Abstract
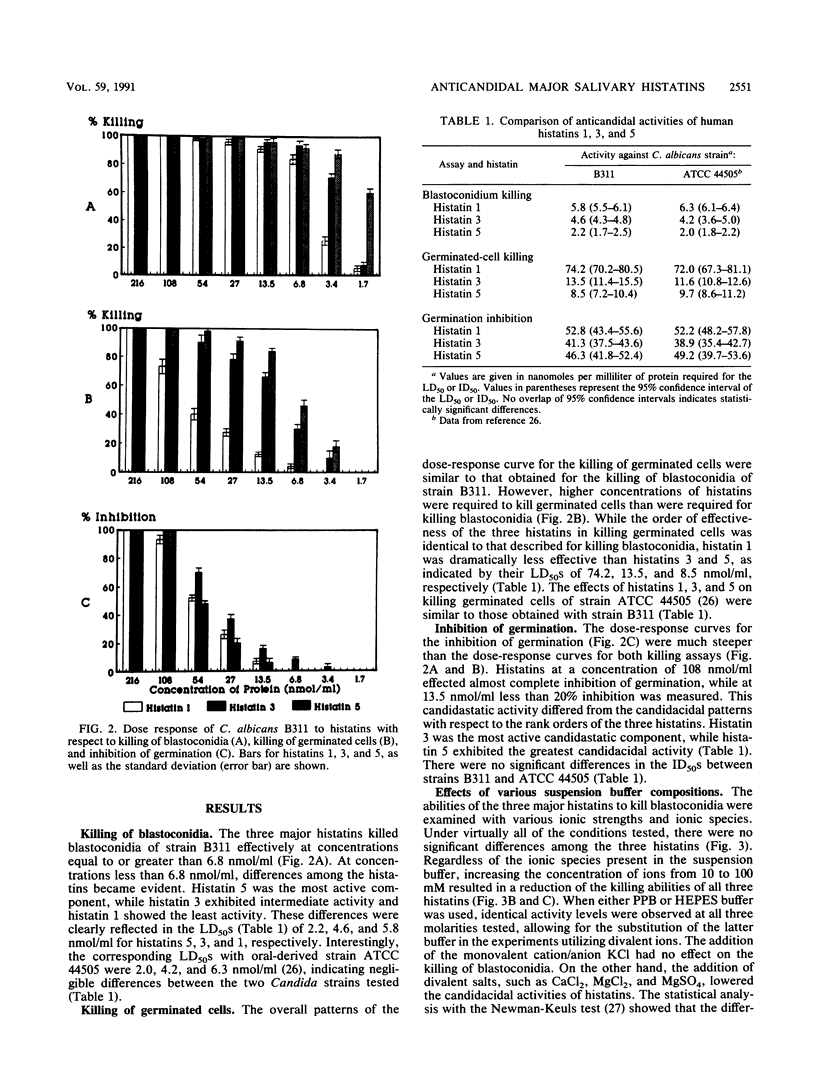
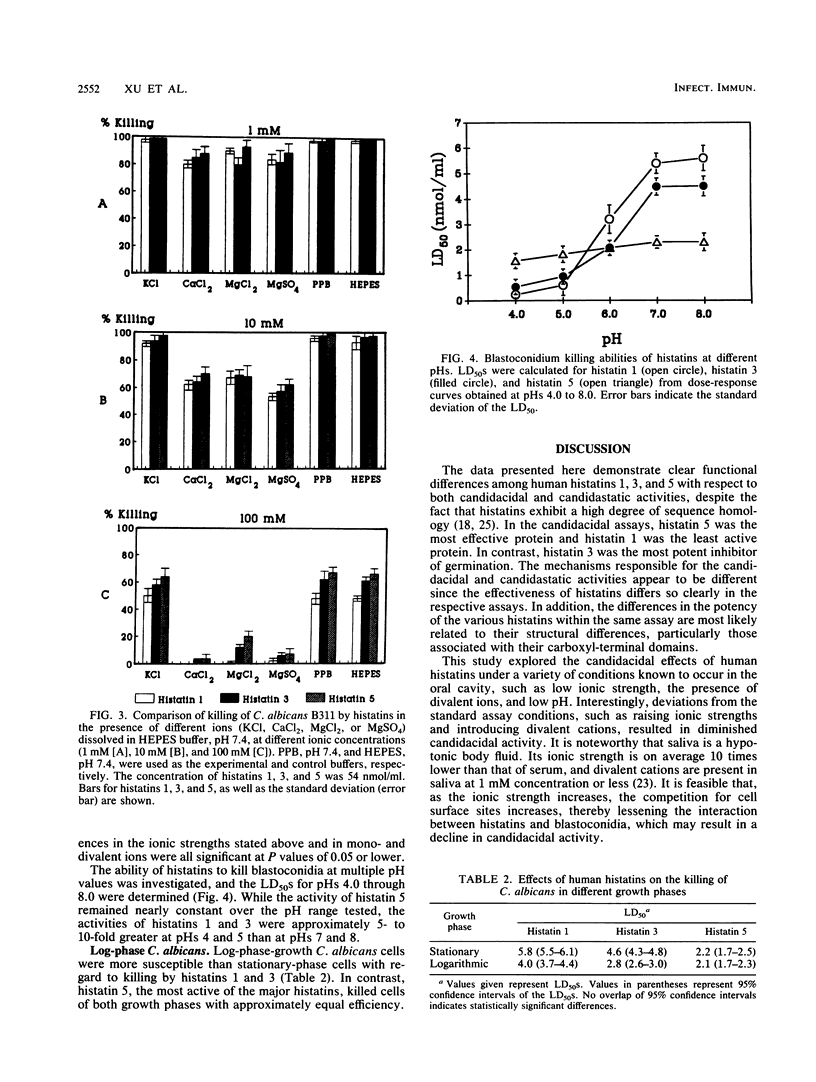
We have previously shown that histatins 1, 3, and 5 are homologous, histidine-rich proteins present in human parotid and submandibular secretions which contain 38, 32, and 24 amino acids, respectively. Interest in these proteins stems from the fact that histatins exhibit candidacidal and candidastatic activities. The goal of the present investigation was a detailed functional characterization of these anticandidal activities of histatins at the levels of killing of blastoconidia, killing of germinated cells, and inhibition of germination by using three bioassays. Candidacidal activities were evaluated at several ionic strengths, in the presence of different mono- and divalent ions, and at multiple pH values. In addition, the susceptibility of Candida albicans in different growth phases to histatins was investigated. While all three major human histatins demonstrated candidacidal activities, they differed in their abilities to kill blastoconidia and germinated cells, with histatin 5 being the most active, histatin 3 showing moderate activity, and histatin 1 exhibiting the lowest level of activity. For the inhibition of germination, however, histatin 3 exhibited more activity than either histatin 1 or histatin 5. The candidacidal activity of histatins was inversely proportional to both the ionic strength and the divalent cation concentration in the medium. Stepwise reduction of the pH of the assay medium enhanced the candidacidal activities of histatins 1 and 3, while the activity of histatin 5 was pH independent over the range of pHs 4 to 8. C. albicans in log-phase growth was more susceptible to histatins 1 and 3 than cells in stationary phase. Cells in either growth phase were still more vulnerable to histatin 5 than to histatins 1 and 3. The results obtained establish the functional relationship of the major histatins with respect to both their fungicidal and fungistatic activities and provide insights into their activities under ionic and pH conditions likely to be encountered in vivo in the oral cavity. Moreover, the data point towards possible mechanisms responsible for the anticandidal activities of histatins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendorf T. M., Walker D. M. The prevalence and intra-oral distribution of Candida albicans in man. Arch Oral Biol. 1980;25(1):1–10. doi: 10.1016/0003-9969(80)90147-8. [DOI] [PubMed] [Google Scholar]
- Atkinson J. C., Yeh C., Oppenheim F. G., Bermudez D., Baum B. J., Fox P. C. Elevation of salivary antimicrobial proteins following HIV-1 infection. J Acquir Immune Defic Syndr. 1990;3(1):41–48. [PubMed] [Google Scholar]
- Epstein J. B., Truelove E. L., Izutzu K. T. Oral candidiasis: pathogenesis and host defense. Rev Infect Dis. 1984 Jan-Feb;6(1):96–106. doi: 10.1093/clinids/6.1.96. [DOI] [PubMed] [Google Scholar]
- Gow N. A., Gooday G. W. Cytological aspects of dimorphism in Candida albicans. Crit Rev Microbiol. 1987;15(1):73–78. doi: 10.3109/10408418709104449. [DOI] [PubMed] [Google Scholar]
- HASENCLEVER H. F., MITCHELL W. O. Production in mice of tolerance to the toxic manifestations of Candida albicans. J Bacteriol. 1962 Sep;84:402–409. doi: 10.1128/jb.84.3.402-409.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. S., Harris C. A., Small C. B., Moll B., Lesser M., Friedland G. H. Oral candidiasis in high-risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984 Aug 9;311(6):354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Selsted M. E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991 Jan 25;64(2):229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Szklarek D., Selsted M. E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988 Jun;81(6):1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Szklarek D., Ganz T., Selsted M. E. Correlation of binding of rabbit granulocyte peptides to Candida albicans with candidacidal activity. Infect Immun. 1985 Jul;49(1):207–211. doi: 10.1128/iai.49.1.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. V., Wilkinson G. R. The oral yeast flora of 10-year-old schoolchildren. Sabouraudia. 1983 Jun;21(2):129–135. doi: 10.1080/00362178385380201. [DOI] [PubMed] [Google Scholar]
- Meunier F. Candidiasis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):438–447. doi: 10.1007/BF01964058. [DOI] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. A simple system for the presumptive identification of Candida albicans and differentiation of strains within the species. Sabouraudia. 1980 Dec;18(4):301–317. [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Oppenheim F. G., Xu T., McMillian F. M., Levitz S. M., Diamond R. D., Offner G. D., Troxler R. F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988 Jun 5;263(16):7472–7477. [PubMed] [Google Scholar]
- Oppenheim F. G., Yang Y. C., Diamond R. D., Hyslop D., Offner G. D., Troxler R. F. The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem. 1986 Jan 25;261(3):1177–1182. [PubMed] [Google Scholar]
- Pollock J. J., Denepitiya L., MacKay B. J., Iacono V. J. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984 Jun;44(3):702–707. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj P. A., Edgerton M., Levine M. J. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990 Mar 5;265(7):3898–3905. [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Ganz T., Lehrer R. I. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985 Jul;49(1):202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper-Jones L. M., Aldred M. J., Walker D. M., Hayes T. M. Candidal infections and populations of Candida albicans in mouths of diabetics. J Clin Pathol. 1981 Jul;34(7):706–711. doi: 10.1136/jcp.34.7.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Offner G. D., Xu T., Vanderspek J. C., Oppenheim F. G. Structural relationship between human salivary histatins. J Dent Res. 1990 Jan;69(1):2–6. doi: 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- Xu T., Telser E., Troxler R. F., Oppenheim F. G. Primary structure and anticandidal activity of the major histatin from parotid secretion of the subhuman primate, Macaca fascicularis. J Dent Res. 1990 Nov;69(11):1717–1723. doi: 10.1177/00220345900690110301. [DOI] [PubMed] [Google Scholar]


