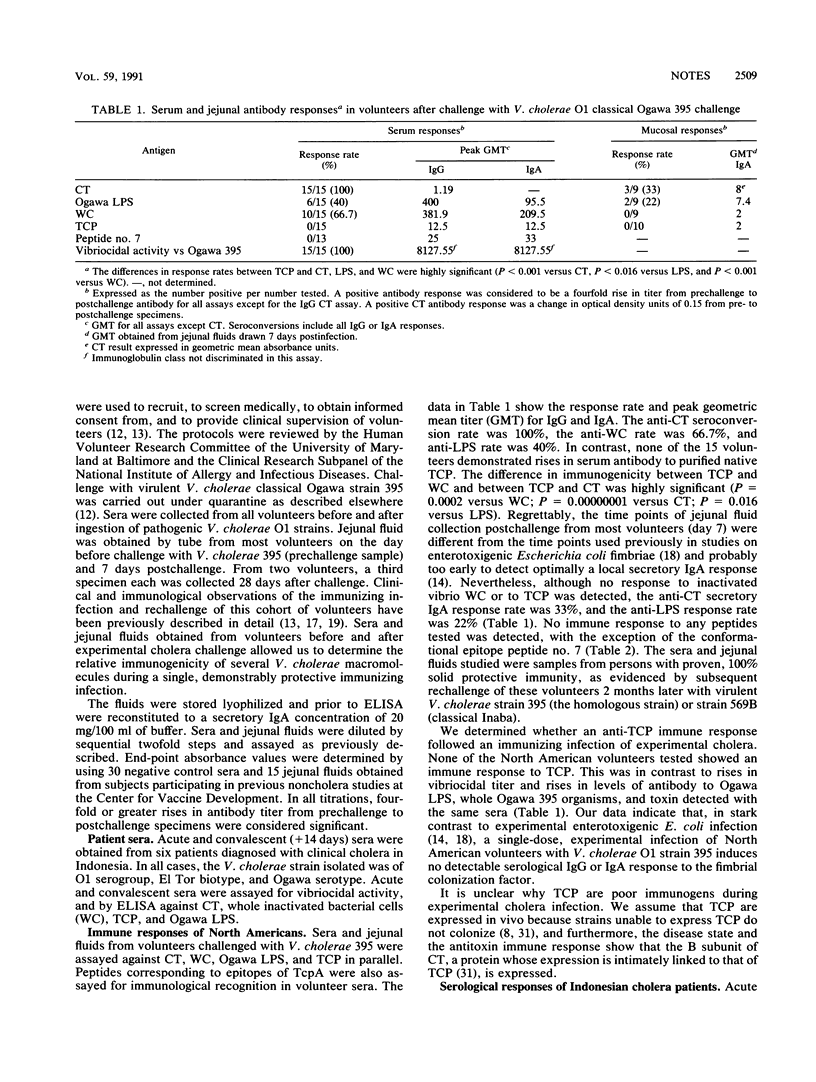
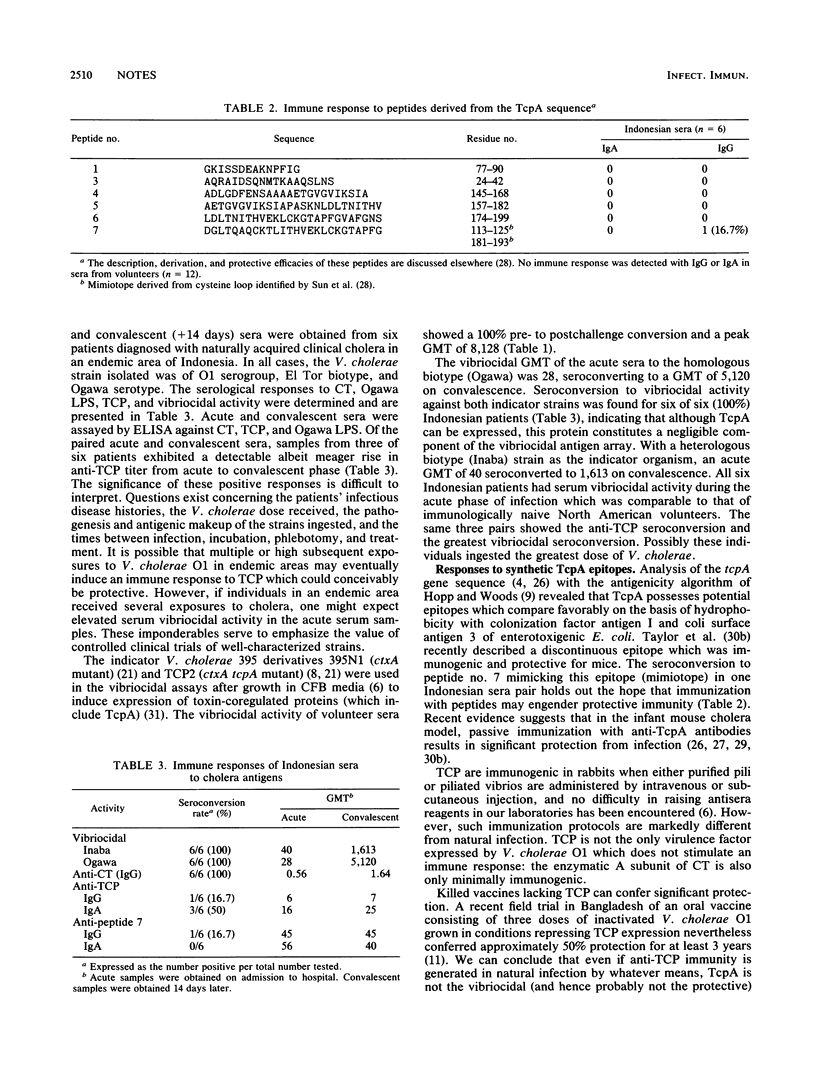
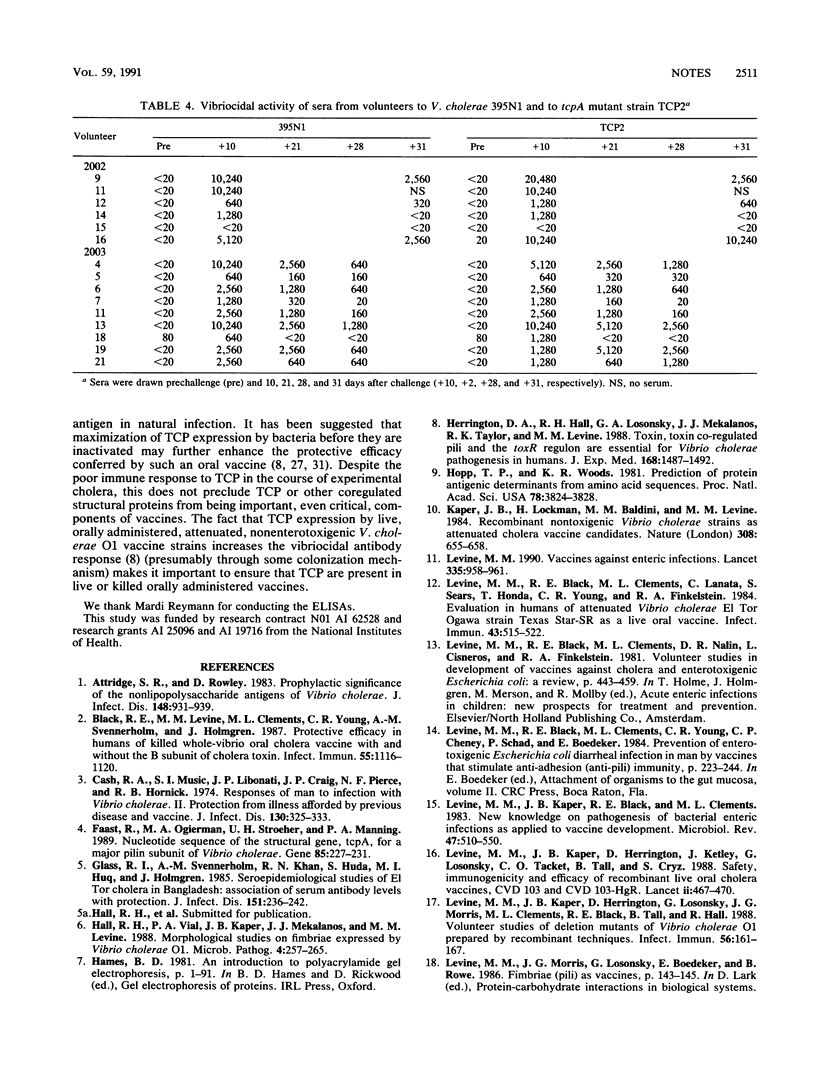
A functional tcpA gene, encoding the major subunit of toxin-coregulated pili (TCP), is necessary for Vibrio cholerae O1 Ogawa strain 395 to colonize the human intestine and confer protective immunity to virulent challenge. The immunogenicity of TCP and other antigens in experimental and naturally acquired cholera was determined. Seroconversion to cholera toxin (CT), whole cell preparations, and to Ogawa lipopolysaccharide but not to purified native TCP or to a TcpA mimiotope was found in volunteers. Local intestinal secretory immunoglobulin A from volunteers showed conversions to cholera toxin and lipopolysaccharide but not to TCP. Cholera patients in Indonesia showed a seroconversion rate to TCP of 3 of 6 and a seroconversion to a TcpA mimiotope of 1 of 6. Volunteer and patient sera showed similar vibriocidal seroconversions when assayed against either TCP-positive and TCP-negative V. cholerae O1 strains, suggesting that TCP do not contribute demonstrably to the vibriocidal antigen. We conclude that although seroconversion to TCP can occur in naturally acquired cholera, solid long-term protection can be engendered in the absence of a detectable anti-TCP immune response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. Prophylactic significance of the nonlipopolysaccharide antigens of Vibrio cholerae. J Infect Dis. 1983 Nov;148(5):931–939. doi: 10.1093/infdis/148.5.931. [DOI] [PubMed] [Google Scholar]
- Black R. E., Levine M. M., Clements M. L., Young C. R., Svennerholm A. M., Holmgren J. Protective efficacy in humans of killed whole-vibrio oral cholera vaccine with and without the B subunit of cholera toxin. Infect Immun. 1987 May;55(5):1116–1120. doi: 10.1128/iai.55.5.1116-1120.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Faast R., Ogierman M. A., Stroeher U. H., Manning P. A. Nucleotide sequence of the structural gene, tcpA, for a major pilin subunit of Vibrio cholerae. Gene. 1989 Dec 21;85(1):227–231. doi: 10.1016/0378-1119(89)90486-1. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Svennerholm A. M., Khan M. R., Huda S., Huq M. I., Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985 Feb;151(2):236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- Hall R. H., Vial P. A., Kaper J. B., Mekalanos J. J., Levine M. M. Morphological studies on fimbriae expressed by Vibrio cholerae 01. Microb Pathog. 1988 Apr;4(4):257–265. doi: 10.1016/0882-4010(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Lockman H., Baldini M. M., Levine M. M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984 Apr 12;308(5960):655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Lanata C., Sears S., Honda T., Young C. R., Finkelstein R. A. Evaluation in humans of attenuated Vibrio cholerae El Tor Ogawa strain Texas Star-SR as a live oral vaccine. Infect Immun. 1984 Feb;43(2):515–522. doi: 10.1128/iai.43.2.515-522.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Ketley J., Losonsky G., Tacket C. O., Tall B., Cryz S. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988 Aug 27;2(8609):467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Herrington D., Losonsky G., Morris J. G., Clements M. L., Black R. E., Tall B., Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988 Jan;56(1):161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Modern vaccines. Enteric infections. Lancet. 1990 Apr 21;335(8695):958–961. doi: 10.1016/0140-6736(90)91013-z. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Young C. R., Black R. E., Takeda Y., Finkelstein R. A. Enzyme-linked immunosorbent assay to measure antibodies to purified heat-labile enterotoxins from human and porcine strains of Escherichia coli and to cholera toxin: application in serodiagnosis and seroepidemiology. J Clin Microbiol. 1985 Feb;21(2):174–179. doi: 10.1128/jcm.21.2.174-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Mosley W. H., McCormack W. M., Ahmed A., Chowdhury A. K., Barui R. K. Report of the 1966-67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population--the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bull World Health Organ. 1969;40(2):187–197. [PMC free article] [PubMed] [Google Scholar]
- Sears S. D., Richardson K., Young C., Parker C. D., Levine M. M. Evaluation of the human immune response to outer membrane proteins of Vibrio cholerae. Infect Immun. 1984 May;44(2):439–444. doi: 10.1128/iai.44.2.439-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D. P., Thomas C., Hall R. H., Levine M. M., Attridge S. R. Significance of toxin-coregulated pili as protective antigens of Vibrio cholerae in the infant mouse model. Vaccine. 1989 Oct;7(5):451–456. doi: 10.1016/0264-410x(89)90161-8. [DOI] [PubMed] [Google Scholar]
- Shaw C. E., Taylor R. K. Vibrio cholerae O395 tcpA pilin gene sequence and comparison of predicted protein structural features to those of type 4 pilins. Infect Immun. 1990 Sep;58(9):3042–3049. doi: 10.1128/iai.58.9.3042-3049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Mekalanos J. J., Taylor R. K. Antibodies directed against the toxin-coregulated pilus isolated from Vibrio cholerae provide protection in the infant mouse experimental cholera model. J Infect Dis. 1990 Jun;161(6):1231–1236. doi: 10.1093/infdis/161.6.1231. [DOI] [PubMed] [Google Scholar]
- Sun D. X., Seyer J. M., Kovari I., Sumrada R. A., Taylor R. K. Localization of protective epitopes within the pilin subunit of the Vibrio cholerae toxin-coregulated pilus. Infect Immun. 1991 Jan;59(1):114–118. doi: 10.1128/iai.59.1.114-118.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Forrest B., Morona R., Attridge S. R., LaBrooy J., Tall B. D., Reymann M., Rowley D., Levine M. M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990 Jun;58(6):1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]


