Abstract
In monocytes, sulfatide, a lipid from Mycobacterium tuberculosis, blocked priming for enhanced release of superoxide (O2-) by the macrophage activating factors lipopolysaccharide, gamma interferon, interleukin-1 beta (IL-1 beta), tumor necrosis factor alpha (TNF-alpha), and muramyl dipeptide. Sulfatide, in the presence of lipopolysaccharide, also caused increased secretion of IL-1 beta and TNF-alpha into monocyte culture medium. Sulfatide altered the pattern of phosphorylation of monocyte proteins. Cell lysates prepared from monocytes treated with sulfatide showed decreased activity of protein kinase C, but sulfatide did not directly inhibit protein kinase C activity when added to lysates. A known inhibitor of protein kinase C, staurosporine, also inhibited O2- release and caused increased secretion of IL-1 beta. Thus, sulfatide appeared to indirectly affect protein kinase C, implicating protein kinase C as part of the mechanism of priming. Because sulfatide blocked priming for enhanced release of O2-, which could interfere with monocyte bactericidal activity, while causing enhanced secretion of IL-1 beta and TNF-alpha, which could promote formation of granulomata, sulfatide might be an important factor in the pathogenesis of M. tuberculosis.
Full text
PDF

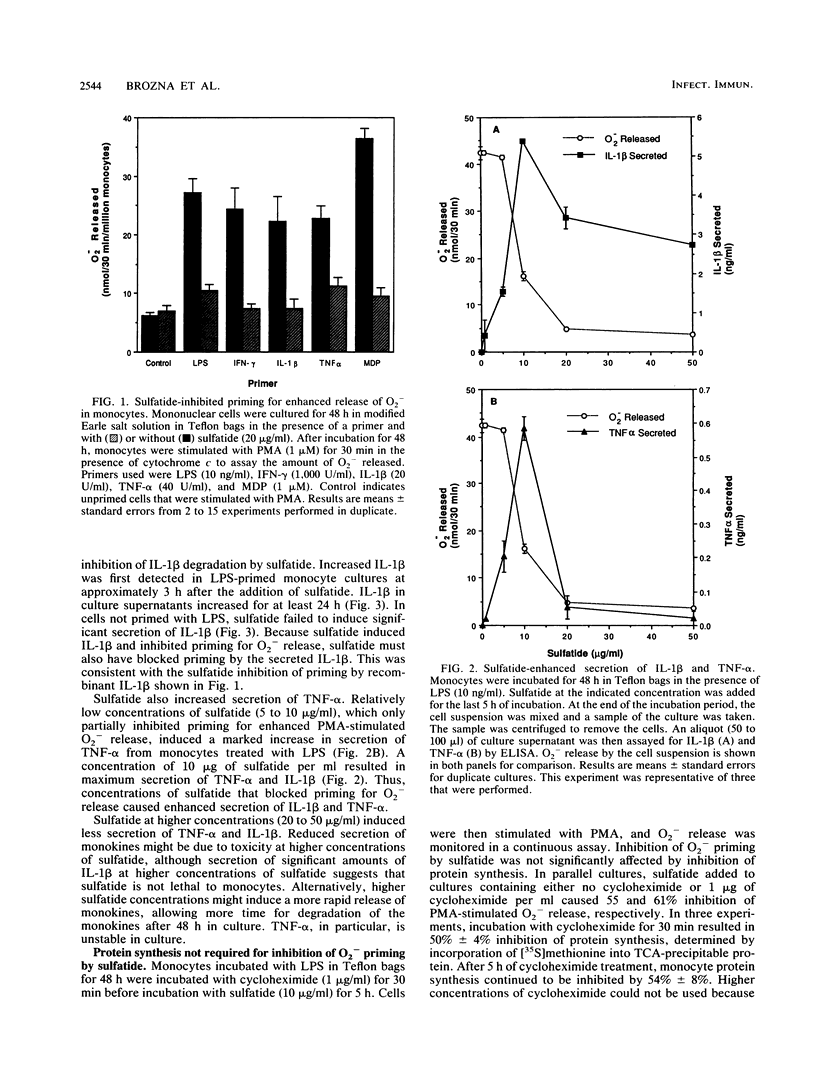
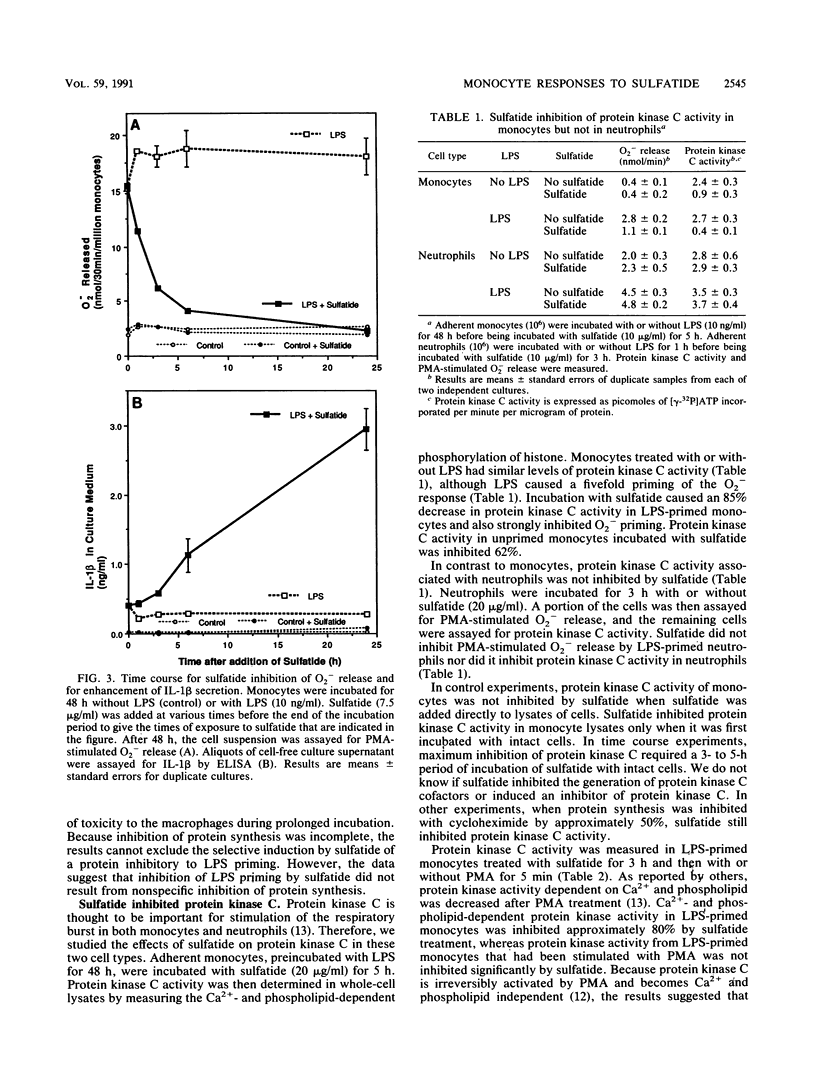
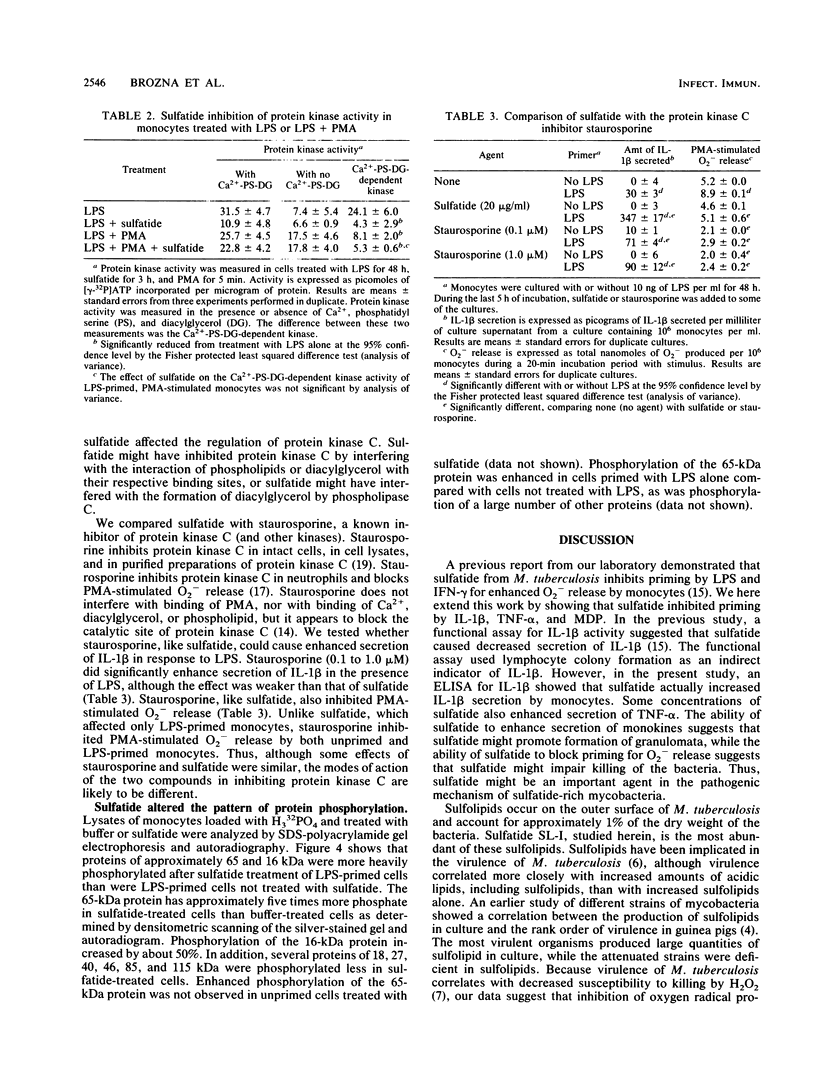


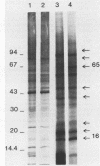
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brozna J. P., Hauff N. F., Phillips W. A., Johnston R. B., Jr Activation of the respiratory burst in macrophages. Phosphorylation specifically associated with Fc receptor-mediated stimulation. J Immunol. 1988 Sep 1;141(5):1642–1647. [PubMed] [Google Scholar]
- Bryant S. M., Guthrie L. A., Pabst M. J., Johnston R. B., Jr Macrophage membrane proteins: possible role in the regulation of priming for enhanced respiratory burst activity. Cell Immunol. 1986 Nov;103(1):216–223. doi: 10.1016/0008-8749(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANGADHARAM P. R., COHN M. L., MIDDLEBROOK G. INFECTIVITY, PATHOGENICITY AND SULPHOLIPID FRACTION OF SOME INDIAN AND BRITISH STRAINS OF TUBERCLE BACILLI. Tubercle. 1963 Dec;44:452–455. doi: 10.1016/s0041-3879(63)80087-2. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Das B. C. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I). Biochemistry. 1976 Jun 29;15(13):2728–2735. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Brokl O., Schaefer W. B. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect Immun. 1974 Jan;9(1):142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Grange J. M., Aber V. R., Allen B. W., Mitchison D. A. Role of lipid content and hydrogen peroxide susceptibility in determining the guinea-pig virulence of Mycobacterium tuberculosis. Br J Exp Pathol. 1982 Dec;63(6):693–700. [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J Biol Chem. 1989 Mar 5;264(7):4088–4092. [PubMed] [Google Scholar]
- Matsushima K., Shiroo M., Kung H. F., Copeland T. D. Purification and characterization of a cytosolic 65-kilodalton phosphoprotein in human leukocytes whose phosphorylation is augmented by stimulation with interleukin 1. Biochemistry. 1988 May 17;27(10):3765–3770. doi: 10.1021/bi00410a037. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Barlow G. H., Sievert H. W., Finley R. A., Yoo A. L. Studies on a lipopolysaccharide from Escherichia coli. Heterogeneity and mechanism of reversible inactivation by sodium deoxycholate. Biochemistry. 1969 Oct;8(10):4063–4067. doi: 10.1021/bi00838a024. [DOI] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Sparatore B., Horecker B. L. The involvement of calpain in the activation of protein kinase C in neutrophils stimulated by phorbol myristic acid. J Biol Chem. 1986 Mar 25;261(9):4101–4105. [PubMed] [Google Scholar]
- Myers M. A., McPhail L. C., Snyderman R. Redistribution of protein kinase C activity in human monocytes: correlation with activation of the respiratory burst. J Immunol. 1985 Nov;135(5):3411–3416. [PubMed] [Google Scholar]
- Nakadate T., Jeng A. Y., Blumberg P. M. Comparison of protein kinase C functional assays to clarify mechanisms of inhibitor action. Biochem Pharmacol. 1988 Apr 15;37(8):1541–1545. doi: 10.1016/0006-2952(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Gross J. M., Brozna J. P., Goren M. B. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J Immunol. 1988 Jan 15;140(2):634–640. [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Sako T., Tauber A. I., Jeng A. Y., Yuspa S. H., Blumberg P. M. Contrasting actions of staurosporine, a protein kinase C inhibitor, on human neutrophils and primary mouse epidermal cells. Cancer Res. 1988 Aug 15;48(16):4646–4650. [PubMed] [Google Scholar]
- Szefler S. J., Norton C. E., Ball B., Gross J. M., Aida Y., Pabst M. J. IFN-gamma and LPS overcome glucocorticoid inhibition of priming for superoxide release in human monocytes. Evidence that secretion of IL-1 and tumor necrosis factor-alpha is not essential for monocyte priming. J Immunol. 1989 Jun 1;142(11):3985–3992. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Warren J. S., Kunkel S. L., Cunningham T. W., Johnson K. J., Ward P. A. Macrophage-derived cytokines amplify immune complex-triggered O2-. responses by rat alveolar macrophages. Am J Pathol. 1988 Mar;130(3):489–495. [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McNally J., Shipman L. J., Godfrey P. P. The action of the protein kinase C inhibitor, staurosporine, on human platelets. Evidence against a regulatory role for protein kinase C in the formation of inositol trisphosphate by thrombin. Biochem J. 1988 Jan 15;249(2):345–350. doi: 10.1042/bj2490345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Goren M. B., Holzer T. J., Andersen B. R. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect Immun. 1988 Nov;56(11):2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]