Abstract
Relapsing fever is a worldwide, endemic disease caused by several spirochetal species belonging to the genus Borrelia. During the recurring fever peaks, borreliae proliferate remarkably quickly compared to the slow dissemination of Lyme disease Borrelia and therefore require efficient nutrient uptake from the blood of their hosts. This study describes the identification and characterization of the first relapsing fever porin, which is present in the outer membranes of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae. The pore-forming protein was purified by hydroxyapatite chromatography and designated Oms38, for outer membrane-spanning protein of 38 kDa. Biophysical characterization of Oms38 was done by using the black lipid bilayer method, demonstrating that Oms38 forms small, water-filled channels of 80 pS in 1 M KCl that did not exhibit voltage-dependent closure. The Oms38 channel is slightly selective for anions and shows a ratio of permeability for cations over anions of 0.41 in KCl. Analysis of the deduced amino acid sequences demonstrated that Oms38 contains an N-terminal signal sequence which is processed under in vivo conditions. Oms38 is highly conserved within the four studied relapsing fever species, sharing an overall amino acid identity of 58% and with a strong indication for the presence of amphipathic β-sheets.
Relapsing fever (RF) is caused by spirochetes of the genus Borrelia, which also includes species responsible for Lyme disease. RF occurs worldwide and often endemically in temperate to tropical and subtropical regions, and in some African communities the incidence is the highest of any bacterial diseases reported (72). The main clinical manifestations are, according to the name, recurring high fever peaks with high densities of Borrelia in the blood (33, 38, 67), which may be accompanied by complications such as spontaneous abortion and perinatal death (19, 37, 40, 71).
RF is caused by approximately 20 species of Borrelia distributed nearly worldwide and is transmitted by hematophagous ticks and lice (20, 33, 67). Four species of spirochetes representing the wide distribution of different RF species are considered the main causes of RF. (i) B. duttonii is present in sub-Saharan Africa and transmitted by the soft-body tick Ornithodoros moubata moubata; this species likely causes more RF cases than anywhere else in the world (38). (ii) B. hermsii is the most common RF-causing pathogen in North America; it is transmitted by the tick Ornithodoros hermsi (31, 62) and has been the focus of antigenic variation research (5, 68). (iii) B. recurrentis occurs endemically in the highlands of Ethiopia, with sporadic cases in Sudan, and is transmitted by the human body louse Pediculus humanus humanus (20). (iv) B. turicatae has been sporadically isolated in Texas, Kansas, and Florida from the tick vector Ornithodoros turicata and dogs; while its exact geographic distribution is not defined (61), B. turicatae is used to study neuroborreliosis (23). These four species were chosen for the study of outer membrane porins.
Borreliae are limited in their metabolic and biosynthetic capacities and therefore are highly dependent on nutrients provided by their hosts (35). Consequently, these parasites need to have an efficient regulation of the nutrient uptake across the cell envelope. The Borrelia cell envelope structure and membrane composition show major differences from those of other gram-negative bacteria (6, 27, 28, 42, 56, 70, 73). Few membrane-spanning proteins are located in the outer membrane (35, 55). The most abundant proteins in the RF outer membrane are the variable major proteins Vmp, lipoproteins that are expressed with different surface epitopes through antigenic variation (2, 5, 68, 69).
In Lyme borreliosis, bacteria slowly disseminate from the infection site, with symptoms often arising weeks or months after the tick bite. In contrast, RF borreliae grow rapidly, reaching high cell densities in the blood in only a few days. To maintain this rapid growth, the bacteria need, in part, to have an extraordinarily efficient nutrient uptake from the plasma. The transport of nutrients and other molecules across the outer membrane is performed by pore-forming proteins, so-called porins. These are integral membrane proteins which form large water-filled pores in the outer membrane of gram-negative bacteria (10) to enable the influx of nutrients and other substances from the environment into the bacterial cell. Porins can be subdivided into two classes: (i) general diffusion pores, such as OmpF of Escherichia coli K12 (9, 10), which sort mainly according to the molecular mass of the solutes, and (ii) pores with a substrate-binding site inside the channel (14, 16, 25, 34, 41). Furthermore, surface-exposed porin loops are potential targets in interaction with other cells (17), bacteriophages (75), and bactericidal compounds (59) and are therefore putative candidates for vaccine development.
Several outer membrane porins, such as P66 (66), Oms28 (65), and P13 (51, 53), were identified recently in the Lyme disease agent B. burgdorferi. Intriguingly, although there are indications of their presence (64), no RF porin has been described in the literature to date. Nevertheless, the analysis of RF porins is of high importance for understanding the spirochetes' nutrient uptake in the blood during their exceptionally fast growth during the fever peaks.
Here, we describe the identification of the first RF porin present in outer membrane fractions (OMFs) of B. duttonii, B. hermsii, and B. recurrentis. The pore-forming proteins were purified by hydroxyapatite chromatography to homogeneity and designated Oms38. Biophysical characterizations of the porins were achieved by using the black lipid bilayer method. They demonstrated that Oms38 porins form water-filled pores with low conductance (80 pS in 1 M KCl) and are selective for anions. Partial sequencing of the protein and searches of genomic DNA sequences from ongoing projects revealed sequences of oms38 in B. duttonii, B. hermsii, B. recurrentis, and B. turicatae. Alignments demonstrated that the deduced amino acid sequences of the different Oms38 porins are highly conserved among the studied RF species and contain β-strands.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The RF strains used in this study were B. duttonii 1120K3, B. hermsii HS1, and B. recurrentis A1. Bacteria were grown in BSKII medium (3) supplemented with 10% rabbit serum and 1.4% gelatin at 37°C until cell densities reached approximately 107 to 108 cells ml−1 and harvested by centrifugation.
Isolation of outer membrane proteins and purification of the 38-kDa protein.
OMFs of the three Borrelia species used in this study were prepared as described previously (46). Purification of the native 38-kDa protein was performed by using a hydroxyapatite Bio-gel (Bio-Rad) column as it has been used previously for the purification of mitochondrial porins (8, 36). One hundred microliters of OMF (approximately 100 ng proteins) was diluted in 400 μl 2% Genapol (Roth) and applied to a hydroxyapatite column made from 0.3 g hydroxyapatite in an Econo-Column (Bio-Rad) with the dimensions of 0.5 by 5 cm and a column volume of 2 ml. The column was washed with 6 column volumes of a buffer containing 2% Genapol and 10 mM Tris-HCl (pH 8.0). For protein elution, 4 column volumes of buffer containing 2% Genapol, 250 mM KCl, and 10 mM Tris-HCl (pH 8.0) were passed through the column; fractions of 2.0 ml were collected.
Protein electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with the Laemmli gel system (43). One hundred microliters of protein samples eluted by hydroxyapatite chromatography was precipitated by the protocol of Wessel and Flügge (74). Proteins were separated by 12% SDS-PAGE under denatured conditions (boiled for 5 min in 4× SDS sample buffer before loading the gel) by using a Bio-Rad electrophoresis system. The gels were stained with silver (18).
Protein sequencing.
For N-terminal sequence analysis, proteins were blotted onto a polyvinylidene difluoride membrane after 12% SDS-PAGE. Microsequencing was performed with a Procise 492 cLC gas phase sequencer (Applied Biosystems GmbH) using the instructions of the manufacturer (32).
Planar lipid bilayer assays.
The methods used for black lipid bilayer experiments have been described previously (12). The instrumentation consisted of a Teflon chamber with two compartments separated by a thin wall and connected by a small circular hole with an area of 0.4 mm2. The membranes were formed by a 1% (wt/vol) solution of diphytanoyl phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) in n-decane. The porin-containing fractions were diluted 1:100 in 1% Genapol (Roth) and added to the aqueous phase after the membrane had turned black. The membrane current was measured with a pair of Ag/AgCl electrodes with salt bridges switched in series with a voltage source and a highly sensitive current amplifier (Keithley 427) while being kept at 20°C throughout.
The voltage dependence of the pores was checked as described elsewhere (48) using membrane potentials of as high as −200 to +200 mV. Zero-current membrane potential measurements were performed by establishing a salt gradient across membranes containing approximately 100 reconstituted channels as described earlier (13, 45). The zero-current membrane potentials were measured with a high-impedance electrometer (Keithley 617).
RESULTS
Pore-forming activities in OMFs of B. duttonii, B. hermsii, and B. recurrentis.
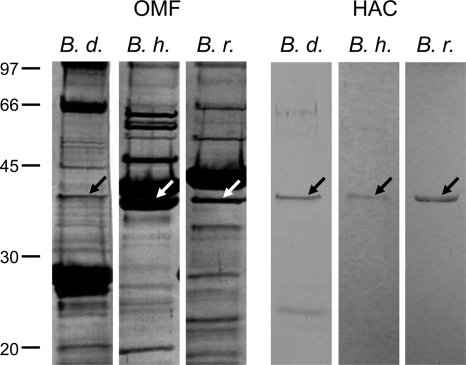
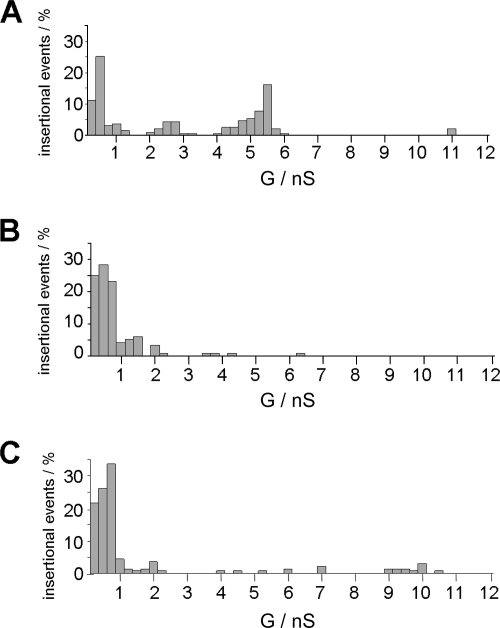
OMFs isolated from the three RF Borrelia species contained numerous proteins as shown by SDS-PAGE (Fig. 1, left panel). Several differences in the outer membrane bands between B. duttonii, B. hermsii, and B. recurrentis are obvious. The B. duttonii OMF exhibited major protein bands at a molecular mass of around 28 kDa, whereas the major bands of the B. hermsii and B. recurrentis OMFs corresponded to a molecular mass of around 40 kDa. These findings are thought to be due to differences in expression and antigenic variation of the numerous variable major proteins known to be present in the outer membranes of RF spirochetes (24, 58, 69). For investigations in the black lipid bilayer, OMFs were highly diluted in 1% Genapol. All OMFs exhibited a broad range of different pore-forming activities (Fig. 2), ranging from 0.1 to 11 nS, caused by different pore-forming proteins reconstituted into the lipid bilayer. A similar broad distribution of channels has been found for detergent-treated OMF of Borrelia burgdorferi (53). A particularly high pore-forming activity was observed within the conductance range from about 10 pS to 0.75 nS for experiments with detergent-treated OMFs of all three RF species. However, the full conductance fluctuation patterns of the single RF species exhibited several remarkable differences.
FIG. 1.
SDS-PAGE analysis of OMFs and the purified 38-kDa pores of B. duttonii, B. hermsii, and B. recurrentis. Approximately 1 to 10 ng of OMF of B. duttonii (B. d.), B. hermsii (B. h.), and B. recurrentis (B. r.) (left panel) or 100 μl of hydroxyapatite chromatography fractions (HAC) containing the purified 38-kDa protein (right panel) was separated by 12% SDS-PAGE and silver stained. The positions of molecular mass standards in kDa are shown at the left.
FIG. 2.
Pore-forming activities in the OMFs of B. duttonii (A), B. hermsii (B), and B. recurrentis (C). The histograms of the single-channel conductance (G) distributions were derived from at least 100 individual single-channel conductance steps derived from at least four different membranes. Highly diluted OMFs treated with 1% Genapol were added to a diphytanoyl phosphatidylcholine-n-decane membrane using 1 M KCl as the electrolyte. The applied voltage was 20 mV and the temperature was 20°C throughout.
The OMF of B. duttonii (Fig. 2A) contained pores with maxima within the histogram of the single-channel conductance centered on 0.5 nS, 2.5 nS, 5.5 nS, and 11 nS. For B. hermsii (Fig. 2B), the highest maximum of the single-channel conductance distribution was about 6.25 nS. Our histogram agrees with the one previously reported (64). The histogram derived from conductance fluctuations observed with the B. recurrentis OMF (Fig. 2C) was similar to those of B. duttonii and B. hermsii, which also had high pore-forming activity of about 2 nS and contained maxima within the histogram around 7 nS and 10 nS.
Purification and identification of a 38-kDa, pore-forming protein in the outer membranes of B. duttonii, B. hermsii, and B. recurrentis.
To identify proteins that are responsible for a defined pore-forming activity in the OMFs, about 100 ng of Borrelia OMF was diluted in 400 μl 2% Genapol-10 mM Tris-HCl (pH 8.0). This sample was applied to a dry hydroxyapatite column, which has been used successfully for the purification of mitochondrial porins (8, 36). After washing of the column with 6 column volumes of a buffer containing 2% Genapol and 10 mM Tris-HCl (pH 8.0), the pore-forming protein was eluted using different protocols. The most efficient one was the addition of 250 mM KCl to the buffer, thus increasing its ionic strength. Some of the fractions (fractions 1 and 2) that eluted from the column showed a high pore-forming activity (see below). To check the purity of the proteins from the pore-forming protein fractions, 100 μl was precipitated by the protocol of Wessel and Flügge (74) and subjected to 12% SDS-PAGE. Pore formation was found exclusively in fractions exhibiting a band that corresponded to a molecular mass of 38 kDa (Fig. 1, right panel). The fractions derived from the OMFs of B. hermsii and B. recurrentis contained exclusively the 38-kDa protein. The fractions derived from the OMF of B. duttonii contained the 38-kDa protein and a second band visible through all fractions, which corresponded to a molecular mass of 27 kDa. Amino acid sequencing identified this band as member of the Vsp lipoprotein family with similarity to the B. burgdorferi OspC (4, 60) (data not shown). The protein was abundant in the OMF of B. duttonii (Fig. 1, left panel); secondary-structure analysis, comparisons with other pore-forming lipoproteins, and the fact that fractions exclusively containing this 27-kDa band did not exhibit pore-forming activity suggested that Vsp is not a pore-forming component. Thus, the 38-kDa protein component was thought to be a putative porin within the fractions.
To identify the gene encoding the 38-kDa protein, we performed partial amino acid sequencing of the protein fractions that contained only the corresponding protein band. Two hundred microliters of the hydroxyapatite chromatography fraction 1 derived from the B. duttonii OMF were separated by 12% SDS-PAGE and blotted onto a polyvinylidene difluoride membrane. N-terminal sequencing provided the first 19 N-terminal amino acids of the native 38-kDa protein of B. duttonii: EEETKQKLETKENSTTKEN. Using this sequence, we searched in the genome sequences from work currently in progress with several RF spirochetes.
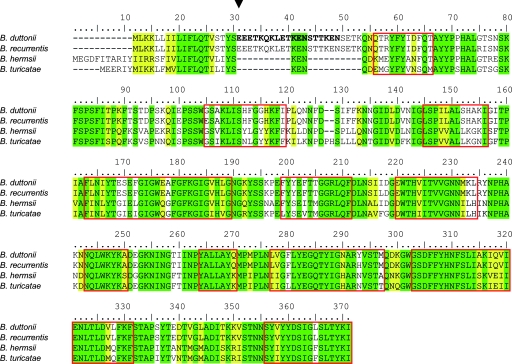
The chromosome sequence of B. duttonii revealed the gene coding for the 38-kDa protein and its complete amino acid sequence (GenBank accession number CP000976) (Fig. 3). According to its molecular mass, the protein was designated Oms38, for outer membrane spanning protein of 38 kDa. Further analyses identified homologues of oms38 in the chromosomes of B. hermsii (GenBank accession number EU660961), B. recurrentis (GenBank accession number CP000993), and B. turicatae (GenBank accession number EU660962).
FIG. 3.
Amino acid sequence alignment of Oms38 of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae (26). Amino acids identical in all four strains are shaded in green; similar amino acids are highlighted in yellow. The sequences of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae Oms38 share an amino acid identity of 58%. B. duttonii and B. recurrentis Oms38 are identical in 98% of their amino acid sequence, B. hermsii and B. turicatae Oms38 in 83%, and B. duttonii and B. hermsii in 67%. The putative N-terminal cleavage site of Oms38 from all four species is marked by an arrowhead (7). The 19-amino-acid sequence derived from the partial sequencing of the native B. duttonii protein is in bold. Predicted β-sheet transmembrane domains concordant in the four proteins are framed in red (1, 39).
Analysis of the amino acid sequences of Oms38 of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae.
Figure 3 shows an alignment of the deduced amino acid sequences of Oms38 of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae (26). The identity of Oms38 of the four species was 58%. B. duttonii- and B. recurrentis-derived Oms38 shared the highest amino acid identity of 98%, B. hermsii and B. turicatae Oms38 were 83% identical, and B. duttonii and B. hermsii Oms38 were 67% identical. The main discrepancies were located in the N terminus, while the remaining parts of the sequences were highly similar. N-terminal sequencing of the native B. duttonii Oms38 identified amino acids 20 to 38 of the full-length protein (Fig. 3). Consequently, the first N-terminal amino acids are removed under in vivo conditions and constitute the putative signal peptide (Fig. 3), as is known for other spirochetal outer membrane proteins and porins (29). The putative N-terminal cleavage sites of B. recurrentis, B. hermsii, and B. turicatae Oms38 are marked in Fig. 3 (7). Computational analyses (1, 39) revealed putative beta-sheet stretches in the secondary structure of Oms38 (Fig. 3). The predicted beta-sheet regions allow structural conceptions of a protein containing 12 to 14 beta-sheets forming a beta-barrel pore, as is known for other well-studied porins (25, 30, 57).
Single-channel measurements of Oms38.
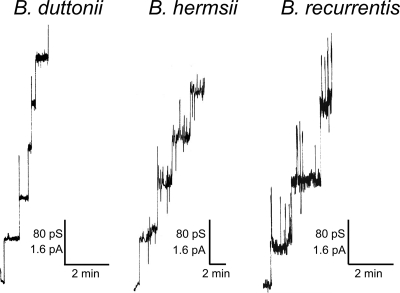
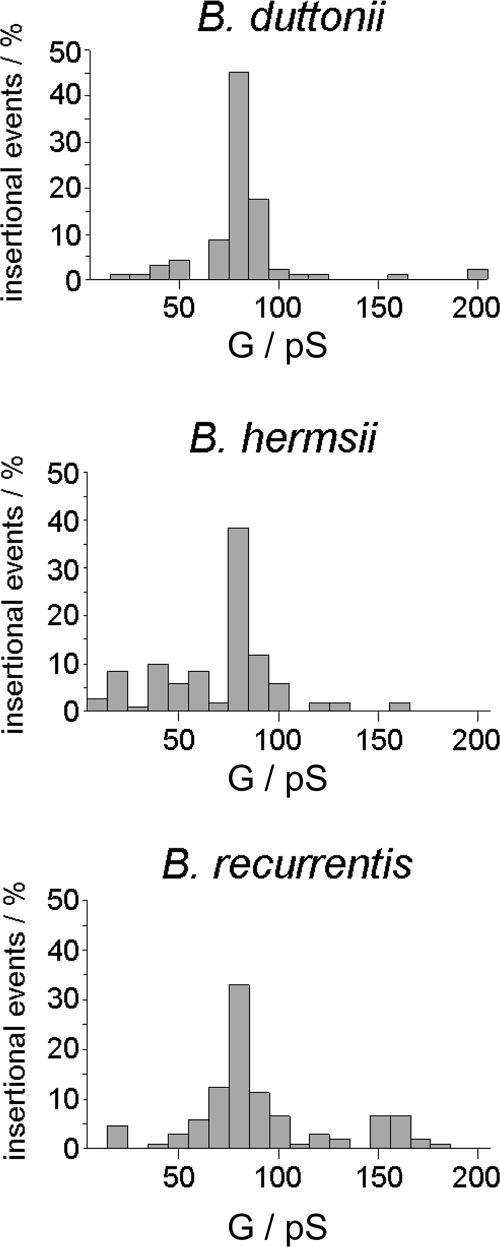
The pores formed by Oms38 were studied in more detail by adding small amounts of Oms38 to black lipid bilayer membranes. The reconstitution of the protein into the membrane caused a substantial conductance increase over several orders of magnitude due to the formation of small ion-permeable channels, similar to that caused by other bacterial porins (10). Under conditions of appropriate amplification and low protein concentration, the records of single reconstitutions into the membranes could be resolved as conductance steps, with an average single-channel conductance of 80 pS in 1 M KCl (Fig. 4). Some single-channel events showed initial sharp spikes during their reconstitution which immediately switched to the stable 80-pS level. This stable level was defined as the single-channel conductance of Oms38. By statistical analysis of at least 50 of these conductance steps, the single-channel conductance of Oms38 was measured as a function of different electrolytes and different concentrations. Figure 5 shows histograms of all current fluctuations observed for B. duttonii, B. hermsii, and B. recurrentis Oms38 in 1 M KCl. The data demonstrate that the Oms38 porins of all three RF species studied consistently exhibited the small average single-channel conductance of 80 pS in 1 M KCl, which is not surprising given the close identity of all three Oms38 proteins. The measurement of Oms38 pores from B. duttonii in different KCl concentrations from 0.1 to 3 M demonstrated that the channel conductance was an almost linear function of the electrolyte concentration (Table 1).
FIG. 4.
Pore-forming activity associated with purified B. duttonii, B. hermsii, and B. recurrentis Oms38. Single-channel insertional events were observed for B. duttonii, B. hermsii, and B. recurrentis Oms38 in a diphytanoyl phosphatidylcholine-n-decane membrane. Highly diluted, purified Oms38 at a concentration of about 10 ng ml−1 was added to the membrane and bathed in 1 M KCl. The applied voltage was 20 mV and the temperature was 20°C throughout.
FIG. 5.
Histograms of individual single-channel events observed for purified Oms38 of B. duttonii, B. hermsii, and B. recurrentis. For each histogram, a total of at least 50 insertions of Oms38 into a diphytanoyl phosphatidylcholine-n-decane membrane were evaluated. The average single-channel conductance (G) for at least 50 conductance steps was 80 pS for Oms38 derived from B. duttonii, B. hermsii, and B. recurrentis OMFs. The aqueous phase contained 1 M KCl; the applied voltage was 20 mV and the temperature was 20°C throughout.
TABLE 1.
Average single-channel conductances (G) of Oms38 of B. duttonii, B. hermsii, and B. recurrentis in different electrolyte solutionsa
| Electrolyte | Concn (M) |
G (pS)
|
||
|---|---|---|---|---|
| B. duttonii | B. hermsii | B. recurrentis | ||
| KCl | 3 | 275 | NM | NM |
| 1 | 80 | 80 | 80 | |
| 0.3 | 30 | NM | NM | |
| 0.1 | 15 | NM | NM | |
| LiCl | 1 | 50 | 75 | 60 |
| KCH3COO (pH 7) | 1 | 30 | 65 | 50 |
The membranes were formed from diphytanoyl phosphatidylcholine dissolved in n-decane. The single-channel conductance was measured in unbuffered electrolyte solutions (approximately pH 6) unless otherwise indicated at an applied voltage of 20 mV and 20°C. The average single-channel conductance, G, was calculated from at least 50 single insertions of Oms38. NM, not measured.
To obtain further information on the properties of the pores formed by Oms38, single-channel experiments were also performed with electrolytes other than KCl. The results are summarized in Table 1 and suggested an anion selectivity of the pore, as derived from single-channel experiments with KCH3COO and LiCl. Replacement of chloride with the large and less mobile acetate resulted in a substantial influence on the single-channel conductance (30 pS for B. duttonii, 65 pS for B. hermsii, and 50 pS for B. recurrentis in 1 M KCH3COO, pH 7), whereas the replacement of potassium by lithium led to a lower influence on the conductance (50 pS, 75 pS, and 60 pS, respectively, in 1 M LiCl).
Strikingly, the single-channel conductances of 80 pS in 1 M KCl of B. duttonii Oms38 and its homologues in B. hermsii and B. recurrentis were identical, whereas the measured conductances in LiCl and KCH3COO differed slightly when comparing Oms38 of the three species. A possible explanation could be different stability states of the proteins derived from different species after the OMF preparation and purification procedure. While B. duttonii Oms38 formed well-defined pores throughout, the channels formed by B. hermsii and B. recurrentis Oms38 were accompanied by flickering and noise, and they were scarcely measurable after about 10 single-channel events. Thus, the purification resulted in an observable percentage of flickering pores in the bilayer and therefore gave a less precise evaluation of the B. hermsii and B. recurrentis pores than for the B. duttonii Oms38. It is noteworthy, however, that channel flicker was not a purification artifact, because different protocols for purification of Oms38 from B. hermsii and B. recurrentis resulted in the same flickering, indicating that the flicker represents an intrinsic property of the channels. Interestingly, Oms38 homologues of all three species agreed in the trend of the single-channel results with LiCl and KCH3COO, in which the change of the anion in the electrolyte had a higher influence on the conductance than the change of the cation, indicating anion selectivity.
Voltage dependence.
Certain bacterial porins exhibit voltage-dependent closure at high voltages despite the fact that no voltage-dependent closure has been observed so far in in vivo experiments (10, 44, 63). To check whether Oms38 is voltage dependent, a multichannel experiment with approximately 100 reconstituted pores was performed. The application of membrane potentials ranging from −200 mV to +200 mV showed no influence on conductance, revealing that Oms38 did not show voltage-dependent closure (data not shown).
Selectivity measurements.
Selectivity measurements allow a quantification of the permeability of the Oms38 pore for anions relative to cations. The selectivity of B. duttonii-derived Oms38 was investigated by multichannel experiments under zero-current conditions in KCl, LiCl, and KCH3COO. Membranes were formed in 100 mM salt solution, and purified Oms38 was added to the aqueous phase when the membranes were in the black state. After the incorporation of approximately 100 pores into the membrane, fivefold salt gradients were established by the addition of small amounts of 3 M salt solution to one side of the membrane. The permeability ratios for cations over anions through Oms38 were calculated using the Goldman-Hodgkin-Katz equation (13) and are listed in Table 2 together with the zero-current membrane potentials. These data support the idea that Oms38 is indeed selective for anions.
TABLE 2.
Zero-current membrane potentials (Vm) of diphytanoyl phosphatidylcholine-n-decane membranes in the presence of B. duttonii Oms38 measured for a fivefold concentration gradient of different electrolytesa
| Electrolyte | Vm (mV) | Pcation/Panion |
|---|---|---|
| KCl | −14.0 | 0.41 |
| LiCl | −22.4 | 0.24 |
| KCH3COO (pH 7) | −8.6 | 0.58 |
Vm is defined as the difference between the potential at the diluted side (100 mM) and the potential at the concentrated side (500 mM). The aqueous electrolyte solutions were buffered with 10 mM Tris-HCl (pH 7.5); the temperature was 20°C. The permeability ratio Pcation/Panion was calculated using the Goldman-Hodgin-Katz equation (13) from at least two individual experiments.
The potential on the more diluted side of the membrane was negative throughout the experiments for KCl (−14.0 mV), LiCl (−22.4 mV), and KCH3COO (−8.6 mV), suggesting preferential movement of anions through the Oms38 pore. Nevertheless, cations may pass the Oms38 pore, because the ratios of the permeability coefficients, Pcation/Panion, were 0.41 (in KCl), 0.24 (in LiCl), and 0.58 (in KCH3COO). These data demonstrate also that the selectivity follows the mobility sequence of the ions, because the decrease in mobility of the anions (KCH3COO) resulted in a decrease of the anion selectivity. Similarly, the cations had a smaller influence on the selectivity because the replacement of K+ by the less mobile Li+ had a smaller influence on the permeability ratio. The results of the selectivity measurements are in agreement with the single-channel measurements, because they also suggested anion selectivity for the Oms38 pore.
DISCUSSION
Lipid bilayer experiments with OMFs of RF species suggest the presence of porins.
In this study we demonstrated that OMFs of the RF species B. duttonii, B. recurrentis, and B. hermsii contained channel-forming proteins, with properties similar to those of porins from other gram-negative bacteria (9, 50, 51, 53, 65, 66). The conductance of these putative porins reconstituted into lipid bilayer membranes was spread over a considerable range, with an obvious accumulation of pores in the low-conductance range from about 10 pS to 0.75 nS. The histogram observed with the OMF of B. hermsii agrees with previously reported data, which revealed a similar distribution of conductance (64). The distribution in all three histograms appears to be similar to that observed with the OMF of B. burgdorferi (53): detergent-treated outer membranes of this Lyme disease-causing species form channels in lipid bilayer membranes that are also spread across a considerable range in terms of conductance. This may indicate that its outer membrane contains a variety of channels (21, 48, 53, 65, 66). From this organism, P13, P66, Oms28, and a TolC-analogous outer membrane channel were identified and characterized in lipid bilayer experiments. All these porins form channels with a conductance in 1 M KCl that is greater than that observed for the channel-forming activity in this study. A channel-forming protein with a molecular mass of about 38 kDa was identified in the RF species, purified to homogeneity, and termed Oms38 in accordance with the nomenclature used for the outer membrane-spanning Oms28. This protein is also a porin, although its pore function has recently been questioned by analysis of its secondary structure, by the observation that Oms28 is not surface exposed, and by Triton X-114 extraction of outer membrane vesicles (49, 65). The Oms38 channels have a single-channel conductance of about 80 pS in 1 M KCl, which is considerably lower than the conductance of all porins that were identified to date in Lyme disease-causing B. burgdorferi.
Purification and identification of Oms38 of B. duttonii, B. hermsii, and B. recurrentis.
Oms38, the first porin identified in any RF Borrelia species, was purified to homogeneity using hydroxyapatite chromatography across a dry hydroxyapatite column. The features responsible for protein purification using this column material are not well understood, but it has been used with great success for the purification of mitochondrial porin and mitochondrial carriers (11, 54). These proteins are deeply buried in the mitochondrial membranes and probably also in the detergent micelles after membrane digestion using detergent treatment. We assume that Oms38 is similarly deeply buried in the outer membrane because of its function as an outer membrane channel. Using the same purification procedure, Oms38 was purified from the OMFs of all three studied RF species. SDS-PAGE revealed a high degree of purity of Oms38 of B. hermsii and B. recurrentis, even under sensitive conditions of silver staining of the proteins separated by SDS-PAGE. The additional, weak 27-kDa protein band visible on the SDS-polyacrylamide gel of the B. duttonii fraction after purification across the hydroxyapatite column could not be responsible for pore formation, because fractions exclusively containing this 27-kDa band did not exhibit pore-forming activity. Mass spectrometry identified this protein as member of the Vsp lipoprotein family, with similarity to OspC of B. burgdorferi (4, 60). Additional secondary-structure analysis and comparison with other pore-forming lipoproteins supported the view that it is not a channel-forming component.
Deduced amino acid sequences of Oms38 of B. duttonii, B. hermsii, B. recurrentis, and B. turicatae.
Partial sequencing of the purified Oms38 of B. duttonii allowed the identification of its gene within the genomes of the three other RF species B. hermsii, B. recurrentis, and B. turicatae. Interestingly, a gene homologous to oms38 is also present in the well-studied Lyme disease agent B. burgdorferi (as “hypothetical protein” bb0418). The function of this gene has not yet been defined, but its presence may indicate the existence of a protein in B. burgdorferi with properties similar to those of Oms38 of RF Borrelia.
The deduced amino acid sequences of Oms38 from the four RF species showed an overall identity of 58%, indicating that the identities between the Oms38 homologues in B. duttonii, B. hermsii, B. recurrentis, and B. turicatae are rather high. B. duttonii and B. recurrentis Oms38 shared an identity of 98%, while those of B. hermsii and B. turicatae shared an identity of 83%. The high identity of the primary structure is in good agreement with the biophysical results, demonstrating that the Oms38 homologues of B. duttonii, B. hermsii, and B. recurrentis exhibited identical biochemical and biophysical properties (see below). From this point of view, we assume that the structures and functions of the Oms38 homologues are identical under in vivo conditions. The N terminus of Oms38 of B. duttonii starts with three glutamic acids. This means that this protein contains a 19-amino-acid, N-terminal signal peptide with a putative recognition sequence for the leader peptidase, similar to those of other spirochetal outer membrane proteins (29). The deduced amino acid sequences of all Oms38 proteins may contain similar N-terminal extensions that are responsible for their transport into the periplasm, as is known for porins of B. burgdorferi (29). The signal peptides are cleaved during the sec-dependent export process, and the protein is able to insert into the outer membrane. Computational analyses predicted several putative beta-sheets in the primary sequences of the different Oms38 porins. This suggests that the protein may form a beta-barrel in the outer membrane, like most gram-negative bacterial porins (10, 25, 30, 57). However, structural predictions for Borrelia porins have to be considered with caution because of the possible presence of alpha-helices in some borrelian porins, as shown by computational analysis for P13 and P66 and by circular dichroism spectroscopy for Oms28 (22, 49, 52). This means that their definitive structure can be deduced only from X-ray analysis of protein crystals.
Biophysical properties of Oms38.
Pure Oms38 porins were obtained by affinity chromatography across a hydroxyapatite column using increasing ionic strength in the elution buffer. The results of lipid bilayer experiments revealed that all the Oms38 porins studied here form channels with the low single-channel conductance of 80 pS in 1 M KCl. To date, a similar pore-forming activity is not known from B. burgdorferi OMF studies, and the value of 80 pS differs clearly from the comparatively huge single-channel conductances of 600 pS (65), 3.5 nS (53), and 9.6 nS (66) associated with well-characterized B. burgdorferi porins. However, Oms38 forms pores with a single-channel conductance in the range of those of certain outer membrane porins of E. coli that have a lower conductance, such as the nucleotide-specific Tsx (10 pS) (47) and the sugar-specific LamB (160 pS) (15). These E. coli channels with comparable single-channel conductance are substrate-specific channels, which means that further investigations have to be done to determine if Oms38 functions as general diffusion pore or as a substrate-specific channel.
Interestingly, in some experiments with Oms38 of B. duttonii we observed initial sharp peaks (Fig. 4), which could be interpreted as additional transient states of the Oms38 channels. These transient states were difficult to resolve fully in some cases; in others, they had approximately the same conductance as the stable state. In experiments with Oms38 from B. hermsii and B. recurrentis, the additional state with a conductance of about 80 pS occurred more frequently and not only during the reconstitution of the channels into the membrane but also as a superposition to the stable 80 pS state (Fig. 4). These results suggest that Oms38 could have at least two states, one transient and one stable. The trigger for the opening of the transient state is not clear, but it was definitely not caused by the purification procedure. In addition, it was also not caused by voltage-dependent gating. In general, no indication for voltage-dependent gating of the Oms38 channels was observed.
Experiments with salts other than KCl demonstrated that the conductance of the Oms38 channels was dependent on the aqueous mobility of the ions (Table 1). Nevertheless, the effect of the anions on the single-channel conductance was more substantial, indicating anion selectivity of the Oms38 channels (Table 1). This is supported by the results of the zero-current potential measurements (see below). The single-channel conductance of the Oms38 channel of B. duttonii was an almost linear function of the KCl concentration. This suggests that the channel does not contain a binding site for the ions used in this study. In addition, the channel probably does not contain net charges that could result in charge effects. The possible anion selectivity of the Oms38 channels was confirmed by selectivity measurements with Oms38 of B. duttonii. For all three salts employed in this study, the more dilute side of the membrane became negative due to the preferential movement of anions over cations (Table 2). This indicates preferential diffusion of anions through the pore.
Taking these results together, we have identified, purified, and characterized the first putative porin in the outer membrane of the RF species B. duttonii, B. hermsii, and B. recurrentis. While detailed structure-function analyses of Oms38 and further RF outer membrane investigations remain to be done, this work represents an important step forward in understanding the outer membrane pathways for nutrient uptake by these strictly host-dependent, pathogenic spirochetes. Furthermore, it provides some knowledge of the outer membrane protein composition and surface-exposed protein domains of RF spirochetes. This could lead to a basis for a successful drug design. Further characterization of RF Borrelia porins could also provide information concerning the physiology of these spirochetes and lead to the discovery of a surface-exposed protein that could function as a potential vaccine candidate.
Acknowledgments
We thank Christian Andersen for critical discussion of the results and Stephen Porcella and Sandra Steward for the help with sequencing.
This work was supported by grants from the joint project between Stint (Sweden) and DAAD (Germany) to S.B. and R.B., from the Fonds der Chemischen Industrie (to R.B.), and from the Swedish Medical Research Council (to S.B.).
Footnotes
Published ahead of print on 29 August 2008.
REFERENCES
- 1.Bagos, P. G., T. D. Liakopoulos, I. C. Spyropoulos, and S. J. Hamodrakas. 2004. A hidden Markov model method, capable of predicting and discriminating beta-barrel outer membrane proteins. BMC Bioinformatics 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 2002. Antigenic variation of relapsing fever Borrelia and other pathogenic bacteria, p. 972-994, Mobile DNA II. American Society for Microbiology, Washington, DC.
- 3.Barbour, A. G. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57521-525. [PMC free article] [PubMed] [Google Scholar]
- 4.Barbour, A. G., C. J. Carter, and C. D. Sohaskey. 2000. Surface protein variation by expression site switching in the relapsing fever agent Borrelia hermsii. Infect. Immun. 687114-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour, A. G., Q. Dai, B. I. Restrepo, H. G. Stoenner, and S. A. Frank. 2006. Pathogen escape from host immunity by a genome program for antigenic variation. Proc. Natl. Acad. Sci. USA 10318290-18295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belisle, J. T., M. E. Brandt, J. D. Radolf, and M. V. Norgard. 1994. Fatty acids of Treponema pallidum and Borrelia burgdorferi lipoproteins. J. Bacteriol. 1762151-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 8.Benz, R. 1994. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim. Biophys. Acta 1197167-196. [DOI] [PubMed] [Google Scholar]
- 9.Benz, R. 2001. Porins—structure and function, p. 227-246. In G. Winkelmann (ed.), Microbial transport systems. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 10.Benz, R. 1994. Solute uptake through bacterial outer membranes, p. 397-423. In G. J. M. Hackenbek (ed.), Bacterial cell wall. Elsevier Science B. V., Amsterdam, The Netherlands.
- 11.Benz, R. 2004. Structure and function of mitochondrial (eukaryotic) porins, p. 259-284. In R. Benz (ed.), Structure and function of prokaryotic and eukaryotic porins. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- 12.Benz, R., K. Janko, W. Boos, and P. Läuger. 1978. Formation of large, ion-permeable membrane channels by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 511305-319. [DOI] [PubMed] [Google Scholar]
- 13.Benz, R., K. Janko, and P. Läuger. 1979. Ionic selectivity of pores formed by the matrix protein (porin) of Escherichia coli. Biochim. Biophys. Acta 551238-247. [DOI] [PubMed] [Google Scholar]
- 14.Benz, R., A. Schmid, C. Maier, and E. Bremer. 1988. Characterization of the nucleoside-binding site inside the Tsx channel of Escherichia coli outer membrane. Reconstitution experiments with lipid bilayer membranes. Eur. J. Biochem. 176699-705. [DOI] [PubMed] [Google Scholar]
- 15.Benz, R., A. Schmid, T. Nakae, and G. H. Vos-Scheperkeuter. 1986. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J. Bacteriol. 165978-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benz, R., A. Schmid, and G. H. Vos-Scheperkeuter. 1987. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J. Membr. Biol. 10021-29. [DOI] [PubMed] [Google Scholar]
- 17.Bernardini, M. L., M. G. Sanna, A. Fontaine, and P. J. Sansonetti. 1993. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect. Immun. 613625-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 893-99. [Google Scholar]
- 19.Brasseur, D. 1985. Tick-borne relapsing fever in a premature infant. Ann. Trop. Paediatr. 5161-162. [DOI] [PubMed] [Google Scholar]
- 20.Bryceson, A. D., E. H. Parry, P. L. Perine, D. A. Warrell, D. Vukotich, and C. S. Leithead. 1970. Louse-borne relapsing fever. Q. J. Med. 39129-170. [PubMed] [Google Scholar]
- 21.Bunikis, I., K. Denker, Y. Östberg, C. Andersen, R. Benz, and S. Bergström. 2008. An RND-type efflux system in Borrelia burgdorferi is involved in virulence and resistance to antimicrobial compounds. PLoS Pathog. 4e1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunikis, J., L. Noppa, and S. Bergström. 1995. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol. Lett. 131139-145. [DOI] [PubMed] [Google Scholar]
- 23.Cadavid, D., P. M. Pennington, T. A. Kerentseva, S. Bergström, and A. G. Barbour. 1997. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect. Immun. 653352-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter, C. J., S. Bergström, S. J. Norris, and A. G. Barbour. 1994. A family of surface-exposed proteins of 20 kilodaltons in the genus Borrelia. Infect. Immun. 622792-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charbit, A. 2003. Maltodextrin transport through lamb. Front Biosci. 8s265-74. [DOI] [PubMed] [Google Scholar]
- 26.Chenna, R., H. Sugawara, T. Koike, R. Lopez, T. J. Gibson, D. G. Higgins, and J. D. Thompson. 2003. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 313497-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox, D. L., D. R. Akins, K. W. Bourell, P. Lahdenne, M. V. Norgard, and J. D. Radolf. 1996. Limited surface exposure of Borrelia burgdorferi outer surface lipoproteins. Proc. Natl. Acad. Sci. USA 937973-7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox, D. L., and J. D. Radolf. 2001. Insertion of fluorescent fatty acid probes into the outer membranes of the pathogenic spirochaetes Treponema pallidum and Borrelia burgdorferi. Microbiology 1471161-1169. [DOI] [PubMed] [Google Scholar]
- 29.Cullen, P. A., D. A. Haake, and B. Adler. 2004. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol. Rev. 28291-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delcour, A. H. 2002. Structure and function of pore-forming beta-barrels from bacteria. J. Mol. Microbiol. Biotechnol. 41-10. [PubMed] [Google Scholar]
- 31.Dworkin, M. S., T. G. Schwan, and D. E. Anderson, Jr. 2002. Tick-borne relapsing fever in North America. Med. Clin. N. Am. 86417-433. [DOI] [PubMed] [Google Scholar]
- 32.Eckerskorn, C., and F. Lottspeich. 1993. Structural characterization of blotting membranes and the influence of membrane parameters for electroblotting and subsequent amino acid sequence analysis of proteins. Electrophoresis 14831-838. [DOI] [PubMed] [Google Scholar]
- 33.Felsenfeld, O. 1971. Borrelia: strains, vectors, human and animal Borreliosis. Green, St. Louis, MO.
- 34.Ferenci, T., M. Schwentorat, S. Ullrich, and J. Vilmart. 1980. Lambda receptor in the outer membrane of Escherichia coli as a binding protein for maltodextrins and starch polysaccharides. J. Bacteriol. 142521-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fuji, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 36.Freitag, H., G. Genchi, R. Benz, F. Palmieri, and W. Neupert. 1982. Isolation of mitochondrial porin from Neurospora crassa. FEBS Lett. 14572-76. [DOI] [PubMed] [Google Scholar]
- 37.Fuchs, P. C., and A. A. Oyama. 1969. Neonatal relapsing fever due to transplacental transmission of Borrelia. JAMA 208690-692. [PubMed] [Google Scholar]
- 38.Goubau, P. F. 1984. Relapsing fevers. A review. Ann. Soc. Belg. Med. Trop. 64335-364. [PubMed] [Google Scholar]
- 39.Gromiha, M. M., S. Ahmad, and M. Suwa. 2004. Neural network-based prediction of transmembrane beta-strand segments in outer membrane proteins. J. Comput. Chem. 25762-767. [DOI] [PubMed] [Google Scholar]
- 40.Guggenheim, J. N., and A. D. Haverkamp. 2005. Tick-borne relapsing fever during pregnancy: a case report. J. Reprod. Med. 50727-729. [PubMed] [Google Scholar]
- 41.Hancock, R. E., and R. Benz. 1986. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim. Biophys. Acta 860699-707. [DOI] [PubMed] [Google Scholar]
- 42.Johnson, R. C. 1977. The spirochetes. Annu. Rev. Microbiol. 3189-106. [DOI] [PubMed] [Google Scholar]
- 43.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 44.Lakey, J. H., and F. Pattus. 1989. The voltage-dependent activity of Escherichia coli porins in different planar bilayer reconstitutions. Eur. J. Biochem. 186303-308. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig, O., V. De Pinto, F. Palmieri, and R. Benz. 1986. Pore formation by the mitochondrial porin of rat brain in lipid bilayer membranes. Biochim. Biophys. Acta 860268-276. [DOI] [PubMed] [Google Scholar]
- 46.Magnarelli, L. A., J. F. Anderson, and A. G. Barbour. 1989. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J. Infect. Dis. 15943-49. [DOI] [PubMed] [Google Scholar]
- 47.Maier, C., E. Bremer, A. Schmid, and R. Benz. 1988. Pore-forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J. Biol. Chem. 2632493-2499. [PubMed] [Google Scholar]
- 48.Mirzabekov, T. A., J. T. Skare, E. S. Shang, D. R. Blanco, J. N. Miller, M. A. Lovett, and B. L. Kagan. 1996. Electrical properties of porins from Borrelia burgdorferi. Biophys. J. 70A205. [Google Scholar]
- 49.Mulay, V., M. J. Caimano, D. Liveris, D. C. Desrosiers, J. D. Radolf, and I. Schwartz. 2007. Borrelia burgdorferi BBA74, a periplasmic protein associated with the outer membrane, lacks porin-like properties. J. Bacteriol. 1892063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson, C. L., H. J. Cooper, K. Hakansson, A. G. Marshall, Y. Östberg, M. Lavrinovicha, and S. Bergström. 2002. Characterization of the P13 membrane protein of Borrelia burgdorferi by mass spectrometry. J. Am. Soc. Mass Spectrom. 13295-299. [DOI] [PubMed] [Google Scholar]
- 52.Noppa, L., Y. Östberg, M. Lavrinovicha, and S. Bergström. 2001. P13, an integral membrane protein of Borrelia burgdorferi, is C-terminally processed and contains surface-exposed domains. Infect. Immun. 693323-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Östberg, Y., M. Pinne, R. Benz, P. Rosa, and S. Bergström. 2002. Elimination of channel-forming activity by insertional inactivation of the p13 gene in Borrelia burgdorferi. J. Bacteriol. 1846811-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmieri, F., C. Indiveri, F. Bisaccia, and R. Kramer. 1993. Functional properties of purified and reconstituted mitochondrial metabolite carriers. J. Bioenerg. Biomembr. 25525-535. [DOI] [PubMed] [Google Scholar]
- 55.Radolf, J. D., K. W. Bourell, D. R. Akins, J. S. Brusca, and M. V. Norgard. 1994. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J. Bacteriol. 17621-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radolf, J. D., M. S. Goldberg, K. Bourell, S. I. Baker, J. D. Jones, and M. V. Norgard. 1995. Characterization of outer membranes isolated from Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 632154-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saier, M. H., Jr. 2000. Families of proteins forming transmembrane channels. J. Membr. Biol. 175165-180. [DOI] [PubMed] [Google Scholar]
- 58.Saint Girons, I., and A. G. Barbour. 1991. Antigenic variation in Borrelia. Res. Microbiol. 142711-717. [DOI] [PubMed] [Google Scholar]
- 59.Sallmann, F. R., S. Baveye-Descamps, F. Pattus, V. Salmon, N. Branza, G. Spik, and D. Legrand. 1999. Porins OmpC and PhoE of Escherichia coli as specific cell-surface targets of human lactoferrin. Binding characteristics and biological effects. J. Biol. Chem. 27416107-16114. [DOI] [PubMed] [Google Scholar]
- 60.Schwan, T. G., and B. J. Hinnebusch. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 2801938-1940. [DOI] [PubMed] [Google Scholar]
- 61.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, P. F. Policastro, J. A. Rawlings, R. S. Lane, E. B. Breitschwerdt, and S. F. Porcella. 2005. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J. Clin. Microbiol. 433851-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwan, T. G., S. J. Raffel, M. E. Schrumpf, and S. F. Porcella. 2007. Diversity and distribution of Borrelia hermsii. Emerg. Infect. Dis. 13436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sen, K., J. Hellman, and H. Nikaido. 1988. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 2631182-1187. [PubMed] [Google Scholar]
- 64.Shang, E. S., J. T. Skare, M. M. Exner, D. R. Blanco, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1998. Isolation and characterization of the outer membrane of Borrelia hermsii. Infect. Immun. 661082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skare, J. T., C. I. Champion, T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1996. Porin activity of the native and recombinant outer membrane protein Oms28 of Borrelia burgdorferi. J. Bacteriol. 1784909-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skare, J. T., T. A. Mirzabekov, E. S. Shang, D. R. Blanco, H. Erdjument-Bromage, J. Bunikis, S. Bergström, P. Tempst, B. L. Kagan, J. N. Miller, and M. A. Lovett. 1997. The Oms66 (p66) protein is a Borrelia burgdorferi porin. Infect. Immun. 653654-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Southern, P., and J. Sanford. 1969. Relapsing fever: a clinical and microbiological review. Medicine 48129-149. [Google Scholar]
- 68.Stoenner, H. G., T. Dodd, and C. Larsen. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med. 1561297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tabuchi, N., K. Tomoda, H. Kawaguchi, H. Iwamoto, and M. Fukunaga. 2006. Immunodominant epitope in the C-terminus of a variable major protein in Borrelia duttonii, an agent of tick-borne relapsing fever. Microbiol. Immunol. 50293-305. [DOI] [PubMed] [Google Scholar]
- 70.Takayama, K., R. J. Rothenberg, and A. G. Barbour. 1987. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 552311-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Holten, J., J. Tiems, and V. H. Jongen. 1997. Neonatal Borrelia duttoni infection: a report of three cases. Trop. Doct. 27115-116. [DOI] [PubMed] [Google Scholar]
- 72.Vial, L., G. Diatta, A. Tall, H. Ba El, H. Bouganali, P. Durand, C. Sokhna, C. Rogier, F. Renaud, and J. F. Trape. 2006. Incidence of tick-borne relapsing fever in west Africa: longitudinal study. Lancet 36837-43. [DOI] [PubMed] [Google Scholar]
- 73.Walker, E. M., L. A. Borenstein, D. R. Blanco, J. N. Miller, and M. A. Lovett. 1991. Analysis of outer membrane ultrastructure of pathogenic Treponema and Borrelia species by freeze-fracture electron microscopy. J. Bacteriol. 1735585-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wessel, D., and U. I. Flügge. 1984. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138141-143. [DOI] [PubMed] [Google Scholar]
- 75.Yu, S., H. Ding, J. Seah, K. Wu, Y. Chang, K. S. Chang, M. F. Tam, and W. Syu. 1998. Characterization of a phage specific to hemorrhagic Escherichia coli O157:H7 and disclosure of variations in host outer membrane protein ompC. J. Biomed Sci. 5370-382. [DOI] [PubMed] [Google Scholar]