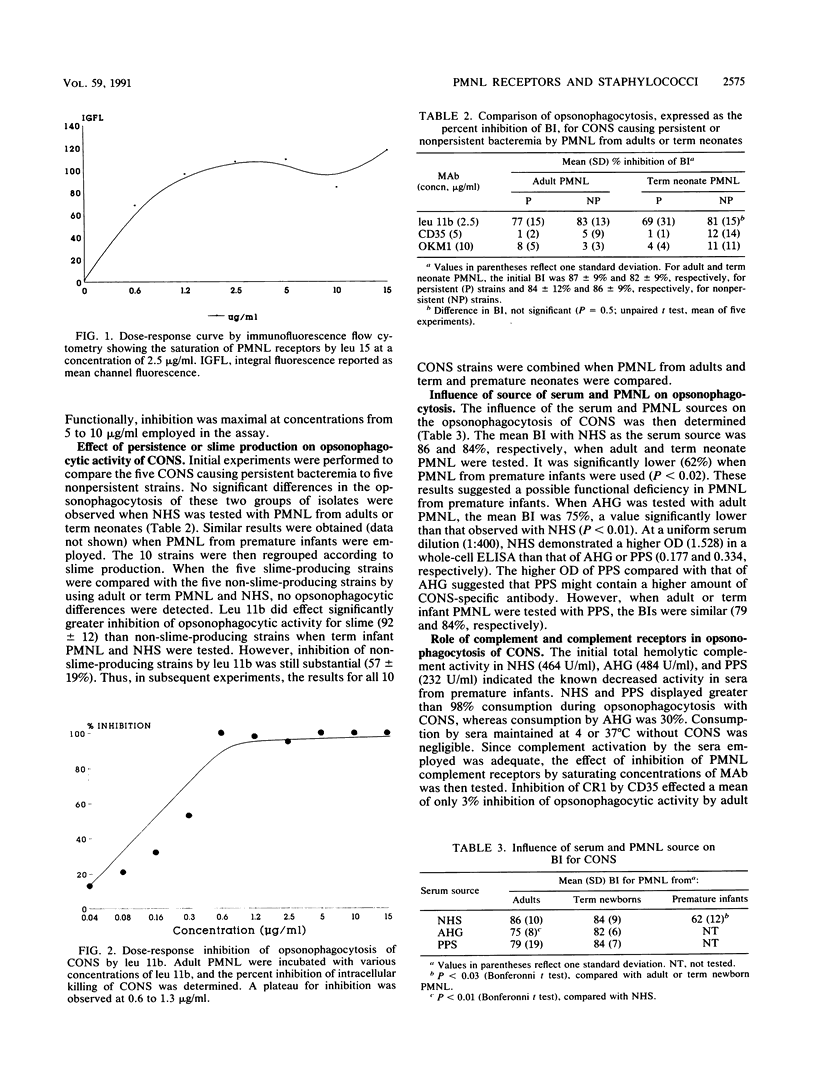
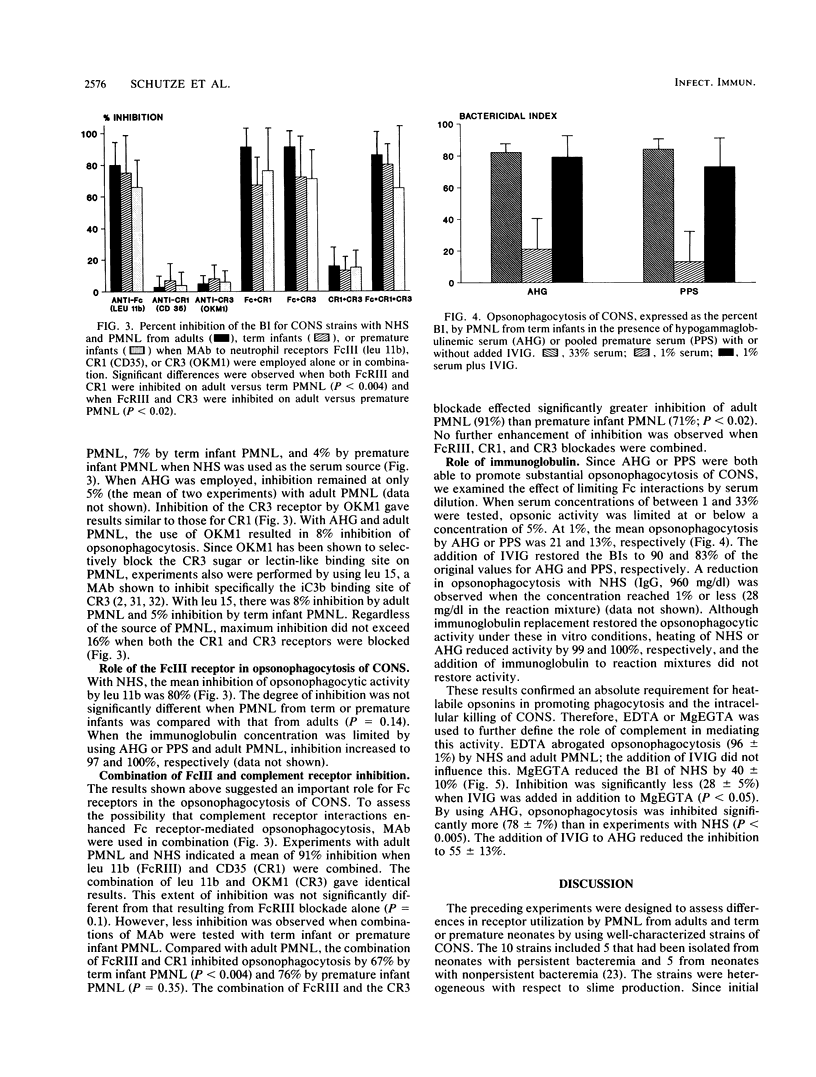
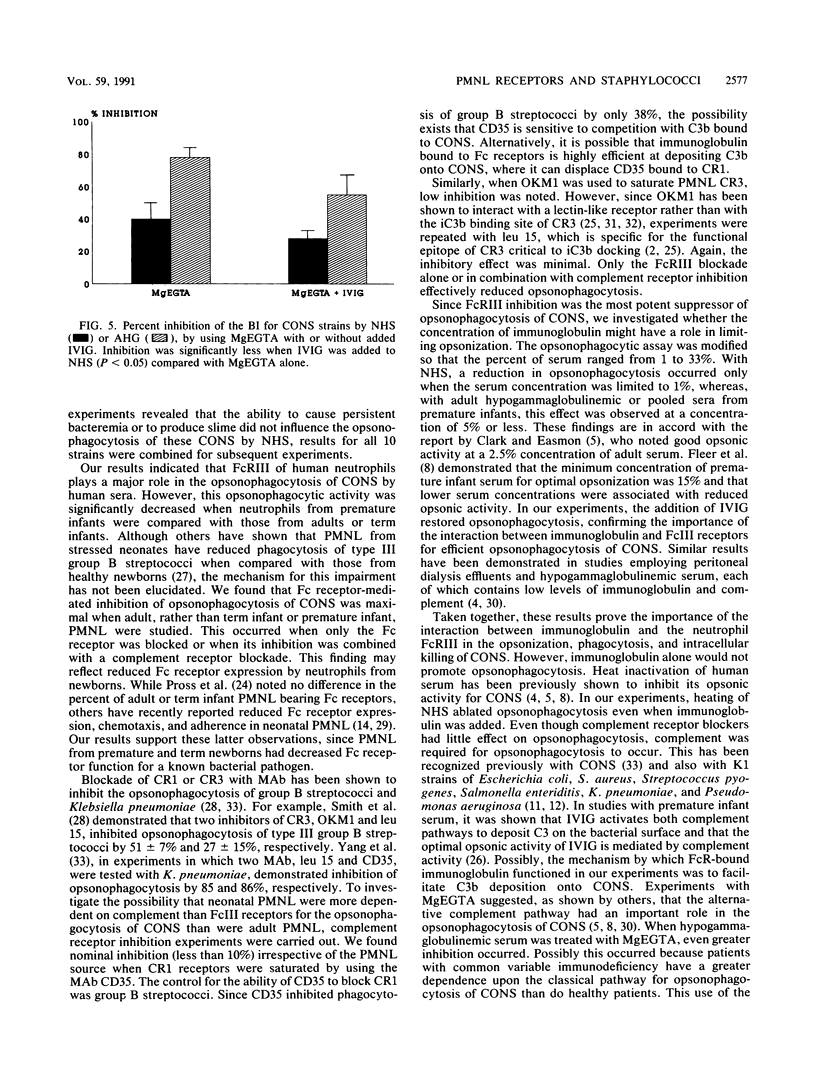
Abstract
The role of neutrophil complement receptors in the opsonophagocytosis of 10 strains of coagulase-negative staphylococci was investigated. Polymorphonuclear leukocytes from adults as well as term and premature newborn infants were tested with normal human serum, adult hypogammaglobulinemic serum, and pooled premature infant serum in an opsonophagocytic assay. Neutrophils from premature infants demonstrated significantly lower killing capacity (62%) than neutrophils from adults (86%) or term infants (84%; P less than 0.02). Maximum inhibition of opsonophagocytosis by adult or infant neutrophils occurred with an FcIII receptor blockade (80%), whereas a blockade of complement receptors produced minimal inhibition. Opsonophagocytic activity for the coagulase-negative staphylococci was not influenced by the serum source but was influenced by reducing the serum concentration below 5%. Abrogation of the complement activity of normal human serum by heating or the addition of ethylenediamine tetraacetate reduced opsonophagocytosis by 100 and 96%, respectively, whereas selective inhibition of the classical complement pathway reduced opsonophagocytosis by only 40%. Thus, opsonophagocytosis of coagulase-negative staphylococci by human sera appears to be mediated primarily by neutrophil Fc receptors, but complement is also required. The inefficiency of these interactions with neutrophils from premature infants may partially explain the enhanced susceptibility of very-low-birth-weight neonates to disseminated, coagulase-negative staphylococcal infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Cain J. A., Newman S. L., Ross G. D. Role of complement receptor type three and serum opsonins in the neutrophil response to yeast. Complement. 1987;4(2):75–86. doi: 10.1159/000463011. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. A., Easmon C. S. Opsonic activity of intravenous immunoglobulin preparations against Staphylococcus epidermidis. J Clin Pathol. 1986 Aug;39(8):856–860. doi: 10.1136/jcp.39.8.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L. A., Easmon C. S. Opsonic requirements of Staphylococcus epidermidis. J Med Microbiol. 1986 Aug;22(1):1–7. doi: 10.1099/00222615-22-1-1. [DOI] [PubMed] [Google Scholar]
- Corry J. M., Polhill R. B., Jr, Edmonds S. R., Johnston R. B., Jr Activity of the alternative complement pathway after splenectomy: comparison to activity in sickle cell disease and hypogammaglobulinemia. J Pediatr. 1979 Dec;95(6):964–969. doi: 10.1016/s0022-3476(79)80284-x. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Baker C. J., Kasper D. L. Opsonic specificity of human antibody to the type III polysaccharide of group B Streptococcus. J Infect Dis. 1979 Dec;140(6):1004–1008. doi: 10.1093/infdis/140.6.1004. [DOI] [PubMed] [Google Scholar]
- Fleer A., Gerards L. J., Aerts P., Westerdaal N. A., Senders R. C., van Dijk H., Verhoef J. Opsonic defense to Staphylococcus epidermidis in the premature neonate. J Infect Dis. 1985 Nov;152(5):930–937. doi: 10.1093/infdis/152.5.930. [DOI] [PubMed] [Google Scholar]
- Gordon D. L., Hostetter M. K. Complement and host defence against microorganisms. Pathology. 1986 Oct;18(4):365–375. doi: 10.3109/00313028609087551. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Shigeoka A. O. Comparative opsonic activity of intravenous gamma globulin preparations for common bacterial pathogens. Am J Med. 1984 Mar 30;76(3A):61–66. doi: 10.1016/0002-9343(84)90321-8. [DOI] [PubMed] [Google Scholar]
- Hill H. R., Bathras J. M. Protective and opsonic activities of a native, pH 4.25 intravenous immunoglobulin G preparation against common bacterial pathogens. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 4):S396–S400. doi: 10.1093/clinids/8.supplement_4.s396. [DOI] [PubMed] [Google Scholar]
- Langley J., Gold R. Sepsis in febrile neutropenic children with cancer. Pediatr Infect Dis J. 1988 Jan;7(1):34–37. doi: 10.1097/00006454-198801000-00008. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Charoenpan P., Ho M., White N. J., Karbwang J., Bunnag D., Harinasuta T. Fatal Plasmodium falciparum malaria after an inadequate response to quinine treatment. J Infect Dis. 1990 Mar;161(3):577–580. doi: 10.1093/infdis/161.3.577. [DOI] [PubMed] [Google Scholar]
- Masuda K., Kinoshita Y., Kobayashi Y. Heterogeneity of Fc receptor expression in chemotaxis and adherence of neonatal neutrophils. Pediatr Res. 1989 Jan;25(1):6–10. doi: 10.1203/00006450-198901000-00002. [DOI] [PubMed] [Google Scholar]
- Meyers J. D. Infection in bone marrow transplant recipients. Am J Med. 1986 Jul 28;81(1A):27–38. doi: 10.1016/0002-9343(86)90511-5. [DOI] [PubMed] [Google Scholar]
- Mollitt D. L., Tepas J. J., Talbert J. L. The role of coagulase-negative Staphylococcus in neonatal necrotizing enterocolitis. J Pediatr Surg. 1988 Jan;23(1 Pt 2):60–63. doi: 10.1016/s0022-3468(88)80542-6. [DOI] [PubMed] [Google Scholar]
- Munson D. P., Thompson T. R., Johnson D. E., Rhame F. S., VanDrunen N., Ferrieri P. Coagulase-negative staphylococcal septicemia: experience in a newborn intensive care unit. J Pediatr. 1982 Oct;101(4):602–605. doi: 10.1016/s0022-3476(82)80718-x. [DOI] [PubMed] [Google Scholar]
- Ohlsson A., Vearncombe M. Congenital and nosocomial sepsis in infants born in a regional perinatal unit: cause, outcome, and white blood cell response. Am J Obstet Gynecol. 1987 Feb;156(2):407–413. doi: 10.1016/0002-9378(87)90294-8. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Hecht D. W. Plasmid profiles in epidemiologic studies of infections by Staphylococcus epidermidis. J Infect Dis. 1980 May;141(5):637–643. doi: 10.1093/infdis/141.5.637. [DOI] [PubMed] [Google Scholar]
- Parisi J. T., Talbot H. W., Skahan J. M. Development of a phage typing set for Staphylococcus epidermidis in the United States. Zentralbl Bakteriol Orig A. 1978 Jul;241(1):60–67. [PubMed] [Google Scholar]
- Patrick C. C. Coagulase-negative staphylococci: pathogens with increasing clinical significance. J Pediatr. 1990 Apr;116(4):497–507. doi: 10.1016/s0022-3476(05)81593-8. [DOI] [PubMed] [Google Scholar]
- Patrick C. C., Kaplan S. L., Baker C. J., Parisi J. T., Mason E. O., Jr Persistent bacteremia due to coagulase-negative staphylococci in low birth weight neonates. Pediatrics. 1989 Dec;84(6):977–985. [PubMed] [Google Scholar]
- Pross S. H., Hallock J. A., Armstrong R., Fishel C. W. Complement and Fc receptors on cord blood and adult neutrophils. Pediatr Res. 1977 Feb;11(2):135–137. doi: 10.1203/00006450-197702000-00011. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Cain J. A., Lachmann P. J. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. J Immunol. 1985 May;134(5):3307–3315. [PubMed] [Google Scholar]
- Shaio M. F., Yang K. D., Bohnsack J. F., Hill H. R. Effect of immune globulin intravenous on opsonization of bacteria by classic and alternative complement pathways in premature serum. Pediatr Res. 1989 Jun;25(6):634–640. doi: 10.1203/00006450-198906000-00016. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Santos J. I., Hill H. R. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979 Sep;95(3):454–460. doi: 10.1016/s0022-3476(79)80535-1. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Campbell D. E., Ludomirsky A., Polin R. A., Douglas S. D., Garty B. Z., Harris M. C. Expression of the complement receptors CR1 and CR3 and the type III Fc gamma receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediatr Res. 1990 Aug;28(2):120–126. doi: 10.1203/00006450-199008000-00009. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Peterson P., Kim Y., Sabath L. D., Quie P. G. Opsonic requirements for staphylococcal phagocytosis. Heterogeneity among strains. Immunology. 1977 Aug;33(2):191–197. [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Hickstein D. D., Schwartz B. R., June C. H., Ochs H. D., Beatty P. G., Klebanoff S. J., Harlan J. M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986 Apr;67(4):1007–1013. [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K. D., Bathras J. M., Shigeoka A. O., James J., Pincus S. H., Hill H. R. Mechanisms of bacterial opsonization by immune globulin intravenous: correlation of complement consumption with opsonic activity and protective efficacy. J Infect Dis. 1989 Apr;159(4):701–707. doi: 10.1093/infdis/159.4.701. [DOI] [PubMed] [Google Scholar]



