Abstract
Enterococcus faecalis is a gram-positive commensal bacterium of the human intestinal tract. Its opportunistic pathogenicity has been enhanced by the acquisition of multiple antibiotic resistances, making the treatment of enterococcal infections an increasingly difficult problem. The extraordinary capacity of this organism to colonize and survive in a wide variety of ecological niches is attributable, at least in part, to signal transduction pathways mediated by two-component systems (TCS). Here, the ability of E. faecalis to utilize ethanolamine as the sole carbon source is shown to be dependent upon the RR-HK17 (EF1633-EF1632) TCS. Ethanolamine is an abundant compound in the human intestine, and thus, the ability of bacteria to utilize it as a source of carbon and nitrogen may provide an advantage for survival and colonization. Growth of E. faecalis in a synthetic medium with ethanolamine was abolished in the response regulator RR17 mutant strain. Transcription of the response regulator gene was induced by the presence of ethanolamine. Ethanolamine induced a 15-fold increase in the rate of autophosphorylation in vitro of the HK17 sensor histidine kinase, indicating that this is the ligand recognized by the sensor domain of the kinase. These results assign a role to the RR-HK17 TCS as coordinator of the enterococcal response to specific nutritional conditions existing at the site of bacterial invasion, the intestinal tract of an animal host.
Enterococci are the third most frequently detected nosocomial pathogens in the United States, with Enterococcus faecalis accounting for the majority of the clinically relevant isolates (12). E. faecalis is a commensal gram-positive nonsporulating bacterium responsible for a wide array of human diseases of various severities, depending on the location of the infection and the host's immune status, including endocarditis, meningitis, and urinary tract infections (34). Infections caused by E. faecalis have increased in the past decade due to the acquisition of multiple antibiotic resistances, which has made some enterococci refractory to all antimicrobial regimens (24). This opportunistic pathogen has an extraordinary capacity to grow under hostile conditions and to colonize and survive in a large range of ecological niches (17).
The ability of most bacteria to sense and adapt to changing conditions is frequently mediated through two-component signal transduction systems (TCS). TCS generally consist of a sensory histidine kinase (HK) and a response regulator (RR). The histidine kinase senses the signal and relays the adaptive response through the transfer of a phosphoryl group to the response regulator, which modulates gene expression by acting as a transcriptional regulator (23).
A total of 17 TCS and one orphan response regulator have been identified on the genome of E. faecalis V583 (18). Bioinformatic analyses on the genomic location of each TCS revealed that the RR-HK17 system is surrounded by genes encoding proteins related to the components of the ethanolamine (EA) utilization pathway in Salmonella enterica (27). This large metabolic pathway must have been maintained by selection, yet no condition is known under which E. faecalis utilizes EA for growth.
EA is an abundant compound in the human intestinal tract (27, 31) and in processed food (2, 10), and it can be utilized as a source of carbon, nitrogen, and energy under aerobic and/or anaerobic conditions. Korbel et al. (28) reported that three of the most hazardous food-borne pathogens, Listeria monocytogenes, Clostridium perfringens, and Salmonella enterica, carried highly similar EA utilization operons, but EA utilization genes are absent in most other prokaryotes. These authors predicted that the EA utilization pathway is an important genomic determinant of pathogenicity associated with food poisoning. The presence of EA utilization genes could promote anaerobic growth in the host and in processed food and, in parallel, may provide an advantage over natural gut bacteria not possessing this function. Consistent with this notion, Salmonella enterica serovar Typhimurium mutant strains unable to grow on EA were found to be attenuated in a mouse model of infection (53).
Several investigations have studied the minimal functions and physiological conditions required for growth of S. enterica serovar Typhimurium on EA. In general, two sequential reactions convert EA into acetyl coenzyme A (Ac-CoA). First, EA is converted to acetaldehyde and free ammonia by the adenosylcobalamin (CoB12)-dependent EA ammonia-lyase encoded by the eutBC genes of the operon (Fig. 1) (13). In the second step, acetaldehyde is oxidized to acetate and activated to Ac-CoA in a step presumably catalyzed by the acetaldehyde dehydrogenase (EutE) (53).
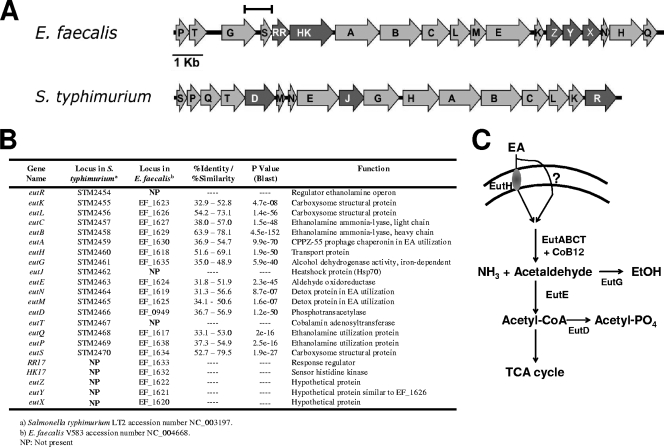
FIG. 1.
eut operon for EA utilization in E. faecalis and Salmonella enterica serovar Typhimurium (S. typhimurium). (A) A diagram of the gene organization in the two operons is shown with the darker shade of gray representing genes that are present in one organism but missing in the other. The bar represents the extent of the fragment cloned in the pTCV-RR17p transcriptional fusion plasmid. (B) Table summarizing gene names, annotation numbers in genome databases, the results of ClustalW and BLAST analyses, and function. (C) Schematic representation of the basic EA metabolism in Salmonella. EA can enter the cell by diffusion either via EutH or by other, unknown routes (38). EutBC are the two subunits of the cobalamin-dependent EA ammonia-lyase. EutE is an acetaldehyde dehydrogenase, EutG is an alcohol dehydrogenase, and EutD is a phosphotransacetylase (6, 27, 39). Notably, the genes for the tricarboxylic acid cycle (TCA) are absent in the genome of E. faecalis V583.
In two additional reactions, acetaldehyde is reduced to ethanol in a reaction catalyzed by EutG with a not-well-defined involvement of EutJ (38). The Ac-CoA is converted to acetyl phosphate in a reaction catalyzed by EutD phosphotransacetylase. Acetyl phosphate is used to conserve energy via substrate level phosphorylation catalyzed by acetate kinase, yielding ATP and acetate. Acetate is excreted into the medium and later recaptured by Ac-CoA synthetase (51). In S. enterica, the anaerobic use of EA provides ATP and an electron sink.
The 17-gene operon encoding the requirements for EA utilization in S. enterica serovar Typhimurium is induced by the simultaneous presence of EA and cobalamin/vitamin B12 (VitB12) and requires the product of the last gene of the operon, eutR. EutR is a transcription factor belonging to the AraC family of DNA-binding proteins (15, 27, 46).
In the present study, we describe a growth condition in which E. faecalis utilizes EA as a carbon source under anaerobic growth conditions in the presence of cobalamin (VitB12). We also show that, in contrast to S. enterica, EA utilization in E. faecalis is dependent upon the RR-HK17 TCS and EA is the direct effector inducing autophosphorylation of the sensor histidine kinase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. faecalis strains and plasmids used in this study are listed in Table 1. Strains were cultured in Todd-Hewitt broth. Notably, the V583T strain was used as a control instead of the parental V583 strain in every experiment that involved the use of mutant strains such as RR15, RR17, and RR15-17 (which carry the TetR plasmid p3TET inserted by single crossing-over integration at the deletion site) which were grown in the presence of tetracycline. To our knowledge, the insertion of p3TET in the V583 strain does not cause any detectable phenotype (19). Escherichia coli DH5α and TB1 were used for plasmid constructions and propagation. Strains were cultivated in Luria-Bertani broth. Antibiotics used for selection in E. coli and E. faecalis were spectinomycin (150 and 750 μg/ml, respectively), ampicillin (100 μg/ml), and tetracycline (15 μg/ml, unless otherwise specified). Enterococcus electroporation was carried out as described previously (11).
TABLE 1.
Enterococcus faecalis strains and plasmids used in this study
| Strain or plasmid | Relevant information | Reference or source(s) |
|---|---|---|
| Strains | ||
| V583 | E. faecalis | Clinical isolate |
| RR15 | RR15 insertion mutant of V583; Tetr | 19 |
| RR17 | RR17 insertion mutant of V583; Tetr | 19 |
| RR15-17 | RR15 RR17 double mutant insertion of V583; Tetr Cmr | This study |
| V583T | Insertion mutant of V583 with p3TET inserted between HK02 and the adjacent open reading frame encoding a putative lipoprotein | 19 |
| V583/pTCV-LacSpec | V583 containing pTCV-LacSpec; Spr | This study |
| V583/pTCV-RR17p | V583 containing pTCV-RR17p; Spr | This study |
| RR17/pTCV-LacSpec | RR17 containing pTCV-LacSpec; Spr Tetr | This study |
| RR17/pTCV-RR17p | RR17 containing pTCV-RR17p; Spr Tetr | This study |
| Plasmids | ||
| pTCV-Lac | Transcriptional lacZ fusion vector | 3 |
| pML28 | pAT28 with aphA3 promoter on a 369-bp EcoRI/BamHI fragment | 20 |
| pML28-RR17 | pML28 derivative containing E. faecalis RR17 coding sequence | This study |
| pTCV-LacSpec | SpcR derivative of pTCV-Lac | This study; Hancock and Perego, unpublished |
| pTCV-RR17p | pTCV-LacSpec derivative containing E. faecalis RR17 promoter; −722 to +122 | This study |
The minimal medium M9HY is base medium (20 mM HEPES [pH 7.0], 2 g/liter yeast extract [final concentration]) to which individually sterilized M9 salts (47), MgSO4, and CaCl2 were added at 1×, 2 mM, and 0.1 mM, respectively. Vitamin and amino acid mixes were also added to achieve a 1× final concentration from 100× stocks. Vitamin and amino acid stocks were prepared essentially as described previously (35). Vitamins at 1× concentration were as follows: thiamine, 100 μg/ml; biotin, 20 μg/ml; panthothenic acid, 20 μg/ml; nicotinic acid, 2 μg/ml; riboflavin, 2 μg/ml; and folic acid, 0.2 μg/ml. Amino acids at 1× concentration were as follows: arginine, 20 μg/ml; glutamate, 20 μg/ml; glycine, 20 μg/ml; histidine, 20 μg/ml; isoleucine, 20 μg/ml; leucine, 20 μg/ml; methionine, 20 μg/ml; tryptophan, 20 μg/ml; and valine, 20 μg/ml. Growth and survival experiments were done at 37°C. The M9HY medium was supplemented with glucose at 100 mM or EA at 40 or 100 mM final concentration. VitB12 and the coenzyme CoB12 (Sigma-Aldrich) were used as exogenous VitB12 sources. All solutions were prepared separately and sterilized by autoclaving for 15 min. Amino acid mix, vitamin mix, and VitB12 derivative solutions were prepared separately and sterilized by filtration.
For growth under normal oxygen conditions, the M9HY medium was inoculated with E. faecalis cells from overnight cultures in M9HY medium with 100 mM glucose. Cells were washed twice in M9HY medium without carbon sources (NCS). Cells were grown at 37°C without agitation and ample air space. This growth condition is referred to as aerobic growth. For the detection of EA utilization, the M9HY medium supplemented with all the necessary components was degassed, inoculated with cells from an overnight culture as described above, and flushed with argon. Argon was flushed in the culture tubes every time a sample was withdrawn. This growth condition is referred to as anaerobic growth throughout this work. Incubations were performed in the dark at 37°C without shaking. Overnight cultures were diluted 1:1,000 (see Fig. 3 and 5), 1:20 (see Fig. 4), or 1:15 (see Fig. 6). The average optical density (OD) at 600 nm of the overnight cultures was ∼0.2 to 0.3.
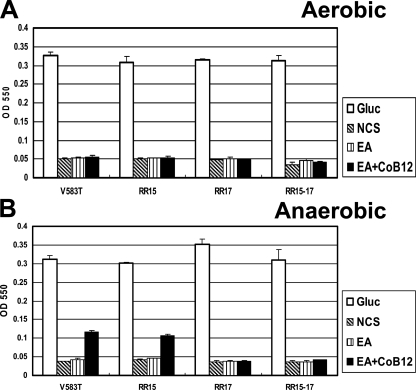
FIG. 3.
Growth of E. faecalis on EA. ODs of E. faecalis cultures after 24 h of growth under aerobic (A) or anaerobic (B) conditions in M9HY medium containing 10 μg/ml of tetracycline. Gluc, M9HY medium plus 100 mM glucose; NCS, M9HY medium without carbon source; EA, M9HY medium plus 100 mM EA; EA + CoB12, M9HY medium plus 100 mM EA and 40 nM CoB12. The error bars represent standard deviations of three independent cultures.
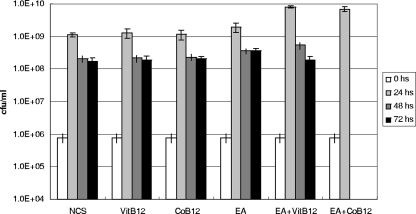
FIG. 5.
Growth and survival of E. faecalis V583 under anaerobic conditions utilizing EA as a carbon source. CFU per ml was measured after 0, 24, 48, and 72 h. NCS, M9HY medium without carbon source; EA, 100 mM EA; CoB12, 40 nM CoB12; VitB12, 40 nM VitB12. The 48-h and 72-h time points of the EA plus CoB12 culture conditions are missing because counts were below the minimal experimental limit set for the experiment. The error bars denote standard deviations calculated from three independent cultures.
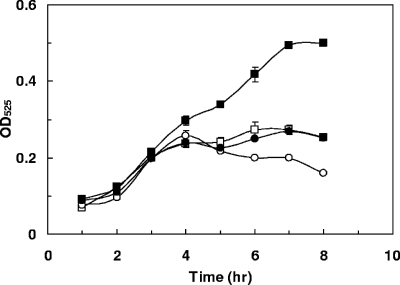
FIG. 4.
Growth curves of E. faecalis parental strain V583T and RR17 mutant strain on EA as the sole carbon source in M9HY medium containing 10 μg/ml of tetracycline. EA was provided at 100 mM and CoB12 at 40 nM. Filled and open symbols represent growth under anaerobic and aerobic conditions, respectively. Squares represent E. faecalis V583T, while circles indicate the RR17 mutant. The error bars represent standard deviations calculated from two independent cultures. OD525, OD at 525 nm.
FIG. 6.
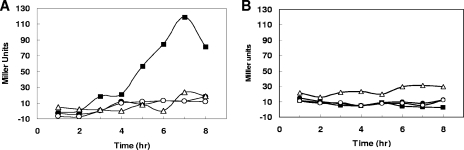
Transcription analysis of the RR17-encoding gene. Time course of β-galactosidase activity was taken from a culture of the parental strain, V583, carrying the pTCV-RR17p transcriptional lacZ fusion construct (A) or the pTCV-LacSpec vector (B). Cells were grown in M9HY medium containing 750 μg/ml spectinomycin and supplemented with the following: 100 mM EA plus 40 mM CoB12 (▪); 100 mM EA (•); NCS plus 40 nM CoB12 (○); and 100 mM glucose plus 40 nM CoB12 (▵).
Transcription analysis.
The transcriptional fusion vector pTCV-LacSpec was used to monitor transcription of the eut operon. The pTCV-LacSpec vector is a derivative of pTCVlac (40) in which the kanamycin resistance marker was replaced by the spectinomycin resistance gene originally from piC333 (52; L. E. Hancock and M. Perego, unpublished data). The region upstream the RR17-encoding gene was amplified by PCR using oligonucleotide primers PduQEco5′ and RR17Bam3′ (Table 2) and was cloned in vector pTCV-LacSpec using EcoRI and BamHI restriction sites to generate a transcriptional fusion to the promoterless lacZ reporter gene (pTCV-RR17p). The 834-bp fragment cloned in pTCV-RR17p included 722 bp upstream the RR17 translational start codon and 112 bp of coding sequence. The sequence of the cloned promoter fragment was confirmed by DNA sequencing analysis.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequencea |
|---|---|
| EFRR175′Nde | 5′-CGTGAAGCATATGGATGGACGAATTGTAATAG-3′ |
| EFRR173′Xho | 5′-AATCGCTCGAGTTATTCATCATCCATTACAATC-3′ |
| EFHK175′Nde | 5′-TGATGAACATATGAAACGATTAGAGCAATTATG-3′ |
| EFHK173′Xho | 5′-CCTTTCTCGAGTCAATGAACAACATCACTGG-3′ |
| RR17Bam25′ | 5′-GGGAGGATCCAAGCGGTGATTGAAGGTTT-3′ |
| RR17SalI3′ | 5′-TTTAGTCGACTGGTGACATAATTGCTCTAATCG-3′ |
| RR17Bam3′ | 5′- CGCGGATCCCAAAACCATCAGCTGCTTCA-3′ |
| PduQEco5′ | 5′-CGCGAATTCCAAATCAAGCAACTCCGTCA-3′ |
Restriction sites are in boldface type.
E. faecalis strains containing pTCV-LacSpec or pTCV-RR17p were grown in M9HY medium in the presence of spectinomycin. Samples were collected at hourly intervals. β-Galactosidase activity was detected by the method described by Poyart and Trieu-Cuot (40), with some modifications. In order to lyse the cells, 10 μl of lysozyme, 10 mg/ml containing 100 U/ml of mutanolysin, were added to 100 μl of sample and incubated at 37°C for 60 min. The β-galactosidase activities were calculated according to Miller (33). Samples were taken and processed in duplicate, and the mean values were used for the calculations.
The RR17-complementing plasmid was constructed in the replicative vector pML28 (20), which is a derivative of pAT28 (55) carrying the aphA3 promoter. The RR17 coding sequence and approximately 100 bp of the upstream region were amplified by PCR using oligonucleotide primers RR17Bam25′ and RR17SalI3′ (Table 2). After digestion by BamHI and SalI, the fragment was cloned in similarly digested pML28.
Expression and purification of RR17-H6 and HK17-H6.
The RR17 and HK17 coding regions were amplified from E. faecalis V583 genomic DNA by using primers EFRR175′NdeI/EFRR173′Xho and EFHK175′Nde/EFHK173′XhoI, respectively (Table 2), and cloned in pET28a(+)-cut NcoI/XhoI, generating a fusion to six-His codons at the 3′ end. The resulting plasmids were transformed in E. coli expression strain BL21(DE3)pLys (Novagen). Protein expression was induced at an OD at 600 nm of 0.5 with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and cells were allowed to grow for 4 h at 28°C. Cells were then harvested, washed in buffer B (50 mM Tris-HCl [pH 7.0 for RR17 and pH 8.0 for HK17], 100 mM NaCl, 1 mM phenylmethylsulfonyl fluoride), resuspended in buffer B with 10 mg/ml lysozyme, incubated 30 min at 4°C, and lysed using sonication. The proteins were then purified by immobilized metal affinity chromatography using the Ni-nitrilotriacetic acid resin (Qiagen, Valencia, CA). The proteins were eluted from the column by a gradient of 30 to 300 mM imidazole in buffer B. Fractions were pooled and dialyzed against 50 mM Tris-HCl (pH 7.0 or pH 8.0), 100 mM NaCl, and 3 mM dithiothreitol. Finally, the proteins were concentrated and stored with 20% glycerol at −20°C.
Protein phosphorylation assay.
The phosphorylation assays were carried out at room temperature in the presence of [γ-32P]ATP (specific activity 6,000 Ci/mmol) and cold ATP (900 μM final concentration) in buffer A (20 mM HEPES [pH 7.5], 50 mM KCl, 2 mM MgCl2, 5 mM CaCl2, 10 mM dithiothreitol, 10 μM bovine serum albumin). The reactions were initiated by the addition of [γ-32P]ATP, terminated by the addition of a 5× sodium dodecyl sulfate sample buffer, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 15% polyacrylamide gels. Proteins and EA concentrations were used as indicated in the figure legends. EA at the concentration used in the assays did not alter the pH of the reaction buffer (data not shown). Gels were dried, and labeled proteins were detected by a PhosphorImager screen (Amersham Molecular Dynamics).
RESULTS
The E. faecalis gene cluster for EA utilization (eut) contains a TCS.
A bioinformatic analysis of the genome context for each of the 17 TCS identified in E. faecalis V583 (19) revealed that the RR-HK17 system (EF1633-EF1632 [GenBank NC_004668]) was embedded within genes encoding proteins with strong similarities to members of the EA utilization pathway of Salmonella enterica serovar Typhimurium (eut) (27) (Fig. 1). The 17 putative eut genes of E. faecalis were found upstream (eutP, -T, -G, and -S) or downstream (eutA, -B, -C, -L, -M, -E, -K, -Z, -Y, -X, -N, -H, and -Q) of TCS-17. As shown in Fig. 1, the gene order is not fully conserved between Enterococcus and Salmonella. Also some genes present in Salmonella are missing in E. faecalis, and vice versa. Most notably, the eutR gene encoding the autoregulator of the eut operon in Salmonella is missing in E. faecalis, which, instead, has the RR-HK17 TCS. Also different from Salmonella, the eutD gene (EF0949) encoding a phosphotransacetylase is not within this cluster of genes but is located in a different chromosomal region. The only gene whose product is not significantly conserved with the product of an eut gene of Salmonella is the EF1637 gene. Nevertheless, the EF1637 protein is annotated as a putative cobalamin adenosyltransferase, making it likely to carry out the same function of EutT (STM2467), which is similarly annotated and shares 11% of identical residues.
The genome sequences of Clostridium perfringens, Listeria monocytogenes, Listeria innocua, and Streptococcus sanguinis also revealed the presence of a highly conserved TCS upstream of the eutA gene. The RR17 and HK17 proteins shared approximately 69% and 47% of identical residues, respectively, with their homologues in all the sequenced Listeria species (data not shown).
The 19 genes present in this E. faecalis cluster may be transcribed from more than one promoter. Consensus sequences compatible with the −35 and −10 recognition sites of σA-containing RNA polymerase are detectable 130 bp upstream of the eutP gene (TTGACA-17 bp-TAAGAT) and in the eutG-eutS intergenic region (TTGACA-17 bp-TATAAT).
Notably, a B12 riboswitch-like element was found in the eutT-eutG intergenic region by the RibEx riboswitch explorer (significance value of 3.16E−10) (1). This riboswitch expands for approximately 170 bp, starting 40 bp from the stop codon of eutT (EF1637) (from nucleotide 1591221 to 1591049, according to Barrick and Breaker [4]). Riboswitches are mRNA control elements that respond to ligand concentration by modulating gene expression via transcription termination or translation mechanisms (59). Similar observations were reported by K. A. Fox, A. Maadani, and D. A. Garsin at the International Conference on Gram-Positive Pathogens in Omaha, NE, in 2006.
Based on GenBank database and BLAST searches, it seems that the E. faecalis V583 genome carries all the genes necessary for EA metabolism.
Structural features of the RR-HK17 TCS.
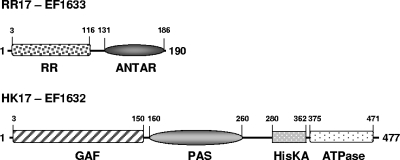
Two-component signal transduction proteins often contain structural features, in addition to the conserved response regulatory, histidine kinase (HisKA), and ATP-binding (ATPase) domains, that may provide an indication of specialized function. Searches for the structural features of the RR17 response regulator and the HK17 histidine kinase were conducted in the PSI-BLAST, Pfam, and FFAS03 databases (14, 26). The results are summarized in Fig. 2. RR17 is a member of the family of regulatory proteins with an ANTAR carboxy-terminal domain (49). The ANTAR domain interacts with RNA instead of DNA, and the first ANTAR response regulator, AmiR of Pseudomonas aeruginosa, was described as an antiterminator of transcription acting on a rho-independent terminator (58). The amino-terminal domain of RR17 is predicted to have a typical response regulator fold, with the aspartate residues at positions 9, 10, and 54 likely to constitute the phosphorylatable active site.
FIG. 2.
Schematic representation of the structural and functional domains of the RR17 response regulator (190 amino acids) and the HK17 histidine kinase (477 amino acids). The numbers delineate the approximate initial and terminal amino acid residues for each domain, as deduced from the Pfam and FFAS03 database analyses.
The HK17 histidine kinase is a cytoplasmic protein of 477 amino acids, with the amino-terminal 150 residues recognized as likely to form a GAF domain (probability of <−9.5 by the FFAS03 search program). GAF domains are ubiquitous motifs found in prokaryotic, eukaryotic, and plant proteins. Originally identified as the noncatalytic binding domain of cyclic GMP in cyclic nucleotide phosphodiesterases (9), it is now often found in sensor histidine kinases, among other proteins. GAF domains, however, may interact with alternative ligands, such as heme, in the Mycobacterium tuberculosis DevS and DosT histidine kinases (48, 50), photopigments in plant phytochromes (30), or nitric oxide (NO) through a nonheme mononuclear iron center as in the E. coli NorR transcription regulator (56). The structure of the GAF domain is very similar to that of the PAS domain, in spite of very limited sequence conservation (22).
A PAS domain is also present in HK17 between the GAF and the HisKA domains. The PAS domain is another ubiquitous structural feature of proteins from bacteria to humans. PAS domains can transduce signals by mediating protein-protein interaction upon sensing signals such as light, oxygen, redox potential, and small ligands (16, 54). These structural features of the HK17 histidine kinase strongly suggest that its activity may be regulated by a ligand such as EA whose metabolic pathway is encoded in the 19-gene cluster comprising TCS-17.
E. faecalis utilizes EA under anaerobic growth conditions.
The use of EA as a source of carbon and nitrogen could be important, since this compound is a constituent of abundant species of lipid present in the intestinal tract. At present, there are no reports of EA utilization in enterococci, and their complex growth requirement has often restricted the use of synthetic media (35).
In order to determine whether E. faecalis could utilize EA as the sole source of carbon, a synthetic medium was devised as a derivative of the M9 minimal salt medium supplemented with HEPES buffer, yeast extract, vitamins, and essential amino acids (see Materials and Methods). The strict E. faecalis requirement for essential amino acids did not really allow us to test the utilization of EA as a source of nitrogen. In the absence of carbon sources, the optical density of the initial inoculum in M9HY medium increased by approximately 2 to 3 orders of magnitude after 24 h of incubation. With the addition of glucose at 100 mM, a poor but highly reproducible growth of the control strain V583T in air at 37°C was observed (Fig. 3A). When glucose was replaced by EA, however, no growth was observed above the level reached in the absence of carbon sources. Because the first enzyme in the EA utilization pathway, the EA ammonia-lyase (the product of the eutBC genes), is dependent upon VitB12 as cofactor and the E. faecalis genome carries genes for VitB12 transport but not for its biosynthesis (43), growth in air was also tested in the presence of EA with either VitB12 or CoB12, but no increase in OD was observed above the level observed for the cultures grown without carbon sources (Fig. 3A and data not shown). Also, no growth stimulation was observed in the presence of VitB12 or CoB12 alone (see Fig. S1 in the supplemental material). The same growth conditions were tested under anaerobic conditions (see Materials and Methods), and the ability of the parental strain to grow poorly but consistently in the presence of VitB12 (data not shown) or CoB12 (Fig. 3B) was observed. This result allowed us to test whether a deletion of the RR17-encoding gene would affect the ability of E. faecalis to utilize EA. As shown in Fig. 3B, the RR17 mutant in the presence of EA and CoB12 or VitB12 did not grow to an OD greater than that of the control culture grown without any carbon sources. Notably, in the absence of EA, neither VitB12 nor CoB12 promoted any growth to a level greater than that of the control culture grown without carbon sources (data not shown; see Fig. S1 in the supplemental material).
The observation that most of the genes from EF1617 (eutQ) to EF1638 (eutP) were highly repressed by the Fsr system in a microarray study (5) prompted us to test whether a deletion of the response regulator FsrA-encoding gene (RR15, or EF1822) affected EA utilization. As shown in Fig. 3B, the RR15 single mutant strain grew as the parental strain under anaerobic conditions and did not overcome the requirement for the RR17 protein in the double mutant strain, thus ruling out a significant role for FsrA in regulating the eut genes under these growth conditions.
A time course of growth of the parental strain, V583T, and the RR17 mutant (Fig. 4) confirmed that EA with CoB12 allowed E. faecalis to grow, albeit poorly, only under anaerobic conditions, and the RR17 response regulator was required for this effect.
Notably, the supernatant of the parental V583T strain grown under anaerobic conditions in the presence of EA and CoB12 acquired a yellow-brown color after more than 24 h of incubation. The color was less intense when the cells were grown with EA and VitB12 (data not shown). The formation of this pigment was dependent upon the presence of bacteria, EA, CoB12, and anaerobiosis. This pigment was soluble in phenol (pH 8.0) and, in an absorption spectrum, showed a maximum in the UV region at an OD of ∼265 nm (see Fig. S2 in the supplemental material). The interference of the yellow-brown pigment to the cell OD at 550 nm was minor and did not affect the conclusion that EA and CoB12 promoted bacterial growth under the assay conditions tested (see Fig. S1 in the supplemental material).
In order to ensure that the increase in OD observed upon incubation in the presence of EA and VitB12 or CoB12 corresponded to an increase in CFU levels (CFU/ml), the V583T strain grown in M9HY medium for 24, 48, or 72 h was plated on Todd-Hewitt agar plates. The results of the colony count shown in Fig. 5 indicated that a small (10-fold) increase in the number of CFU/ml was consistently observed in the presence of EA and either VitB12 or CoB12 after 24 h of incubation, thus confirming the ability of E. faecalis to grow with these supplements.
Nevertheless, prolonged incubation (48 and 72 h) resulted in a reproducible, drastic loss of viability, as measured by CFU/ml, particularly in the cultures containing CoB12 (Fig. 5), suggesting that a product of EA utilization may be toxic to the cells.
A promoter upstream of RR17 is induced by EA-CoB12 in anaerobiosis.
The 17-gene operon for EA utilization in S. enterica serovar Typhimurium is transcribed from a main promoter, located upstream of eutS, and from a minor promoter, adjacent to the eutR gene that provides a low constitutive level of EutR regulator sufficient to initiate induction of the main promoter.
In order to test whether in E. faecalis, as in Salmonella, the regulator of eut expression was immediately preceded by a promoter, plasmid pTCV-RR17p was constructed by fusing a fragment ranging from position −722 to +112, relative to the RR17 start codon, to the promoterless lacZ reporter gene in pTCV-LacSpec. This construct and the vector itself were transformed by electroporation into the V583T parental strain, and the β-galactosidase activities were analyzed in M9HY medium under anaerobic growth conditions. As shown in Fig. 6A, induction of transcription was observed in the parental strain grown in the presence of EA and CoB12, but when cells were grown in the presence of only EA, or CoB12 or VitB12, the level of transcription was not significantly different from the level detected in the parental strain V583T carrying the vector pTCV-LacSpec alone (Fig. 6B). Also, no induction of transcription was observed when cells were grown in the presence of EA, CoB12, and glucose at 100 mM (data not shown), suggesting that carbon catabolite repression may regulate this promoter, as previously shown in Salmonella for the main promoter of the eut operon (46).
The inability of the RR17 mutant strain to grow under these assay conditions did not allow us to test the role of the RR17 response regulator on the transcription generated by this lacZ fusion construct.
These results, together with the observation that RR17 is required for E. faecalis growth in the presence of EA, indicate that the RR-HK17 TCS is likely expressed from an autoregulated promoter in response to EA and VitB12 but subjected to repression by a carbon catabolite repression mechanism. This promoter is likely located in the eutG-eutS intergenic region where putative −10 (TATAAT) and −35 (TTGACA) consensus sequences separated by 17 bp and compatible with recognition by RNA polymerase containing a σA-type sigma factor are located approximately 150 bp upstream of the eutS translational start site.
Autophosphorylation of HK17 is stimulated by EA.
The EutR protein of S. enterica serovar Typhimurium induces the expression of the eut operon in response to EA and VitB12, but binding of either or both effectors has not been demonstrated experimentally.
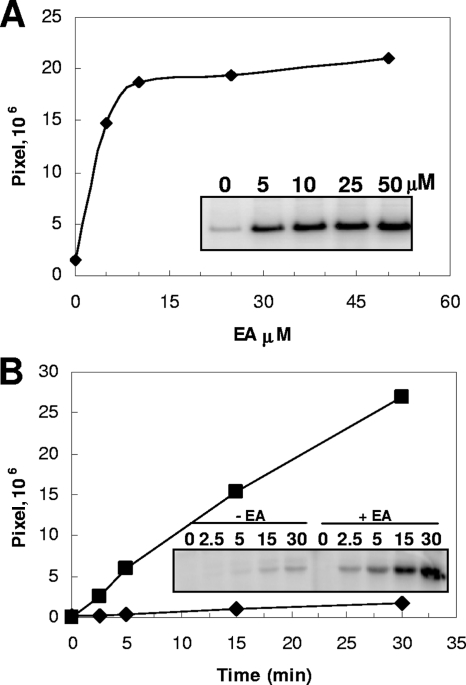
In order to test whether EA could induce the autophosphorylation of the HK17 sensor histidine kinase, an in vitro assay was carried out with the protein purified from an overexpressing E. coli strain (see Materials and Methods). As shown in Fig. 7A, the presence of an equimolar concentration of EA stimulated the enzyme autophosphorylation activity by approximately 10-fold. With a 2:1 stoichiometry of ligand versus enzyme, the autophosphorylation activity essentially reached a plateau. No stimulation of autokinase activity was observed when VitB12 or CoB12 was added to the reaction (see Fig. S3 in the supplemental material), and the presence of CoB12 did not affect the stimulation brought about by EA (data not shown). A time course analysis of HK17 autophosphorylation in the presence and absence of EA revealed an approximately 15-fold increase in the rate of the reaction in the presence of a saturating concentration of effector (Fig. 7B).
FIG. 7.
EA stimulated HK17 autophosphorylation activity. (A) Concentration-dependent activation of the autophosphorylation activity of the HK17 histidine kinase (5 μM) was measured after 30 min of incubation in the presence of EA concentrations shown on the x axis. (B) Time course of HK17 (5 μM) autophosphorylation in the presence (▪) or absence (⧫) of EA (25 μM).
In the presence of purified RR17 protein, the autophosphorylated HK17 sensor kinase was able to efficiently transfer phosphoryl groups to its mated response regulator, thus confirming the functionality of the TCS in sensing EA and responding to its presence by activating the transcription factor (see Fig. S4 in the supplemental material).
DISCUSSION
For the lifestyle of E. faecalis in the digestive tract of its host, it may be important to be able to use EA as a source of carbon and nitrogen. EA is, in the form of phosphatidylethanolamine, an essential component in the membranes of both prokaryotic and eukaryotic organisms (42), and it is a constituent of an abundant class of lipids available to gut inhabitants as part of the host's dietary intake (2, 10). Therefore, it is not surprising that EA utilization occurs in enterobacteria (such as Escherichia coli and Salmonella species) and that S. enterica serovar Typhimurium mutants defective in EA utilization were shown to be slightly attenuated in a mouse model of infection (53).
Here, we reported the identification of the E. faecalis gene cluster encoding the components for EA utilization and provided evidence for EA as the direct effector of a TCS required for this metabolic function. The RR-HK17 TCS was found to be embedded within genes encoding proteins with strong similarity to the EA utilization components of S. enterica serovar Typhimurium, and the addition of EA in an in vitro assay induced the autophosphorylation rate of the HK17 histidine approximately 15-fold from a low but measurable basal level. Autophosphorylation activity of HK17 reached a plateau at a 2:1 molar ratio between effector and kinase, supporting a physiological significance for EA as inducer of this histidine kinase.
The HK17 sensor histidine kinase is one of the few TCS kinases whose molecular environmental effector is now known. Furthermore, EA is only one of two small molecule ligands, along with toluene for the TodS histidine kinase of Pseudomonas putida, shown to be directly responsible for activation of autophosphorylation activity of a full-length, cytoplasmic kinase in vitro (29). The possibility that the GAF and/or the PAS domains in HK17 are the target for EA binding resulting in kinase activation is currently being investigated.
Phosphorylated HK17 specifically transferred its phosphoryl groups to the response regulator RR17 but no phosphotransfer was observed to the FsrA response regulator (RR15) used as a control (data not shown). Phosphorylation of RR17 by HK17 distinguishes this response regulator from AmiR, the first response regulator identified with an ANTAR output domain. In fact, AmiR lacks the residues essential for phosphotransfer, and its activation occurs via ligand disruption of its sequestering complex with the AmiC sensor protein (36).
Phosphorylation of RR17 by the EA-activated HK17 kinase provides the functional link between ligand detection and transcription activation for bacterial adaptation to growth in the presence of EA as the sole source of carbon.
The presence of EA in the medium as the sole carbon source induced transcriptional activity within a fragment located 722 bp upstream of the RR17 translational start site, suggesting the presence of an autoregulated promoter, because this induction was not observed in the absence of EA and growth of E. faecalis on EA required RR17. The inability of the RR17 mutant strain to grow in the medium containing only EA and CoB12 did not allow us to make a direct correlation between RR17 and its requirement for transcriptional induction, but the indirect evidence strongly supports this hypothesis. This promoter is likely located in the eutG-eutS intergenic region and must be required for the transcription of the genes downstream of the RR17-encoding gene, because expression of the RR17 response regulator from the constitutive aphA3 promoter in the multicopy plasmid pML28 (20) failed to complement the RR17 deletion and restore growth on EA in this mutant strain (data not shown). Nevertheless, the presence of an additional promoter(s) downstream of the RR17-encoding gene cannot be ruled out at this time. An additional promoter for the transcription of eut genes may also be present upstream of the eutP gene (EF1638) as suggested by the presence of putative −10 and −35 recognition sequences for σA-containing RNA polymerase, although read-through from an upstream putative ABC transport operon (EF1641-1639) could occur. Also, in the eutT-eutG intergenic region that contains the putative B12 riboswitch, the presence of a promoter cannot be excluded.
The presence of a putative B12 riboswitch, together with the VitB12 cofactor requirement for the activity of the EA ammonia lysase, indicates that a complex regulatory mechanism must exist for the expression of the EA utilization pathway. An additional B12 element has been identified by Barrick and Breaker (4) at position 1595486 to 1595326 on the E. faecalis V583 genome corresponding to the region upstream of EF1641, the first gene of a three-gene operon annotated as “iron compound ABC transporter,” whose transcription could read through to the eutP and eutT genes. The product of the EF1641 gene shares 24% identical residues (BLAST E value, 3e−08) with BtuF of S. enterica serovar Typhimurium, the substrate-binding protein that, with the BtuC permease and the BtuD ATPase, constitutes the ABC transporter for cobalamin (25, 57).
Bacterial riboswitches are regulatory elements located in the 5′ untranslated regions of mRNAs and induce the formation of a terminator hairpin that prematurely terminates transcription or the formation of a structure at the ribosome binding site that blocks ribosome binding. Most riboswitches block the production of biosynthetic enzymes or transporters in the presence of the ligand that is the final product of the regulated pathway. Other riboswitches activate the expression of a salvage or degradation pathway when their effector molecule is present in excess (4).
The locations of the putative riboswitches identified upstream of eutG and EF1641 are both suggestive of a transcription termination regulatory mechanism. This implies that the expression of the eut gene cluster in the presence of EA as the sole carbon source, which occurs only in the concomitant presence of EA and cobalamin, requires a mechanism counteracting the transcription termination effect brought about by the B12 riboswitch. The transcription antitermination role must be played by the ANTAR-containing RR17 response regulator upon activation by EA of the mated HK17 histidine kinase. Detailed characterization of this possible new mechanism of riboswitch regulation of gene expression through a TCS mechanism is ongoing in order to define what seems like a complex pathway for EA utilization in E. faecalis.
Curiously, the eutD gene encoding a phosphotransacetylase (Pta), located within the eut operon in S. enterica serovar Typhimurium (Fig. 1) (6), is likely to correspond to the EF0949 monocistronic gene located outside the E. faecalis eut gene cluster and possibly encoding the only Pta in this organism. EutD is responsible for the conversion of Ac-CoA to acetyl-PO4 (6). In Salmonella, the Ac-CoA that is not converted in acetyl-PO4 by EutD enters the tricarboxylic acid cycle, but this cycle is absent in E. faecalis (37). This suggests that the eutD gene may be located outside the eut operon in order to be regulated differently and/or not exclusively by the presence of EA and thus avoid the accumulation of Ac-CoA.
The regulation of eut gene expression by a TCS and the genome organization of this pathway is not unique to E. faecalis but is also found in S. sanguinis, Listeria species, Clostridium species, and Paenibacillus larvae; in these organisms, the highly conserved TCS is also located upstream of the gene annotated eutA. Because the sources of EA in the environment are largely man-made (e.g., toothpaste, mouthwash, and antifreeze), while it is naturally abundant in the human gut (27, 31) and in processed food (2, 10) and its utilization is dependent upon VitB12, it is possible that this large gene cluster may have been selected in these bacterial pathogens by exposure to the host environment. Interestingly, McBride et al. have searched for specific sets of genes in order to identify genes common to maximally diverse strains of E. faecalis, in order to define a core genome, and they found that TCS-17 was present in all strains tested (32).
In Enterococcus, EA degradation has not yet been studied, and the role of the eut genes within the context of enterococcal metabolism is still unknown. It was previously reported that salmonellae can use EA as a sole source of carbon and nitrogen under aerobic and anaerobic conditions (41, 44, 45). Of the 17 genes in the Salmonella eut operon, only 6 (eutA, -B, -C, -D, -E and -R) are required under standard conditions (37°C, pH 7.0). Five of the extra genes (eutM, -N, -L, -K, and -G) become necessary under conditions that favor loss of the volatile intermediate acetaldehyde, which escapes as a gas during growth on EA.
Acetaldehyde is highly toxic to the cells, and we cannot exclude the possibility that the limited growth of E. faecalis in the M9HY medium supplemented with EA and CoB12 is due to the accumulation of this compound (39). The alternative possibility that the OD would not increase because of the lack of an electron acceptor in the medium was tested by growing the cells in the supplemented M9HY medium under anaerobic conditions in the presence of potassium nitrate (41), but no improvement in growth was detected (data not shown).
The anaerobic growth of E. faecalis in the M9HY medium supplemented with EA and CoB12 is accompanied by the production of a yellow-brown pigment which is indicative of active metabolism and is likely the result of bacterium-dependent alteration of the corrin nucleus of VitB12 (8). This pigment strongly resembles the pigments derived from VitB12 by anaerobic growth of Pseudomonas rubescens or aerobic growth of Aerobacter aerogenes that have been characterized in the past (7, 21). It cannot be ruled out that it is the accumulation of this pigment that limited bacterial growth and reduced cell viability after prolonged incubation, as its production or characterization in E. faecalis, to our knowledge, has never been reported.
Supplementary Material
Acknowledgments
We thank James A. Hoch for helpful suggestions, Lynn E. Hancock for the construction of plasmid pTCV-LacSpec, and Jaiweon Hwang for the construction of the RR15-17 strain.
The present work was supported by grant AI052289 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USPHS. M.F.D.P. is a member of CONICET. Oligonucleotide synthesis and DNA sequencing were supported in part by the Stein Beneficial Trust.
This is manuscript number 19602 from The Scripps Research Institute.
Footnotes
Published ahead of print on 5 September 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abreu-Goodger, C., and E. Merino. 2005. RibEx: a web server for locating riboswitches and other conserved bacterial regulatory elements. Nucleic Acids Res. 33W690-W692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. 1988. Biogenic amines in lactic acid-fermented vegetables. Lebensm. Wiss. Technol. 2168-69. [Google Scholar]
- 3.Arantes, O., and D. Lereclus. 1991. Construction of cloning vectors for Bacillus thuringiensis. Gene 108115-119. [DOI] [PubMed] [Google Scholar]
- 4.Barrick, J. E., and R. R. Breaker. 2007. The distributions, mechanisms, and structures of metabolite-binding riboswitches. Genome Biol. 8R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgogne, A., S. G. Hilsenbeck, G. M. Dunny, and B. E. Murray. 2006. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J. Bacteriol. 1882875-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinsmade, S. R., and J. C. Escalante-Semerena. 2004. The eutD gene of Salmonella enterica encodes a protein with phosphotransacetylase enzyme activity. J. Bacteriol. 1861890-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgus, R. C., J. B. Hufham, W. M. Scott, and J. J. Pfeiffner. 1964. Microbial degradation of corrinoids. III. Pigments derived from vitamin B12 by Pseudomonas rubescens. J. Bacteriol. 881139-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgus, R. C., J. B. Hufham, W. M. Scott, and J. J. Pfeiffner. 1965. Microbial degradation of corrinoids. IV. Alteration of the corrin nucleus. Arch. Biochem. Biophys. 110490-495. [DOI] [PubMed] [Google Scholar]
- 9.Charbonneau, H., R. K. Prusti, H. LeTrong, W. K. Sonnenburg, P. J. Mullaney, K. A. Walsh, and J. A. Beavo. 1990. Identification of a noncatalytic cGMP-binding domain conserved in both the cGMP-stimulated and photoreceptor cyclic nucleotide phosphodiesterases. Proc. Natl. Acad. Sci. USA 87288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier, P. D., D. D. O. Cromiue, and A. P. Davies. 1991. Mechanisms of formation of chloropropanols present in protein hydrolysates. J. Am. Oil Chem. Soc. 68785-790. [Google Scholar]
- 11.Cruz-Rodz, A. L., and M. S. Gilmore. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224152-154. [DOI] [PubMed] [Google Scholar]
- 12.Edmond, M. B., S. E. Wallace, D. K. McClish, M. A. Pfaller, R. N. Jones, and R. P. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29239-244. [DOI] [PubMed] [Google Scholar]
- 13.Faust, L. R., J. A. Connor, D. M. Roof, J. A. Hoch, and B. M. Babior. 1990. Cloning, sequencing, and expression of the genes encoding the adenosylcobalamin-dependent ethanolamine ammonia-lyase of Salmonella typhimurium. J. Biol. Chem. 26512462-12466. [PubMed] [Google Scholar]
- 14.Finn, R. D., J. Mistry, B. Schuster-Bockler, S. Griffiths-Jones, V. Hollich, T. Lassmann, S. Moxon, M. Marshall, A. Khanna, R. Durbin, S. R. Eddy, E. L. Sonnhammer, and A. Bateman. 2006. Pfam: clans, web tools and services. Nucleic Acids Res. 34D247-D251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallegos, M. T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galperin, M. Y. 2004. Bacterial signal transduction network in a genomic perspective. Environ. Microbiol. 6552-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilmore, M. S., D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice. 2002. The enterococci: pathogenesis, molecular biology, antibiotic resistance, and infection control. ASM Press, Washington, DC.
- 18.Hancock, L. E., and M. Perego. 2002. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 1845819-5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, L. E., and M. Perego. 2004. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J. Bacteriol. 1867951-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock, L. E., and M. Perego. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 1865629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helgeland, K., J. Jonsen, and S. Laland. 1961. New pigments derived from vitamin B12 by a strain of Aerobacter aerogenes present in river mud. Biochem. J. 81260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho, Y. S., L. M. Burden, and J. H. Hurley. 2000. Structure of the GAF domain, a ubiquitous signaling motif and new class of cyclic GMP receptor. EMBO J. 195288-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoch, J. A., and T. J. Silhavy. 1995. Two-component signal transduction. American Society for Microbiology, Washington, DC.
- 24.Huycke, M. M., D. F. Sahm, and M. S. Gilmore. 1998. Multiple-drug resistant enterococci: the nature of the problem and an agenda for the future. Emerg. Infect. Dis. 4239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hvorup, R. N., B. A. Goetz, M. Niederer, K. Hollenstein, E. Perozo, and K. P. Locher. 2007. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD-BtuF. Science 3171387-1390. [DOI] [PubMed] [Google Scholar]
- 26.Jaroszewski, L., L. Rychlewski, Z. Li, W. Li, and A. Godzik. 2005. FFAS03: a server for profile-profile sequence alignments. Nucleic Acids Res. 33W284-W288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kofoid, E., C. Rappleye, I. Stojiljkovic, and J. Roth. 1999. The 17-gene ethanolamine (eut) operon of Salmonella typhimurium encodes five homologues of carboxysome shell proteins. J. Bacteriol. 1815317-5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korbel, J. O., T. Doerks, L. J. Jensen, C. Perez-Iratxeta, S. Kaczanowski, S. D. Hooper, M. A. Andrade, and P. Bork. 2005. Systematic association of genes to phenotypes by genome and literature mining. PLoS Biol. 3e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lacal, J., A. Busch, M. E. Guazzaroni, T. Krell, and J. L. Ramos. 2006. The TodS-TodT two-component regulatory system recognizes a wide range of effectors and works with DNA-bending proteins. Proc. Natl. Acad. Sci. USA 1038191-8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamparter, T. 2004. Evolution of cyanobacterial and plant phytochromes. FEBS Lett. 5731-5. [DOI] [PubMed] [Google Scholar]
- 31.Lawhon, S. D., J. G. Frye, M. Suyemoto, S. Porwollik, M. McClelland, and C. Altier. 2003. Global regulation by CsrA in Salmonella typhimurium. Mol. Microbiol. 481633-1645. [DOI] [PubMed] [Google Scholar]
- 32.McBride, S. M., V. A. Fischetti, D. J. Leblanc, R. C. Moellering, Jr., and M. S. Gilmore. 2007. Genetic diversity among Enterococcus faecalis. PLoS ONE 2e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Assays of beta-galactosidase, p. 352-355. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 34.Murray, B. 1990. Testing for high-level aminoglycoside resistance in enterococcal infections. Eur. J. Clin. Microbiol. Infect. Dis. 9633-634. [DOI] [PubMed] [Google Scholar]
- 35.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 1755216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Hara, B. P., R. A. Norman, P. T. Wan, S. M. Roe, T. E. Barrett, R. E. Drew, and L. H. Pearl. 1999. Crystal structure and induction mechanism of AmiC-AmiR: a ligand-regulated transcription antitermination complex. EMBO J. 185175-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 1996. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 2992071-2074. [DOI] [PubMed] [Google Scholar]
- 38.Penrod, J. T., C. C. Mace, and J. R. Roth. 2004. A pH-sensitive function and phenotype: evidence that EutH facilitates diffusion of uncharged ethanolamine in Salmonella enterica. J. Bacteriol. 1866885-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penrod, J. T., and J. R. Roth. 2006. Conserving a volatile metabolite: a role for carboxysome-like organelles in Salmonella enterica. J. Bacteriol. 1882865-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to β-galactosidase in gram-positive bacteria. FEMS Microbiol. Lett. 156193-198. [DOI] [PubMed] [Google Scholar]
- 41.Price-Carter, M., J. Tingey, T. A. Bobik, and J. R. Roth. 2001. The alternative electron acceptor tetrathionate supports B12-dependent anaerobic growth of Salmonella enterica serovar Typhimurium on ethanolamine or 1,2-propanediol. J. Bacteriol. 1832463-2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Randle, C. L., P. W. Albro, and J. C. Dittmer. 1969. The phosphoglyceride composition of gram-negative bacteria and the changes in composition during growth. Biochim. Biophys. Acta 187214-220. [DOI] [PubMed] [Google Scholar]
- 43.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J. Biol. Chem. 27841148-41159. [DOI] [PubMed] [Google Scholar]
- 44.Roof, D. M., and J. R. Roth. 1988. Ethanolamine utilization in Salmonella typhimurium. J. Bacteriol. 1703855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roof, D. M., and J. R. Roth. 1989. Functions required for vitamin B12-dependent ethanolamine utilization in Salmonella typhimurium. J. Bacteriol. 1713316-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roof, D. M., and J. R. Roth. 1992. Autogenous regulation of ethanolamine utilization by a transcriptional activator of the eut operon in Salmonella typhimurium. J. Bacteriol. 1746634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 48.Sardiwal, S., S. L. Kendall, F. Movahedzadeh, S. C. Rison, N. G. Stoker, and S. Djordjevic. 2005. A GAF domain in the hypoxia/NO-inducible Mycobacterium tuberculosis DosS protein binds haem. J. Mol. Biol. 353929-936. [DOI] [PubMed] [Google Scholar]
- 49.Shu, C. J., and I. B. Zhulin. 2002. ANTAR: an RNA-binding domain in transcription antitermination regulatory proteins. Trends Biochem. Sci. 273-5. [DOI] [PubMed] [Google Scholar]
- 50.Sousa, E. H., J. R. Tuckerman, G. Gonzalez, and M. A. Gilles-Gonzalez. 2007. DosT and DevS are oxygen-switched kinases in Mycobacterium tuberculosis. Protein Sci. 161708-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starai, V. J., and J. C. Escalante-Semerena. 2004. Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 3401005-1012. [DOI] [PubMed] [Google Scholar]
- 52.Steinmetz, M., and R. Richter. 1994. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J. Bacteriol. 1761761-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stojiljkovic, I., A. J. Baumler, and F. Heffron. 1995. Ethanolamine utilization in Salmonella typhimurium: nucleotide sequence, protein expression, and mutational analysis of the cchA cchB eutE eutJ eutG eutH gene cluster. J. Bacteriol. 1771357-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor, B. L., and I. B. Zhulin. 1999. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 63479-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in gram-positive bacteria. Nucleic Acids Res. 184296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tucker, N. P., B. D'autreaux, S. Spiro, and R. Dixon. 2006. Mechanism of transcriptional regulation by the Escherichia coli nitric oxide sensor NorR. Biochem. Soc. Trans. 34191-194. [DOI] [PubMed] [Google Scholar]
- 57.Van, B. M., C. Bradbeer, N. Clark, and J. R. Roth. 1999. A new class of cobalamin transport mutants (btuF) provides genetic evidence for a periplasmic binding protein in Salmonella typhimurium. J. Bacteriol. 1815539-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson, S. A., S. J. Wachira, R. A. Norman, L. H. Pearl, and R. E. Drew. 1996. Transcription antitermination regulation of the Pseudomonas aeruginosa amidase operon. EMBO J. 155907-5916. [PMC free article] [PubMed] [Google Scholar]
- 59.Winkler, W. C., and R. R. Breaker. 2005. Regulation of bacterial gene expression by riboswitches. Annu. Rev. Microbiol. 59487-517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.