Abstract
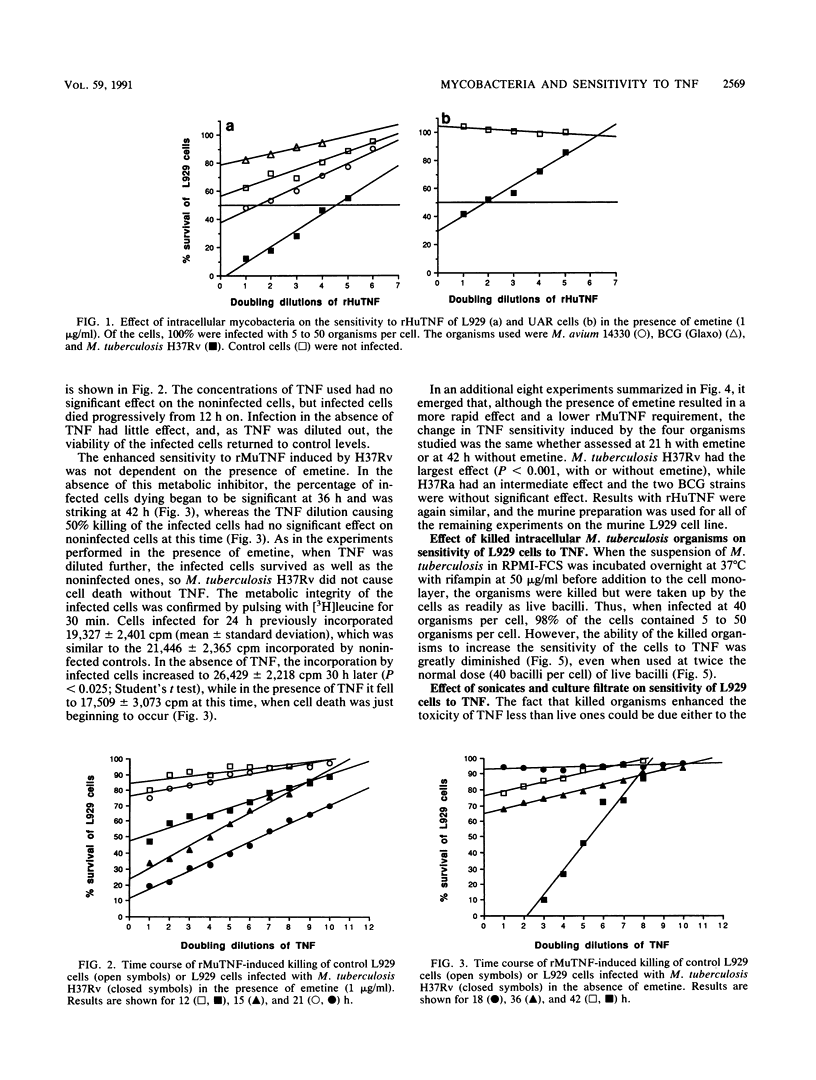
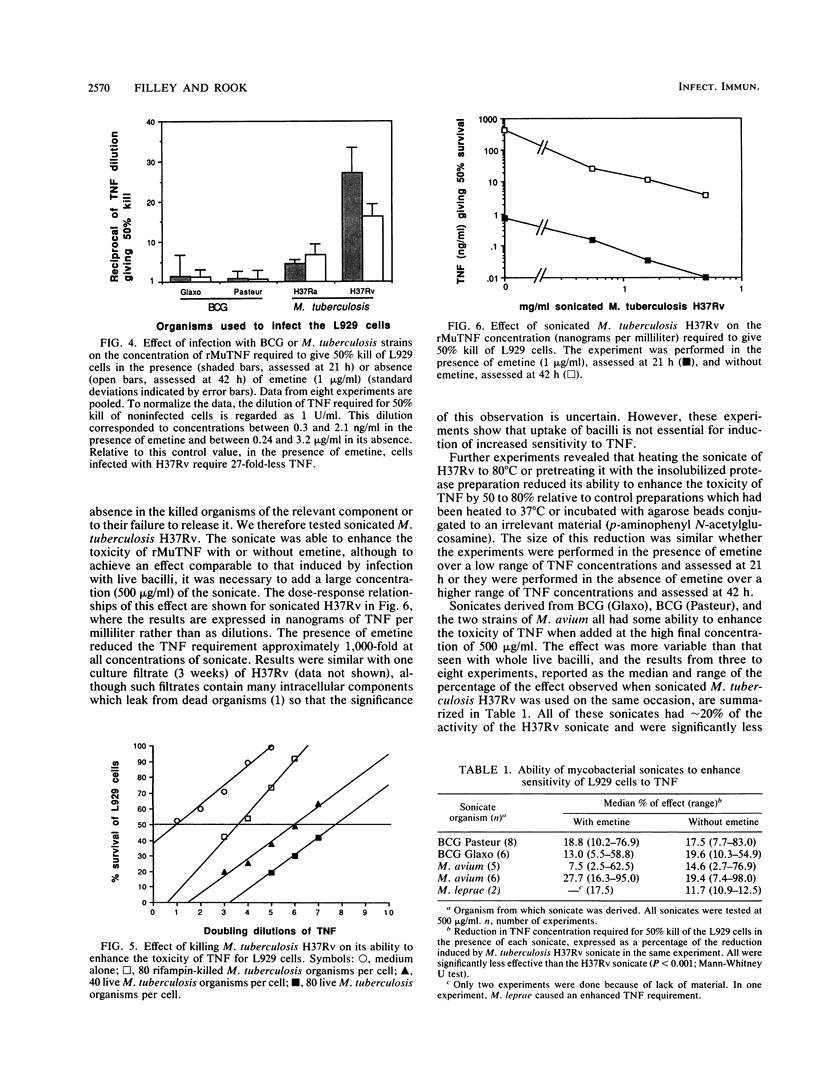
Unlike Mycobacterium leprae, Mycobacterium tuberculosis is not found inside cells other than macrophages and polymorphonuclear cells in vivo, yet previous work has revealed that in vitro it readily enters all cell lines tested. Moreover, these cells are not killed by the intracellular mycobacteria. We report here that when fibroblasts take up live (but not killed) M. tuberculosis H37Rv, they develop greatly increased sensitivity to the toxic effects of tumor necrosis factor (TNF) whether the cell line is inherently sensitive to TNF or not. Ultrasonically disrupted M. tuberculosis also has this property. The increased sensitivity is seen in the absence of metabolic inhibitors, although addition of emetine, an inhibitor of protein synthesis, causes the effect to manifest itself earlier and at a lower concentration of TNF. In contrast, infection with Mycobacterium bovis bacillus Calmette-Guérin induces little or no increased sensitivity to TNF, whereas Mycobacterium avium and M. tuberculosis H37Ra have intermediate sensitivities. We discuss the possibility that virulent tuberculosis strains produce a factor which distorts the normal protective function of TNF, rendering it toxic to host tissues and leading to the classical immunopathology of tuberculous lesions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Smith I., Grange J. M., Ratliff T. L., Steele J., Rook G. A. The secreted antigens of Mycobacterium tuberculosis and their relationship to those recognized by the available antibodies. J Gen Microbiol. 1988 Feb;134(2):531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- Boddingius J. The occurrence of Mycobacterium leprae within axons of peripheral nerves. Acta Neuropathol. 1974 Mar 26;27(3):257–270. doi: 10.1007/BF00687635. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Weddell A. G., Rees R. J. Infection of murine striated muscle with Mycobacterium leprae: a study by light and electron microscopy. J Pathol. 1972 Feb;106(2):73–80. doi: 10.1002/path.1711060203. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Levy L. Ultrastructural changes in cells of the mouse footpad infected with Mycobacterium leprae. Infect Immun. 1972 Feb;5(2):238–247. doi: 10.1128/iai.5.2.238-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley N., Lambert C., McNicol M., Johnson N., Rook G. A. An inhibitor of the toxicity of tumour necrosis factor in the serum of patients with sarcoidosis, tuberculosis and Crohn's disease. Clin Exp Immunol. 1990 Jun;80(3):395–399. doi: 10.1111/j.1365-2249.1990.tb03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Klimpel G. R., Shaban R., Niesel D. W. Bacteria-infected fibroblasts have enhanced susceptibility to the cytotoxic action of tumor necrosis factor. J Immunol. 1990 Jul 15;145(2):711–717. [PubMed] [Google Scholar]
- Koff W. C., Fann A. V. Human tumor necrosis factor-alpha kills herpesvirus-infected but not normal cells. Lymphokine Res. 1986 Summer;5(3):215–221. [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Soma G., Mizuno D., Yamamoto N., Kobayashi N. Cytocidal effect of tumor necrosis factor on cells chronically infected with human immunodeficiency virus (HIV): enhancement of HIV replication. J Virol. 1989 Jun;63(6):2504–2509. doi: 10.1128/jvi.63.6.2504-2509.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Neale M. L., Fiera R. A., Matthews N. Tumour cells which develop resistance to cytolysis by tumour necrosis factor have a different glycoform of a 105-kDa glycoprotein and lose the capacity to invade and metastasize. Int J Cancer. 1990 Jan 15;45(1):203–208. doi: 10.1002/ijc.2910450136. [DOI] [PubMed] [Google Scholar]
- Paul R. C., Stanford J. L., Carswell J. W. Multiple skin testing in leprosy. J Hyg (Lond) 1975 Aug;75(1):57–68. doi: 10.1017/s0022172400047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Mechanisms of pathogenicity in mycobacteria. Biochimie. 1988 Aug;70(8):1101–1120. doi: 10.1016/0300-9084(88)90272-6. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Foley N. M., Meager A. What mediates the immunopathological component of the immune response to Mycobacterium tuberculosis? Can it be switched off? Bull Int Union Tuberc Lung Dis. 1990 Jun-Sep;65(2-3):23–26. [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The role of vitamin D in tuberculosis. Am Rev Respir Dis. 1988 Oct;138(4):768–770. doi: 10.1164/ajrccm/138.4.768. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C. A comparison of the growth of selected mycobacteria in HeLa, monkey kidney, and human amnion cells in tissue culture. J Exp Med. 1958 Feb 1;107(2):237–246. doi: 10.1084/jem.107.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spofford B. T., Daynes R. A., Granger G. A. Cell-mediated immunity in vitro: a highly sensitive assay for human lymphotoxin. J Immunol. 1974 Jun;112(6):2111–2116. [PubMed] [Google Scholar]
- Taverne J., Matthews N., Depledge P., Playfair J. H. Malarial parasites and tumour cells are killed by the same component of tumour necrosis serum. Clin Exp Immunol. 1984 Aug;57(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]


