Abstract
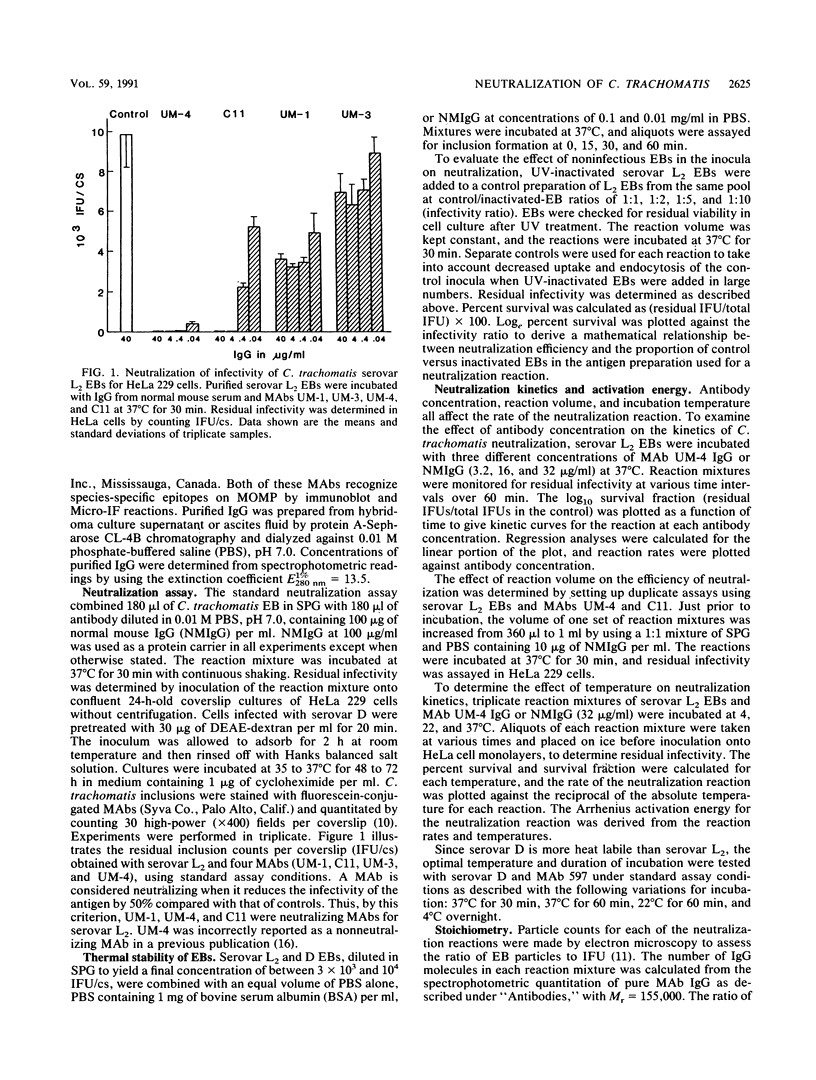
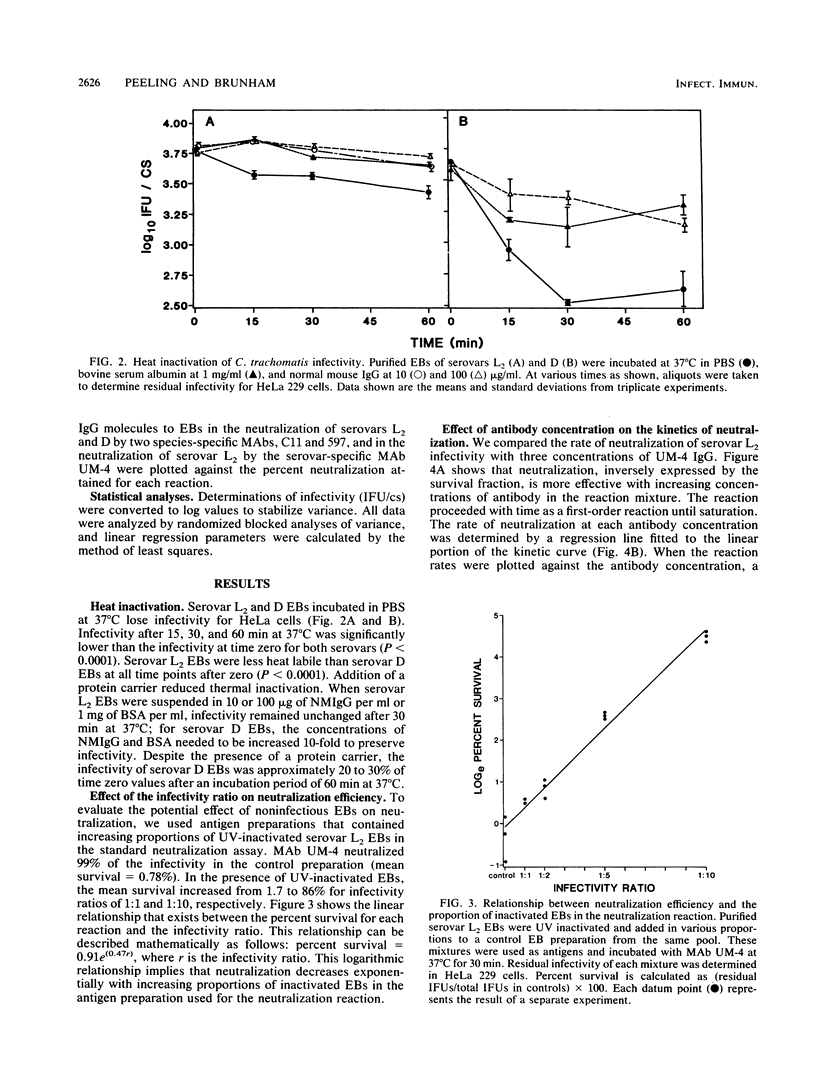
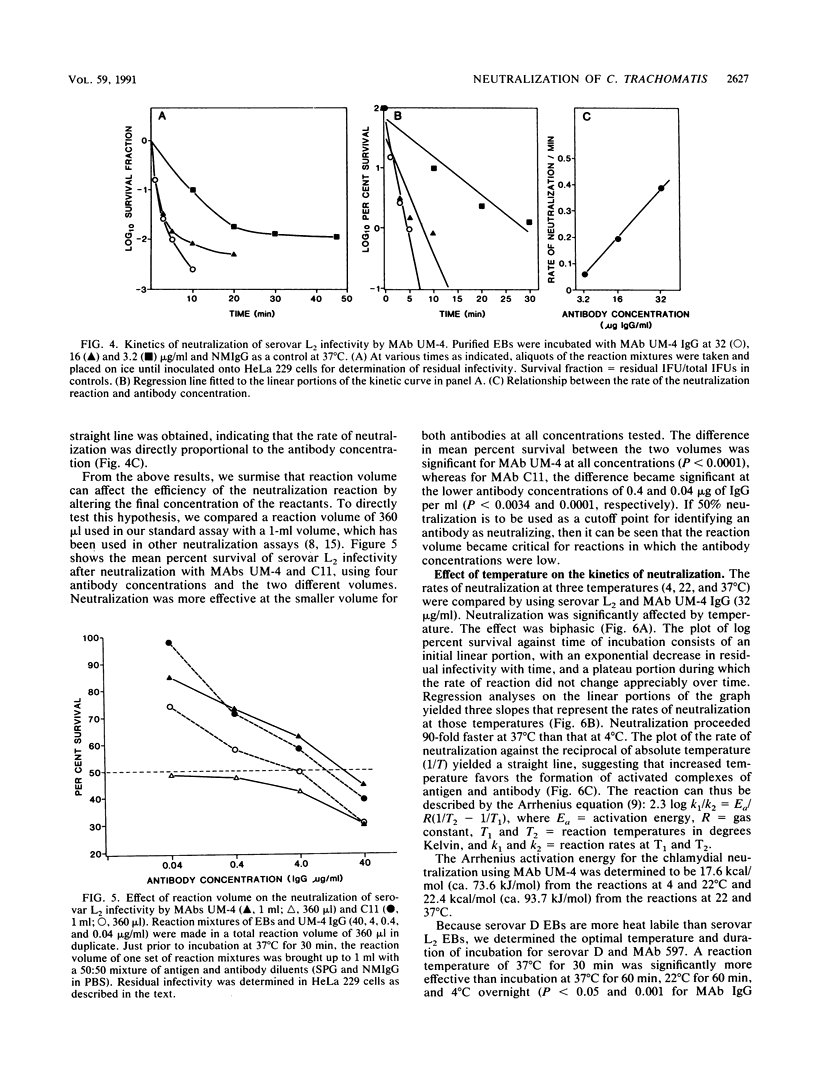
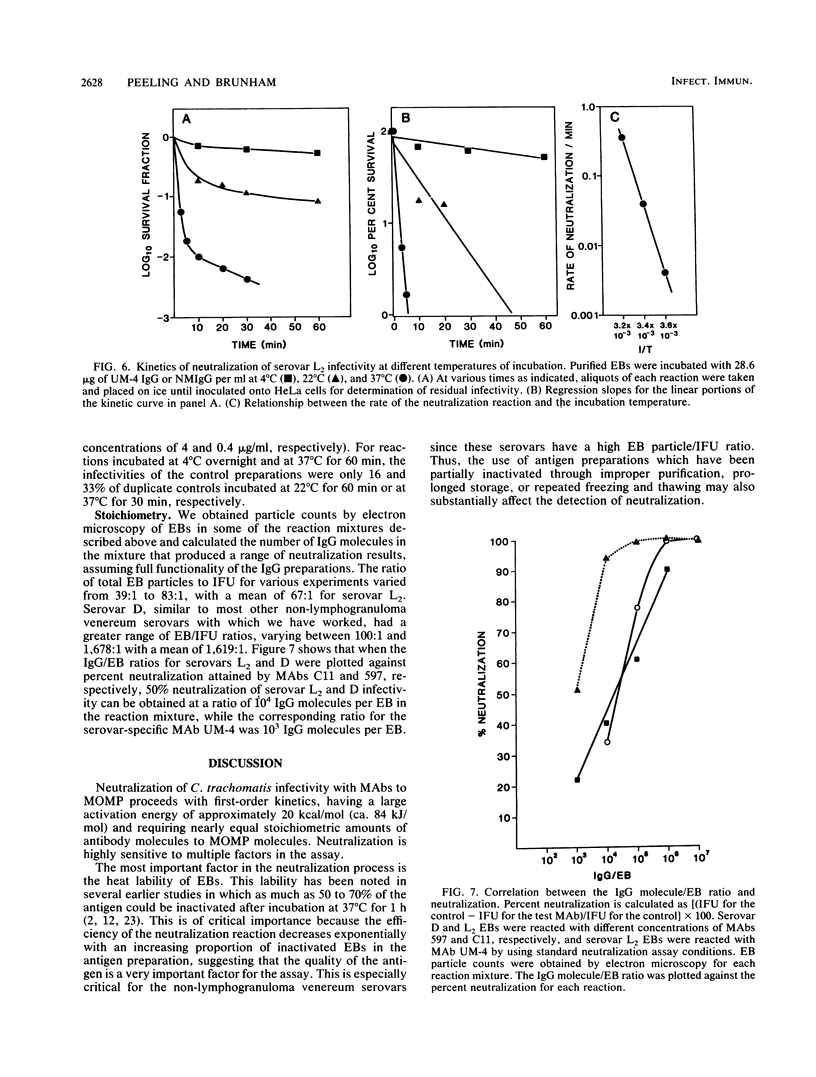
Monoclonal antibodies to the major outer membrane protein of Chlamydia trachomatis were used to neutralize C. trachomatis infectivity in HeLa 229 cells and to determine the kinetics and stoichiometry of the reaction. In vitro neutralization of C. trachomatis infectivity proceeded as a first-order reaction and required an activation energy of approximately 20 kcal/mol (ca. 84 kJ/mol). The rate of neutralization was linear with respect to antibody concentration and reaction temperature. The efficiency of neutralization decreased exponentially as the ratio of noninfective to infective chlamydiae increased in the antigen preparation. The neutralization assay was also significantly affected by reaction parameters such as the reaction volume and the duration of incubation. Stoichiometric calculations showed that an average ratio of 10(3) and 10(4) immunoglobulin molecules per chlamydial particle was required to yield 50% neutralization by monoclonal antibodies specifying serovar-specific and species-specific epitopes, respectively. The implications of these findings for vaccine design and for the role of the major outer membrane protein in the pathogenesis of chlamydial infections are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLYTH W. A., REEVE P., GRAHAM D. M., TAVERNE J. The production of antisera that neutralize inclusion blennorrhoea virus. Br J Exp Pathol. 1962 Jun;43:340–343. [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Picornaviruses are no longer black boxes. Science. 1985 Sep 27;229(4720):1366–1367. doi: 10.1126/science.2994219. [DOI] [PubMed] [Google Scholar]
- Banks J., Eddie B., Sung M., Sugg N., Schachter J., Meyer K. F. Plaque reduction technique for demonstrating neutralizing antibodies for Chlamydia. Infect Immun. 1970 Oct;2(4):443–447. doi: 10.1128/iai.2.4.443-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barenfanger J., MacDonald A. B. The role of immunoglobulin in the neutralization of trachoma infectivity. J Immunol. 1974 Nov;113(5):1607–1617. [PubMed] [Google Scholar]
- Blyth W. A., Taverne J. Neutralization of TRIC organisms by antibody: enhancement by antisera prepared against immunoglobulins. J Hyg (Lond) 1974 Feb;72(1):129–134. doi: 10.1017/s0022172400023299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Peeling R., Maclean I., McDowell J., Persson K., Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987 Apr;155(4):749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Nachamkin I., Schatzki P. F., Dalton H. P. Localization of distinct surface antigens on Chlamydia trachomatis HAR-13 by immune electron microscopy with monoclonal antibodies. Infect Immun. 1982 Dec;38(3):1273–1278. doi: 10.1128/iai.38.3.1273-1278.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M., STRICKLAND A. G. A study of the basic aspects of neutralization of two animal viruses, western equine encephalitis virus and poliomyelitis virus. Virology. 1956 Apr;2(2):162–205. doi: 10.1016/0042-6822(56)90017-4. [DOI] [PubMed] [Google Scholar]
- FURNESS G., GRAHAM D. M., REEVE P. The titration of trachoma and inclusion blennorrhoea viruses in cell cultures. J Gen Microbiol. 1960 Dec;23:613–619. doi: 10.1099/00221287-23-3-613. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Hazelton P. R., Chuang I., Klisko B. Improved detection of viruses by electron microscopy after direct ultracentrifuge preparation of specimens. J Clin Microbiol. 1981 Aug;14(2):210–221. doi: 10.1128/jcm.14.2.210-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V. Neutralization of Chlamydia trachomatis in cell culture. Infect Immun. 1975 Apr;11(4):698–703. doi: 10.1128/iai.11.4.698-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenogle J., Shiwen H., Duke G., Gilbert S., Rueckert R., Anderegg J. Neutralization of poliovirus by a monoclonal antibody: kinetics and stoichiometry. Virology. 1983 Jun;127(2):412–425. doi: 10.1016/0042-6822(83)90154-x. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Osborn M. F., Rowntree S., Thomas B. J., Taylor-Robinson D. A study of inactivation of Chlamydia trachomatis by normal human serum. Br J Vener Dis. 1983 Dec;59(6):369–372. doi: 10.1136/sti.59.6.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero M. E., Kuo C. C. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect Immun. 1985 Nov;50(2):595–597. doi: 10.1128/iai.50.2.595-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean I. W., Peeling R. W., Brunham R. C. Characterization of Chlamydia trachomatis antigens with monoclonal and polyclonal antibodies. Can J Microbiol. 1988 Feb;34(2):141–147. doi: 10.1139/m88-028. [DOI] [PubMed] [Google Scholar]
- Mandel B. Neutralization of animal viruses. Adv Virus Res. 1978;23:205–268. doi: 10.1016/S0065-3527(08)60101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megran D. W., Stiver H. G., Peeling R., Maclean I. W., Brunham R. C. Complement enhancement of neutralizing antibody to the structural proteins of Chlamydia trachomatis. J Infect Dis. 1988 Sep;158(3):661–663. doi: 10.1093/infdis/158.3.661. [DOI] [PubMed] [Google Scholar]
- Nichols R. L., Oertley R. E., Fraser E. C., MacDonald A. B., McComb D. E. Immunity to chlamydial infections of the eye. VI. Homologous neutralization of trachoma infectivity for the owl monkey conjuctivae by eye secretions from humans with trachoma. J Infect Dis. 1973 Apr;127(4):429–432. doi: 10.1093/infdis/127.4.429. [DOI] [PubMed] [Google Scholar]
- Peeling R. W., Peeling J., Brunham R. C. High-resolution 31P nuclear magnetic resonance study of Chlamydia trachomatis: induction of ATPase activity in elementary bodies. Infect Immun. 1989 Nov;57(11):3338–3344. doi: 10.1128/iai.57.11.3338-3344.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Gordon F. B., Quan A. L. Fluorescent antibody responses to chlamydial infection in patients with lymphogranuloma venereum and urethritis. J Immunol. 1974 Jun;112(6):2126–2134. [PubMed] [Google Scholar]
- REEVE P., GRAHAM D. M. A neutralization test for trachoma and inclusion blennorrhoea viruses grown in HeLa cell cultures. J Gen Microbiol. 1962 Jan;27:177–180. doi: 10.1099/00221287-27-1-177. [DOI] [PubMed] [Google Scholar]
- Su H., Watkins N. G., Zhang Y. X., Caldwell H. D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990 Apr;58(4):1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL D. A., HORSFALL F. L., Jr Neutralization of viruses by homologous immune serum. I. Quantitative studies on factors which affect the neutralization reaction with Newcastle disease, influenza A, and bacterial virus T3. J Exp Med. 1953 Jun;97(6):845–861. doi: 10.1084/jem.97.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J Infect Dis. 1974 Oct;130(4):388–397. doi: 10.1093/infdis/130.4.388. [DOI] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]



