Abstract
Lsr2 is a small, basic protein present in Mycobacterium and related actinomycetes. Our previous in vitro biochemical studies showed that Lsr2 is a DNA-bridging protein, a property shared by H-NS-like proteins in gram-negative bacteria. Here we present in vivo evidence based on genetic complementation experiments that Lsr2 is a functional analog of H-NS, the first such protein identified in gram-positive bacteria. We show that lsr2 can complement the phenotypes related to hns mutations in Escherichia coli, including β-glucoside utilization, mucoidy, motility, and hemolytic activity. We also show that Lsr2 binds specifically to H-NS-regulated genes and the repression of hlyE by Lsr2 can be partially eliminated by overexpression of slyA, suggesting that the molecular mechanisms of Lsr2 repression and depression are similar to those of H-NS. The functional equivalence of these two proteins is further supported by the ability of hns to complement the lsr2 phenotype in Mycobacterium smegmatis. Taken together, our results demonstrate unequivocally that Lsr2 is an H-NS-like protein.
The bacterial nucleoid-associated proteins are low-molecular-weight, basic proteins that bind nucleic acids (29). Also referred to as the histone-like proteins, they play an integral role in organizing and condensing the bacterial chromosome and they also have roles in replication, recombination, and gene regulation (29). H-NS (encoded by hns) is one of the most abundant nucleoid-associated proteins in the Enterobacteriaceae and has been extensively studied in Escherichia coli and Salmonella enterica serovar Typhimurium, mainly on the basis of its interactions with DNA molecules (17). H-NS exists essentially as a homodimer and binds preferentially to AT-rich, curved DNA often occurring at promoters (36, 40). Recently, high-affinity binding sites have also been reported, with the current paradigm being that they serve as initiation sites for nucleation of H-NS to form higher-order nucleoprotein structures (6). Multiple studies have demonstrated that H-NS is a global regulator that controls the expression of a large number of genes (>400) in E. coli (39) or Salmonella (33, 36), most of which are derived from foreign sources via horizontal gene transfer. Many H-NS-repressed genes are regulated by environmental conditions such as pH, osmolarity, and temperature or are involved in bacterial virulence (17, 35). The effects of H-NS on gene expression are largely inhibitory, which is partially explained by the ability of H-NS to bridge adjacent helices of DNA (12, 13), causing either the trapping or occlusion of RNA polymerase in the promoter regions (10, 17). Numerous phenotypes associated with hns mutations have been described, including derepression of β-glucoside metabolism (14), increased resistance to low pH and high osmolarity (27), and a loss of motility (4, 26). H-NS also regulates virulence genes in other gram-negative pathogens, including Vibrio cholerae (38, 45), Yersinia (18, 25), and Shigella flexneri (1, 15, 20, 41).
A number of H-NS homologues have been found in gammaproteobacteria (46). They share a high level of sequence conservation and have been identified on the basis of sequence homology (46). Less well conserved H-NS homologues, termed H-NS-like or H-NS-related proteins, have been identified outside of this group by in vivo complementation, such as BpH3 of Bordetella pertussis (22) and HvrA of Rhodobacter capsulatus (2), which belong to the alpha and beta subdivisions of the proteobacteria, respectively. As such, it was suggested that the H-NS-like proteins are widespread in gram-negative bacteria (46). However, H-NS-related proteins have not been identified in bacteria phylogenetically distant from proteobacteria, such as in gram-positive bacteria, either by in silico analysis of genomes or by in vivo complementation using a genomic library, as in the case of Bacillus subtilis (46).
Mycobacteria belong to high G+C gram-positive bacteria often called the actinomycetes. Lsr2 is a small (∼12-kDa), basic protein found in all mycobacterial genomes that have been sequenced so far. Lsr2 homologues are also present in other actinomycetes such as Streptomyces, Nocardia, and Rhodococcus. Previous studies by others and by us showed that Lsr2 is a regulatory protein involved in multiple cellular processes, including cell wall biosynthesis and antibiotic resistance (7, 9, 32). Recently, we obtained in vitro biochemical evidence that Lsr2 is a DNA-bridging protein (8), which suggests that Lsr2 is an H-NS-like protein. Here, we present in vivo evidence based on genetic complementation experiments that Lsr2 is functionally homologous to H-NS. This study, together with our previous findings, has identified Lsr2 as the first H-NS-like protein in gram-positive bacteria.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The E. coli K-12 strain MC4100 was used as the wild-type (WT) strain for most of our experiments. WN582 is the hns mutant strain of MC4100 (E. coli K-12 MC4100 Δhns::Cm), which was kindly provided by Henry Rosen (University of Washington). Another E. coli K-12 strain, N99, and its hns mutant were used in the motility assay. The hns mutant of N99 was created by P1rev6 phage transduction (48). P1rev6 phage was grown on a WN582 background to obtain an hns gene disrupted by a chloramphenicol cassette. The resulting phage lysate was incubated with N99 for 30 min in the presence of P1 salts (10 mM CaCl2-5 mM MgSO4). LB and sodium citrate were then added to prevent secondary infection and incubated for 1 h at 37°C with shaking. The cells were then pelleted and resuspended in LB and plated onto LB agar plates with 20 μg/ml chloramphenicol. Chloramphenicol-resistant colonies were then verified for the Δhns::Cm genotype by PCR (data not shown). Mycobacterium smegmatis strain mc2155 and its lsr2 mutant, previously described (7), were used for complementation experiments. E. coli strains were grown in LB at 37°C with appropriate antibiotics, and M. smegmatis strains were grown in Middlebrook 7H9 broth or 7H11 agar supplemented with 10% OADC (oleic acid-albumin-dextrose-catalase) and appropriate antibiotics.
Molecular cloning.
The open reading frame of lsr2 of Mycobacterium tuberculosis including the 200-bp upstream sequence was PCR amplified using the forward primer 5′-CAGTCTAGAAACCGGAATGGGTATCGA-3′ and the reverse primer 5′-TTTTTAAGCTTCTAAGCGTAGTCTGGGACGTCGTATGGGTAGGTCGCCGCGTGGTATGCGTCGAT-3′, which included a C-terminal hemagglutinin (HA) tag (YPYDVPDYA). The resulting PCR product was digested with XbaI and HindIII and cloned into pNBV1 pretreated with the same enzymes to yield pLSR2-HA. A second lsr2 construct with a C-terminal FLAG tag (DYKDDDDK) was also constructed for use later in the chromatin immunoprecipitation (ChIP) experiments described below. The same forward primer as used above was used with the reverse primer 5′-GCTCTAGAAGCTTACTACTTGTCATCGTCGTCCTTGTAGTCGGTCGCCGCGTGGTATGC-3′, which included the FLAG tag. The resulting PCR product was digested with XbaI and HindIII and cloned into pNBV1 to yield pLSR2-FLAG.
The hns-containing plasmid pWN426 served as the template for PCR amplification of the hns gene of S. enterica serovar Typhimurium with the forward primer 5′-TTGGATCCGACGACAAACCGATACGAGAG-3′ and the reverse primer 5′-TTTTTAAGCTTTTATGCGTAGTCTGGTACGTCATAAGGGTA-3′. The PCR product included the 800-bp upstream sequence of the hns open reading frame as well as a C-terminal HA tag. The hns PCR product was digested with BamHI and HindIII and cloned into pNBV1, yielding pHNS-HA.
The slyA gene of S. enterica serovar Typhimurium was PCR amplified using the forward primer 5′-AAAAGAATTCTTATAAGGAGATGAAATTGGAATCGCCACTAGGTT-3′ and the reverse primer 5′-AAAACTCGAGACAAGGAAATACGCGTTTCTCGGC-3′. The PCR product was digested with EcoRI and XhoI and cloned into the arabinose-inducible expression vector pLC2002 (gift from Zhou Yu, University of Toronto, and Leslie Cuthbertson, McMaster University) pretreated with the same enzymes to generate pSLYA. All constructs were confirmed by DNA sequencing.
β-Glucoside fermentation assay.
To assay for fermentation of aryl-β,d-glucosides, we employed MacConkey agar (Difco) supplemented with 0.4% salicin. Bacteria were streaked on the plates and incubated at 37°C for 24 h. MacConkey agar is an indicator medium containing small peptides allowing for growth of gram-negative bacteria regardless of their ability to utilize a sugar that is supplemented into the agar. If bacteria can ferment the sugar, they will produce acidic by-products, causing a drop in pH which gives rise to red/pink colonies. If the bacteria are unable to ferment the sugar they will grow using the peptone and produce ammonia as a by-product of amino acid metabolism. In turn this leads to an increase in pH, resulting in white colonies.
Motility assay.
The motility phenotype was assessed on tryptone swarm plates containing 1% Bacto tryptone, 0.5% NaCl, and 0.3% Bacto agar. Colonies were stabbed into the plates and incubated at 37°C for 5 h.
Hemolysis assay.
Hemolytic activity was assessed on blood agar plates containing 3% tryptic soy broth, 1.5% agar, and 5% defibrinated sheep blood (Rockland). Three independent colonies per strain were stabbed into the plates and incubated at 37°C for 24 h. An area of clearing around a stab culture was indicative of hemolytic activity.
ChIP assay.
Cultures of WN582 harboring pLSR2-HA or pLSR2-FLAG were grown to mid-logarithmic phase (optical density at 600 nm of 0.4 to 0.6) and treated with 1% formaldehyde for 15 min at room temperature. The cross-linking reaction was then quenched with 1.25 mM glycine for 10 min. Cells were washed twice with ice-cold phosphate-buffered saline and sonicated to generate DNA fragments of ∼500 bp. Cell lysates were precipitated with an anti-HA antibody (Sigma H9658) using agarose protein G beads (Calbiochem). The LSR2 protein tagged with the FLAG epitope served as a negative control, since it does not interact with the HA antibody.
Quantitative RT-PCR.
Quantitative real-time PCR (RT-PCR) analyses were performed using the SYBR green mix from Sigma (S4438) according to the manufacturer's instructions. Each primer set was done in triplicate. Primer pairs used for this analysis were as follows: forward 5′-ACACTGTTAACCGCCAGGAAGACA-3′ and reverse 5′-GGATGAAAGCAAAGCGCAAGCAGA-3′ for bglG; forward 5′-AGTTCCGTGCAGGAAGAGAACCTT-3′ and reverse 5′-TGGTTACGTCGCTTTCGGCTTACT-3′ for yjcF; forward 5′-AATATTTGGCGAGCATCCACAGCG-3′ and reverse 5′-TTTACCCGAGCCGGATAATCCCAT-3′ for proV; forward 5′-GCAATCGACGCGATTCTTCCATCAAG-3′ and reverse 5′-GCAGCGCGTTTAAATATGTCTCAGCC-3′ for xapR; forward 5′-GCGCCATTGGCTGGAATGATTGTA-3′ and reverse 5′-ATGCTGATCTTCTGCGGTTGTGTC-3′ for narZ; and forward 5′-CGAACAGCATGGCGAAGACCATTT-3′ and reverse 5′-AGTGGCAGGATTACCTCACCGAAA-3′ for phnE.
Reverse transcriptase quantitative PCR analysis of hlyE transcript.
The E. coli strains MC4100 and WN582/pLSR2-HA were transformed with the arabinose-inducible pSLYA plasmid. Cultures of MC4100, MC4100/pSLYA, WN582, WN582/pLSR2-HA, and WN582/pLSR2-HA+pSLYA were grown in LB media supplemented with 0.2% arabinose to mid-log phase (optical density at 600 nm of 0.5). The cells (0.5 ml) were mixed with 1 ml of RNAprotect bacterial reagent (Qiagen) and incubated for 15 min at room temperature. Subsequent RNA preparations were performed using the Aurum total RNA minikit (Bio-Rad 732-6820). Reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen). The cDNA generated was used for quantitative RT-PCR analysis as described above. The transcript of hlyE was analyzed by using the primers 5′-TCCCTGGTAAGCTCACAAAGT-3′ (forward) and 5′-ACCGGCATATGCTTCCTCCTGAT-3′ (reverse). The transcript of gyrB, a gene not regulated by H-NS (36), was also analyzed and used as an internal standard for normalization among different samples. The primers for gyrB are 5′-CACTTTCACGGAAACGACCGCAAT-3′ (forward) and 5′-TTACCAACAACATTCCGCAGCGTG-3′ (reverse). Cultures grown in the absence of arabinose were used as the negative control.
RESULTS
lsr2 of M. tuberculosis complements various phenotypes of the E. coli hns mutant.
Our previous biochemical studies indicate that Lsr2 is a DNA-bridging protein (8), a characteristic shared by H-NS-like proteins (11). Given similar biochemical properties but a low level of sequence homology (<20% identity in amino acid sequence) between Lsr2 and H-NS, it was of interest to determine whether Lsr2 could functionally mimic H-NS and complement its deficiency in an E. coli hns mutant. For this purpose, we cloned the lsr2 gene of M. tuberculosis and hns of S. enterica serovar Typhimurium into the mycobacterial-E. coli shuttle vector pNBV1 (28). We chose to study the lsr2 of M. tuberculosis since it is functionally interchangeable with lsr2 of M. smegmatis (32). The H-NS proteins of enteric bacteria are also functionally interchangeable (3). For complementation experiments in E. coli, we first transformed the pLSR2-HA construct into an hns mutant strain of E. coli WN582 by standard procedures and examined the expression of Lsr2 in the E. coli recombinant strain. Western blot analysis using an anti-HA antibody indicated that Lsr2 protein was expressed in the E. coli strain harboring pLSR2-HA (Fig. 1).
FIG. 1.

Western blot analysis of Lsr2 expression in E. coli. Lane 1, hns mutant WN582; lane 2, hns mutant WN582 with pNBV1; lanes 3 to 7, five randomly picked colonies of WN582 with pLSR2; lane M, molecular weight marker.
In E. coli and Salmonella, mutations of hns are highly pleiotropic. Several phenotypes associated with hns mutations have been well characterized, including mucoidy (24, 43), β-glucoside utilization (14), hemolytic activity (21), and loss of motility (4, 26). The mechanisms of hns effects on the corresponding genes involved in these phenotypes are well documented. As such, these phenotypes have been routinely employed for identification of new hns-like genes by in vivo complementation experiments (22) or for structure-function relationship studies of H-NS proteins (2). To determine whether Lsr2 is an H-NS-like protein, we first examined whether lsr2 could complement these various phenotypes normally associated with hns mutations.
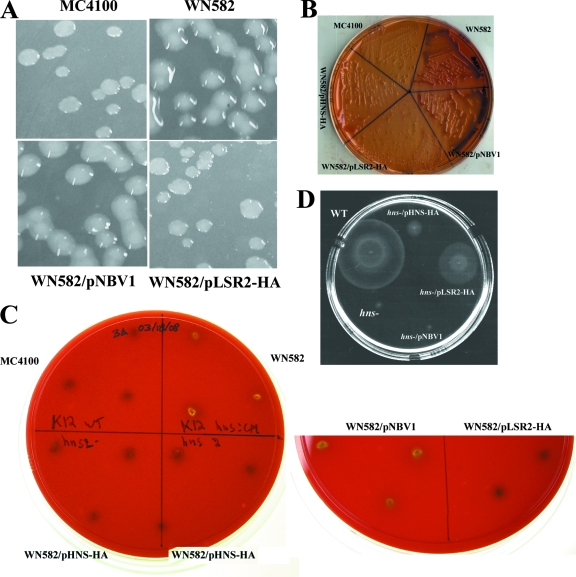
The hns gene is involved in the regulation of colanic acid biosynthesis (24, 43). When E. coli or Salmonella overproduces colanic acid, colonies growing on agar plates are mucoid. Consistent with previous findings, the E. coli hns mutant strain WN582 used in this study took on a mucoid appearance on LB agar plates (Fig. 2A). Interestingly, colonies of the recombinant WN582 strain harboring pLSR2-HA (Fig. 2A) or pHNS-HA (not shown) became nonmucoid and resembled the WT, parental strain MC4100. As a control, WN582 transformed with the cloning vector pNBV1 remained mucoid (Fig. 2A).
FIG. 2.
Lsr2 complements various phenotypes in an E. coli hns mutant. (A) Complementation of the mucoidy phenotype of the hns mutant by lsr2. Bacterial strains were plated on LB agar. MC4100, WT strain; WN582, hns mutant; WN582/pNBV1, WN582 transformed with pNBV1; WN582/pLSR2-HA, WN582 transformed with pLSR2-HA. (B) Assay for fermentation of salicin. Bacterial strains were plated on MacConkey agar plates supplemented with 0.4% salicin. (C) Assay for hemolytic activity. Hemolytic activity was assessed on blood agar plates (see the text). (D) Assay for motility. Motility was assayed on tryptone swarm plates containing 0.3% Bacto agar. WT, WT strain N99; hns−, the hns mutant of N99; hns−/pHNS-HA, the hns mutant of N99 transformed with pHNS-HA; hns−/pLSR2-HA, the hns mutant of N99 transformed with pLSR2-HA; hns−/pNBV1, the hns mutant of N99 transformed with pNBV1.
We next examined whether lsr2 could replace hns in the regulation of β-glucoside metabolism. In WT E. coli cells, the bgl operon encoding the gene products necessary for the uptake and fermentation of aryl-β,d-glucosides is cryptic, primarily due to its repression by H-NS (16). As such, the ability to metabolize aryl-β,d-glucosides such as salicin has been employed as a positive selection assay for hns mutants (30). To assay for fermentation of aryl-β,d-glucosides, we employed MacConkey agar plates supplemented with 0.4% salicin as an indicator medium. As expected, the WT strain MC4100, unable to ferment salicin, grew as white colonies, and the hns mutant strain WN582 grew as red colonies, since the fermentation of salicin produces acidic by-products, causing a drop in pH which gives rise to red/pink colonies (Fig. 2B). Interestingly, the WN582 strain transformed with pLSR2-HA gave rise to white colonies, whereas the negative control WN582 transformed with the cloning vector pNVB1 remained red (Fig. 2B). As expected, transformation of WN582 with pHNS-HA yielded white colonies.
The third hns-related phenotype we examined was hemolytic activity. H-NS represses the expression of hlyE (50, 52), which encodes a pore-forming toxin in E. coli. Consequently, WT cells of E. coli are nonhemolytic, whereas an hns mutant strain has a hemolytic phenotype (21). Hemolytic activity was assessed on blood agar plates. As expected, the WT strain MC4100 exhibited no hemolytic activity, whereas the hns mutant strain WN582 had a translucent halo of clearing (Fig. 2C). Transformation of WN582 with pLSR2-HA or pHNS-HA restored the WT, i.e., nonhemolytic, phenotype, whereas WN582 harboring the cloning vector had obvious hemolytic activity (Fig. 2C).
Lastly, we examined whether Lsr2 could complement the motility phenotype associated with hns mutations. Mutations in hns result in the loss of motility in Salmonella and E. coli due to the lack of flagella biogenesis (4, 26). This is explained by the finding that H-NS is a repressor of hdfR, which negatively regulates the flagellar master-regulator operon, flhDC (31). The motility phenotype was assessed on tryptone swarm plates. Unexpectedly, the E. coli WT strain MC4100 in our collection was nonmotile, which is likely caused by laboratory-acquired mutations affecting this phenotype. To overcome this problem, we used a different E. coli K-12 WT strain, N99, for this experiment. This strain was indeed motile (Fig. 2D), and an hns mutant strain was created by P1rev6 phage transduction (48). As expected, the hns mutant of N99 was nonmotile (Fig. 2D), and transformation of this strain with pLSR2-HA or pHNS-HA restored the motility (Fig. 2D), although not to the WT level. This partial complementation of motility phenotype by hns on a plasmid had been observed previously by different groups and is thought to be caused by the dosage effect of the hns gene (3, 4, 22). Importantly, the hns mutant strain transformed with the cloning vector pNVB1 was nonmotile (Fig. 2D), confirming that the lsr2 gene by itself was responsible for the reversion of the swarming behavior of the hns mutant. Collectively, our results demonstrate that Lsr2 is capable of complementing the various phenotypes of E. coli related to hns mutations, which provides in vivo evidence that Lsr2 is an H-NS-like protein.
The hns gene complements the phenotype of an M. smegmatis lsr2 mutant.
To further test the functional equivalence of Lsr2 and H-NS, we examined whether hns could complement the phenotype of an lsr2 mutant of M. smegmatis. We previously showed that the lsr2 mutant of M. smegmatis exhibited a dramatic change of colony morphology: the mutant colonies are smooth, wet, and round, in contrast to the dry, rough, and rugose morphology of the WT mc2155 strain (7). Although the underlying molecular mechanism remains incompletely understood, transformation of the WT lsr2 gene from M. smegmatis or M. tuberculosis fully restored the morphological phenotype (7, 32). Taking advantage of this simple phenotype, we transformed the lsr2 mutant strain of M. smegmatis (7) with the pHNS-HA plasmid by electroporation and examined the morphology of the resulting recombinant strain. Indeed, the lsr2 mutant strain containing the hns plasmid reverted to the WT colony morphology (Fig. 3C), whereas the lsr2 mutant harboring the cloning vector pNVB1 still exhibited the smooth and round colony morphology (Fig. 3B). Transformation of the lsr2 mutant strain with pLSR2-HA restored the colony morphology (not shown). These data, together with the above results, clearly demonstrate that Lsr2 and H-NS are functionally interchangeable at least for the phenotypes we have examined thus far, suggesting that they are truly functional homologues.
FIG. 3.
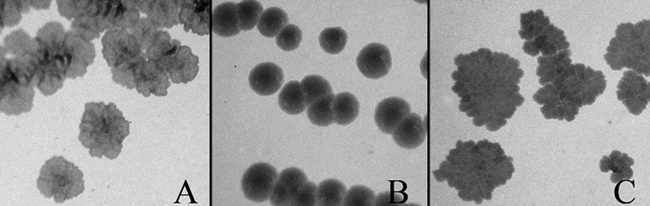
Complementation of the colony morphology of the lsr2 mutant of M. smegmatis by hns. Bacterial strains were plated on 7H11 agar and incubated at 37°C for 3 days. (A) WT M. smegmatis strain mc2155. (B) The lsr2 mutant of mc2155 transformed with pNBV1. (C) The lsr2 mutant of mc2155 transformed with pHNS-HA.
Lsr2 binds specifically to H-NS-regulated genes.
To determine whether repression by Lsr2 was specific to H-NS-regulated genes, we assayed the binding of epitope-tagged Lsr2 to E. coli genes by performing ChIP followed by quantitative RT-PCR. Two well-characterized H-NS-regulated genes, proV (40) and bglG (16), and two H-NS-recognized genes (yjcF and xapR) identified by ChIP-on-chip experiments (23) were chosen as the positive H-NS binding sites. For negative H-NS binding sites, narZ and phnE were selected, both of which were not recognized by H-NS in the ChIP-on-chip experiments (23) and have a relatively low AT content: 45% and 37%, respectively. Cells of the WN582 strain harboring pLSR2-HA were fixed with formaldehyde and subsequently sonicated to lyse the cells and shear the chromosomal DNA to fragments of approximately 500 bp. An anti-HA antibody was then used to precipitate DNA fragments associated with Lsr2 protein. For a negative control, the WN582 strain harboring pLSR2-FLAG, in which Lsr2 was expressed as a FLAG-tagged protein, was used and the cell lysates made in the same way were precipitated with the anti-HA antibody. Quantitative RT-PCR analyses with gene-specific primers were then performed to determine the enrichment of target sequences.
As expected, HA-tagged Lsr2 was found to coprecipitate with all four known H-NS binding sites (proV, bglG, yjcF, and xapR) with significantly higher levels of enrichment observed than for the two negative binding sites (narZ and phnE) (Fig. 4). These data indicate that Lsr2 binds specifically to the H-NS-regulated loci in vivo.
FIG. 4.

Specific binding of Lsr2 to H-NS-regulated genes. The figure shows results from ChIP and quantitative RT-PCR analyses. Cultures of WN582 (hns mutant) harboring pLSR2-HA were subjected to ChIP experiments, and selective genes were analyzed by RT-PCR. Genes regulated by H-NS (bglG, proV, xapR, and yjcF) are highly enriched compared to genes not regulated by H-NS (narZ and phnE).
Repression of hlyE by Lsr2 is partially relieved by overexpression of slyA.
To further characterize the functional synergy of Lsr2 and H-NS, we investigated whether mechanisms of derepression of Lsr2 are the same as for H-NS. As mentioned above, H-NS represses the expression of hlyE (50, 52). Overexpression of slyA results in the derepression of hlyE (34, 52) due to the fact that SlyA binds to a site at the hlyE promoter that overlaps with an H-NS binding site (52). We examined whether overexpression of slyA could also eliminate the repression of hlyE by Lsr2. For this purpose, the slyA gene was cloned into the arabinose-inducible pLC2002 plasmid and transformed into the WT and the hns mutant WN582 harboring pLSR2-HA, and the hlyE transcript levels in these strains were measured by performing reverse transcriptase quantitative RT-PCR.
As expected, the hns mutant strain WN582 exhibited a much higher level of hlyE expression than the WT strain (Fig. 5, lane 2). Consistent with our phenotypic complementation studies described above, transformation of WN582 with pLSR2-HA restored the hlyE transcript to the WT level (Fig. 5, lane 3). Interestingly, overexpression of slyA in WN582 harboring pLSR2-HA resulted in a significant increase of the hlyE transcript (Fig. 5, compare lanes 3 and 4). The same strains grown in the absence of arabinose served as the negative control, and no enhancement of hlyE expression was observed (Fig. 5, compare lanes 6 and 7). For the positive control, overexpression of slyA in the WT strain resulted in a 3.5-fold increase of the hlyE expression level (Fig. 5, lane 1). Taken together, these data indicate that overexpression of slyA partially antagonizes the repression of hlyE by Lsr2, presumably by the same mechanism as it antagonizes H-NS.
FIG. 5.

Overexpression of slyA causes partial derepression of hlyE. The figure shows n-fold hlyE transcript levels determined from reverse transcriptase quantitative PCR analysis. The data were normalized to the hlyE transcript level of WT strain MC4100 to obtain n-fold expression of hlyE in various strains. The transcript level of gyrB was used as an internal control for normalization among different samples. Lane 1, WT/pSLYA; lane 2, WN582; lane 3, WN582/pLSR2-HA; lane 4, WN582/pLSR2-HA+pSLYA; lane 5, WT/pSLYA; lane 6, WN582/pLSR2-HA; lane 7, WN582/pLSR2-HA+pSLYA. +Ara, in the presence of arabinose; −Ara, in the absence of arabinose.
DISCUSSION
In this study, we obtained direct evidence through in vivo complementation experiments that Lsr2 is a functional homologue of H-NS. The lsr2 gene is able to complement all the phenotypes related to hns mutations that we have examined, including β-glucoside utilization, mucoidy, motility, and hemolytic activity. Since the various phenotypes resulted from the effects of H-NS on the expression of several unrelated genes, these findings suggest that Lsr2 functions as a global regulator in E. coli, just like H-NS. We also showed that Lsr2 binds specifically to H-NS-regulated genes and the repression of hlyE by Lsr2 can be eliminated partially by overexpression of slyA, suggesting that the molecular mechanisms of Lsr2 repression and depression are similar to those of H-NS. The functional equivalence of these two proteins is further supported by the ability of hns to complement the lsr2 phenotype in M. smegmatis. Taken together, our results demonstrate unequivocally that Lsr2 is an H-NS-like protein, a conclusion that is borne out by our previous in vitro biochemical studies of Lsr2 (8). We previously demonstrated that Lsr2 binds to DNA in a relatively sequence-independent manner but exhibits a preference for AT-rich sequences. Analysis by atomic force microscopy revealed that Lsr2 has the ability to bridge distant DNA segments. Furthermore, we showed that Lsr2 exists as a homodimer in vivo and that DNA binding and protein oligomerization both are essential for the normal function of Lsr2 in vivo (8). All of these characteristics corresponding to Lsr2 are shared by H-NS, which prompted us to examine in the current study whether lsr2 and hns are functionally equivalent by performing in vivo complementation experiments. Our results have confirmed previous findings and demonstrated that Lsr2 is indeed an H-NS-like protein. It remains to be determined whether Lsr2 could play an equivalent role for other H-NS-related functions in gram-negative bacteria, e.g., maintaining the loop structure of the bacterial chromosome, contributing to nucleoid compaction, and silencing expression of foreign DNA (36, 37). However, given the similar biochemical properties and in vivo functional equivalences of these two proteins that we have uncovered so far, it is likely that such functional conservation between Lsr2 and H-NS could also occur. Our results therefore have strong implications for the role of Lsr2 in mycobacterial physiology and genome maintenance. Like H-NS, Lsr2 is likely to be a global regulator influencing multiple genes involved in stress responses and virulence. Supporting this, previous studies showed that expression of lsr2 is induced by environmental stress conditions, including nutrient availability, growth temperature, and antibiotic exposures (9, 44, 51). Lsr2 has been experimentally shown to be involved in several unrelated cellular processes, including cell wall lipid biosynthesis (7, 32) and antibiotic resistance (9). A number of genes putatively regulated by Lsr2 have also been identified in M. smegmatis by microarray analysis (9).
It is remarkable that Lsr2 is functionally interchangeable with H-NS, as demonstrated by in vivo complementation experiments, considering the lack of a significant level of sequence homology and the phylogenetic distribution of these two proteins. H-NS-like proteins have been identified only in gram-negative bacteria thus far, mostly in gammaproteobacteria, and have not been described in bacteria outside of this group. On the other hand, a mouse protein complements the phenotypes of an E. coli hns mutant (49), suggesting that H-NS analogs are widespread and could be too divergent to be identified on the basis of sequence similarity (46). Thus far, Lsr2 proteins have only been found in actinomycetes, which are phylogenetically distant from gram-negative bacteria. Among mycobacteria, Lsr2 exhibits a high level of sequence conservation (Fig. 6). Less-conserved homologues are present in related actinomycetes (Fig. 6). The exclusive distribution of Lsr2 in actinomycetes mirrors the distribution of H-NS in gram-negative bacteria, which may represent an example of convergent evolution. Supporting this notion, considerable sequence divergences among H-NS-like proteins have been noted, e.g., BpH3 of B. pertussis (22) and HvrA of R. capsulatus (2), which share low levels of (20 to 30%) sequence identity with H-NS of E. coli. Nevertheless, all H-NS-like proteins are predicted to be organized into two functional domains separated by a flexible linker, the N-terminal oligomerization domain and the C-terminal DNA binding domain (2). The nuclear magnetic resonance (NMR) structures of the N-terminal domain of E. coli H-NS reveals that it contains three α-helical segments; the third and longest α-helix forms the core of a coiled-coil configuration, whereas the two remaining stabilize the structure (5, 19). Although the N-terminal portion is not conserved in amino acid sequences, all the H-NS-like proteins are predicted to adopt a similar coiled-coil conformation. In contrast, more-conserved sequences are found in the C-terminal domain, and the NMR structure of the C-terminal domain of E. coli H-NS shows that it is composed of an antiparallel β-sheet, an α-helix, and a 310-helix structure forming a hydrophobic core (42). The two-module organization of the functional domains accounts for the ability of different H-NS-like proteins to restore the WT phenotype of E. coli hns mutants, as well as the experimental results using chimeric proteins between different H-NS proteins (47). Lsr2 exhibits a low level of sequence homology (<20% identity in amino acid sequence) and has a predicted secondary structure different from that of H-NS (Fig. 6). However, our previous studies suggest that the Lsr2 protein might also be organized into two functional domains: the C-terminal DNA binding domain and N-terminal oligomerization domain (8). We previously found that an Arg residue at the C terminus (residue 86 of M. smegmatis Lsr2 or 84 of M. tuberculosis Lsr2) is involved in DNA binding. Replacing this residue with Ala reduces the DNA binding affinity but does not affect the protein oligomerization (8). Two other mutations, R45A and D28A, likely affecting protein oligomerization were identified in the N-terminal domain (8). Furthermore, our preliminary data show that a C-terminally truncated Lsr2 protein (retaining the N-terminal residues 1 to 50) exhibits a dominant-negative effect in a WT strain of M. smegmatis, suggesting that the N-terminal part of Lsr2 is involved in protein oligomerization (L. Wang and J. Liu, unpublished data). It appears that although Lsr2 and H-NS do not share a similar sequence or even structural fold, they each contain two functional domains that are equivalent between these two proteins, i.e., for DNA binding and protein oligomerization. Since both functions are necessary for the normal functions of Lsr2 (8) and H-NS (17) in vivo, it is possible that they could each achieve the same goal by preferential binding of AT-rich sequences on the genome and bridging of DNA segments via protein oligomerization. Future studies of the structure of Lsr2 to test this hypothesis are warranted.
FIG. 6.
Sequence alignment and secondary structure prediction of Lsr2 proteins in various actinomycetes. The alignment was produced using the CLUSTALW program. The secondary structure was analyzed by using the Jpred program (http://www.compbio.dundee.ac.uk/∼www-jpred/). The arrows represent β-strands and ovals represent α-helices. Species abbreviations: M. tb., Mycobacterium tuberculosis; M. marinum, Mycobacterium marinum; M. ulcerans, Mycobacterium ulcerans; M. avium, Mycobacterium avium; M. paratb, Mycobacterium avium subsp. paratuberculosis; M. leprae, Mycobacterium leprae; M. flavescens, Mycobacterium flavescens; M. gilvum, Mycobacterium gilvum; M. vanbaalenii, Mycobacterium vanbaalenii; M. smegmatis, Mycobacterium smegmatis; M. abscessus, Mycobacterium abscessus; N. farcinica, Nocardia farcinica; R. RHA1, Rhodococcus sp. strain RHA1; S. erythraea, Saccharopolyspora erythraea; S. avermitilis, Streptomyces avermitilis; S. coelicolor, Streptomyces coelicolor; S. griseus, Streptomyces griseus subsp. griseus; J. HTCC, Janibacter sp. strain HTCC2649; A. aurescens, Arthrobacter aurescens; R. salmoninarum, Renibacterium salmoninarum; F. alni, Frankia alni; M. luteus, Micrococcus luteus; Cjw1, Mycobacterium phage Cjw1; C. sepedonicus, Clavibacter michiganensis subsp. sepedonicus; K. rhizophila, Kocuria rhizophila; T. fusca, Thermobifida fusca; Omega, Mycobacterium phage Omega. The H-NS of E. coli is included in the alignment (bottom), but only the predicted secondary structure of Lsr2 is shown.
Acknowledgments
This work was supported by funding from the Canadian Institutes of Health Research (CIHR) (MOP-15107 to J.L. and MOP-86683 to W.W.N.).
Footnotes
Published ahead of print on 5 September 2008.
REFERENCES
- 1.Beloin, C., and C. J. Dorman. 2003. An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol. Microbiol. 47825-838. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, P., N. Benhabiles, E. Krin, C. Laurent-Winter, C. Tendeng, E. Turlin, A. Thomas, A. Danchin, and R. Brasseur. 1999. The structural and functional organization of H-NS-like proteins is evolutionarily conserved in gram-negative bacteria. Mol. Microbiol. 31319-329. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, P., F. Hommais, E. Krin, O. Soutourina, C. Tendeng, S. Derzelle, and A. Danchin. 2001. H-NS and H-NS-like proteins in gram-negative bacteria and their multiple role in the regulation of bacterial metabolism. Biochimie 83235-241. [DOI] [PubMed] [Google Scholar]
- 4.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 1765537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch, V., Y. Yang, E. Margeat, A. Chavanieu, M. T. Auge, B. Robert, S. Arold, S. Rimsky, and M. Kochoyan. 2003. The H-NS dimerization domain defines a new fold contributing to DNA recognition. Nat. Struct. Biol. 10212-218. [DOI] [PubMed] [Google Scholar]
- 6.Bouffartigues, E., M. Buckle, C. Badaut, A. Travers, and S. Rimsky. 2007. H-NS cooperative binding to high-affinity sites in a regulatory element results in transcriptional silencing. Nat. Struct. Mol. Biol. 14441-448. [DOI] [PubMed] [Google Scholar]
- 7.Chen, J. M., G. J. German, D. C. Alexander, H. Ren, T. Tan, and J. Liu. 2006. Roles of Lsr2 in colony morphology and biofilm formation of Mycobacterium smegmatis. J. Bacteriol. 188633-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J. M., H. Ren, J. E. Shaw, Y. J. Wang, M. Li, A. S. Leung, V. Tran, N. M. Berbenetz, D. Kocincova, C. M. Yip, J. M. Reyrat, and J. Liu. 2008. Lsr2 of Mycobacterium tuberculosis is a DNA-bridging protein. Nucleic Acids Res. 362123-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colangeli, R., D. Helb, C. Vilcheze, M. H. Hazbon, C. G. Lee, H. Safi, B. Sayers, I. Sardone, M. B. Jones, R. D. Fleischmann, S. N. Peterson, W. R. Jacobs, and D. Alland. 2007. Transcriptional regulation of multi-drug tolerance and antibiotic-induced responses by the histone-like protein Lsr2 in M. tuberculosis. PLoS Pathog. 3e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dame, R. T. 2005. The role of nucleoid-associated proteins in the organization and compaction of bacterial chromatin. Mol. Microbiol. 56858-870. [DOI] [PubMed] [Google Scholar]
- 11.Dame, R. T., M. S. Luijsterburg, E. Krin, P. N. Bertin, R. Wagner, and G. J. Wuite. 2005. DNA bridging: a property shared among H-NS-like proteins. J. Bacteriol. 1871845-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dame, R. T., M. C. Noom, and G. J. Wuite. 2006. Bacterial chromatin organization by H-NS protein unravelled using dual DNA manipulation. Nature 444387-390. [DOI] [PubMed] [Google Scholar]
- 13.Dame, R. T., C. Wyman, and N. Goosen. 2000. H-NS mediated compaction of DNA visualised by atomic force microscopy. Nucleic Acids Res. 283504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defez, R., and M. De Felice. 1981. Cryptic operon for beta-glucoside metabolism in Escherichia coli K12: genetic evidence for a regulatory protein. Genetics 9711-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deighan, P., C. Beloin, and C. J. Dorman. 2003. Three-way interactions among the Sfh, StpA and H-NS nucleoid-structuring proteins of Shigella flexneri 2a strain 2457T. Mol. Microbiol. 481401-1416. [DOI] [PubMed] [Google Scholar]
- 16.Dole, S., V. Nagarajavel, and K. Schnetz. 2004. The histone-like nucleoid structuring protein H-NS represses the Escherichia coli bgl operon downstream of the promoter. Mol. Microbiol. 52589-600. [DOI] [PubMed] [Google Scholar]
- 17.Dorman, C. J. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2391-400. [DOI] [PubMed] [Google Scholar]
- 18.Ellison, D. W., and V. L. Miller. 2006. H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J. Bacteriol. 1885101-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esposito, D., A. Petrovic, R. Harris, S. Ono, J. F. Eccleston, A. Mbabaali, I. Haq, C. F. Higgins, J. C. Hinton, P. C. Driscoll, and J. E. Ladbury. 2002. H-NS oligomerization domain structure reveals the mechanism for high order self-association of the intact protein. J. Mol. Biol. 324841-850. [DOI] [PubMed] [Google Scholar]
- 20.Falconi, M., B. Colonna, G. Prosseda, G. Micheli, and C. O. Gualerzi. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 177033-7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Gomez, J. M., J. Blazquez, F. Baquero, and J. L. Martinez. 1996. Hns mutant unveils the presence of a latent haemolytic activity in Escherichia coli K-12. Mol. Microbiol. 19909-910. [DOI] [PubMed] [Google Scholar]
- 22.Goyard, S., and P. Bertin. 1997. Characterization of BpH3, an H-NS-like protein in Bordetella pertussis. Mol. Microbiol. 24815-823. [DOI] [PubMed] [Google Scholar]
- 23.Grainger, D. C., D. Hurd, M. D. Goldberg, and S. J. Busby. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 344642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison, J. A., D. Pickard, C. F. Higgins, A. Khan, S. N. Chatfield, T. Ali, C. J. Dorman, C. E. Hormaeche, and G. Dougan. 1994. Role of hns in the virulence phenotype of pathogenic salmonellae. Mol. Microbiol. 13133-140. [DOI] [PubMed] [Google Scholar]
- 25.Heroven, A. K., G. Nagel, H. J. Tran, S. Parr, and P. Dersch. 2004. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol. Microbiol. 53871-888. [DOI] [PubMed] [Google Scholar]
- 26.Hinton, J. C., D. S. Santos, A. Seirafi, C. S. Hulton, G. D. Pavitt, and C. F. Higgins. 1992. Expression and mutational analysis of the nucleoid-associated protein H-NS of Salmonella typhimurium. Mol. Microbiol. 62327-2337. [DOI] [PubMed] [Google Scholar]
- 27.Hommais, F., E. Krin, C. Laurent-Winter, O. Soutourina, A. Malpertuy, J. P. Le Caer, A. Danchin, and P. Bertin. 2001. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol. Microbiol. 4020-36. [DOI] [PubMed] [Google Scholar]
- 28.Howard, N. S., J. E. Gomez, C. Ko, and W. R. Bishai. 1995. Color selection with a hygromycin-resistance-based Escherichia coli-mycobacterial shuttle vector. Gene 166181-182. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, R. C., M. J. Johnson, J. W. Schmidt, and J. F. Gardner. 2005. Major nucleoid proteins in the structure and function of the Escherichia coli chromosome, p. 65-132. In N. P. Higgins (ed.), The bacterial chromosome. ASM Press, Washington, DC.
- 30.Kharat, A. S., and S. Mahadevan. 2000. Analysis of the beta-glucoside utilization (bgl) genes of Shigella sonnei: evolutionary implications for their maintenance in a cryptic state. Microbiology 1462039-2049. [DOI] [PubMed] [Google Scholar]
- 31.Ko, M., and C. Park. 2000. H-NS-dependent regulation of flagellar synthesis is mediated by a LysR family protein. J. Bacteriol. 1824670-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocincova, D., A. K. Singh, J. L. Beretti, H. Ren, D. Euphrasie, J. Liu, M. Daffe, G. Etienne, and J. M. Reyrat. 2008. Spontaneous transposition of IS1096 or ISMsm3 leads to glycopeptidolipid overproduction and affects surface properties in Mycobacterium smegmatis. Tuberculosis (Edinburgh) 88390-398. [DOI] [PubMed] [Google Scholar]
- 33.Lucchini, S., G. Rowley, M. D. Goldberg, D. Hurd, M. Harrison, and J. C. Hinton. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig, A., C. Tengel, S. Bauer, A. Bubert, R. Benz, H. J. Mollenkopf, and W. Goebel. 1995. SlyA, a regulatory protein from Salmonella typhimurium, induces a haemolytic and pore-forming protein in Escherichia coli. Mol. Gen. Genet. 249474-486. [DOI] [PubMed] [Google Scholar]
- 35.Navarre, W. W., M. McClelland, S. J. Libby, and F. C. Fang. 2007. Silencing of xenogeneic DNA by H-NS-facilitation of lateral gene transfer in bacteria by a defense system that recognizes foreign DNA. Genes Dev. 211456-1471. [DOI] [PubMed] [Google Scholar]
- 36.Navarre, W. W., S. Porwollik, Y. Wang, M. McClelland, H. Rosen, S. J. Libby, and F. C. Fang. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313236-238. [DOI] [PubMed] [Google Scholar]
- 37.Noom, M. C., W. W. Navarre, T. Oshima, G. J. Wuite, and R. T. Dame. 2007. H-NS promotes looped domain formation in the bacterial chromosome. Curr. Biol. 17R913-R914. [DOI] [PubMed] [Google Scholar]
- 38.Nye, M. B., J. D. Pfau, K. Skorupski, and R. K. Taylor. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 1824295-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima, T., S. Ishikawa, K. Kurokawa, H. Aiba, and N. Ogasawara. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13141-153. [DOI] [PubMed] [Google Scholar]
- 40.Owen-Hughes, T. A., G. D. Pavitt, D. S. Santos, J. M. Sidebotham, C. S. Hulton, J. C. Hinton, and C. F. Higgins. 1992. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell 71255-265. [DOI] [PubMed] [Google Scholar]
- 41.Porter, M. E., and C. J. Dorman. 1994. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J. Bacteriol. 1764187-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shindo, H., T. Iwaki, R. Ieda, H. Kurumizaka, C. Ueguchi, T. Mizuno, S. Morikawa, H. Nakamura, and H. Kuboniwa. 1995. Solution structure of the DNA binding domain of a nucleoid-associated protein, H-NS, from Escherichia coli. FEBS Lett. 360125-131. [DOI] [PubMed] [Google Scholar]
- 43.Soutourina, O. A., E. Krin, C. Laurent-Winter, F. Hommais, A. Danchin, and P. N. Bertin. 2002. Regulation of bacterial motility in response to low pH in Escherichia coli: the role of H-NS protein. Microbiology 1481543-1551. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, G. R., L. Wernisch, R. Stabler, J. A. Mangan, J. Hinds, K. G. Laing, D. B. Young, and P. D. Butcher. 2002. Dissection of the heat-shock response in Mycobacterium tuberculosis using mutants and microarrays. Microbiology 1483129-3138. [DOI] [PubMed] [Google Scholar]
- 45.Tendeng, C., C. Badaut, E. Krin, P. Gounon, S. Ngo, A. Danchin, S. Rimsky, and P. Bertin. 2000. Isolation and characterization of vicH, encoding a new pleiotropic regulator in Vibrio cholerae. J. Bacteriol. 1822026-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tendeng, C., and P. N. Bertin. 2003. H-NS in gram-negative bacteria: a family of multifaceted proteins. Trends Microbiol. 11511-518. [DOI] [PubMed] [Google Scholar]
- 47.Tendeng, C., E. Krin, O. A. Soutourina, A. Marin, A. Danchin, and P. N. Bertin. 2003. A novel H-NS-like protein from an Antarctic psychrophilic bacterium reveals a crucial role for the N-terminal domain in thermal stability. J. Biol. Chem. 27818754-18760. [DOI] [PubMed] [Google Scholar]
- 48.Thomason, L. C., N. Costantino, and D. L. Court. 2007. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 791.17.1-1.17.8. [DOI] [PubMed] [Google Scholar]
- 49.Timchenko, T., A. Bailone, and R. Devoret. 1996. Btcd, a mouse protein that binds to curved DNA, can substitute in Escherichia coli for H-NS, a bacterial nucleoid protein. EMBO J. 153986-3992. [PMC free article] [PubMed] [Google Scholar]
- 50.Westermark, M., J. Oscarsson, Y. Mizunoe, J. Urbonaviciene, and B. E. Uhlin. 2000. Silencing and activation of ClyA cytotoxin expression in Escherichia coli. J. Bacteriol. 1826347-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong, D. K., B.-Y. Lee, M. A. Horwitz, and B. W. Gibson. 1999. Identification of Fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect. Immun. 67327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyborn, N. R., M. R. Stapleton, V. A. Norte, R. E. Roberts, J. Grafton, and J. Green. 2004. Regulation of Escherichia coli hemolysin E expression by H-NS and Salmonella SlyA. J. Bacteriol. 1861620-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]