Abstract
Previous studies identified irvA as a normally repressed but highly inducible transcription regulator capable of repressing mutacin I gene expression in Streptococcus mutans. In this study, we aimed to identify and characterize the regulator(s) responsible for repressing the expression of irvA. An uncharacterized open reading frame (SMU.1398) located immediately adjacent to irvA and annotated as a putative transcription repressor was identified as a likely candidate. The results of mutation studies confirmed that the expression of irvA was greatly increased in the SMU.1398 background. Mutation of SMU.1398 (“irvR”) abolished genetic competence and reduced the expression of the late competence genes/operons comEA, comY, and dprA without affecting the expression of the known competence regulators comC, comED, or comX. In addition, irvR was found to be a potent negative regulator of dextran-dependent aggregation (DDAG) and gbpC expression. Each of these irvR mutant phenotypes could be rescued with a double mutation of irvA or complemented by introducing a wild-type copy of irvR on a shuttle vector. These data indicate that the repression of irvA is critically dependent upon irvR and that irvA repression is essential for the development of genetic competence and the proper control of DDAG in S. mutans.
Streptococcus mutans is a gram-positive oral commensal species found in human dental plaque and is primarily associated with the initiation of caries development (tooth decay) (3, 8, 28, 30, 38, 41, 45). Certain species, such as S. mutans, have a much greater capacity to both excrete acidic metabolites (acidogenic) and proliferate in an acidic environment (aciduric) and thus, can gain a competitive advantage over nonaciduric species (2, 29). Yet, examinations of oral plaque samples and carious lesions have identified numerous other aciduric species (4, 9), which suggests that the success of S. mutans as a dental pathogen cannot be solely attributed to its acid tolerance. Biofilm formation, natural competence, and bacteriocin production are also recognized as virulence factors that are necessary for the persistence of S. mutans in the presence of numerous environmental stresses and fierce interspecies competition (2, 21, 29).
Studies of genetic factors that regulate these processes in S. mutans have found a surprising variety of genetic mutations that each affect multiple virulence factors and stress tolerances simultaneously (7, 14-18, 20, 22-24, 26, 33, 36, 39, 43). This implies that a large overlap must exist between the pathways responsible for the regulation of persistence-related abilities. For example, our laboratory and others have observed that a mutation in the S. mutans ortholog of luxS creates altered oxidative and acid stress tolerances, as well as defects in biofilm formation, natural competence, and bacteriocin (mutacin I) production. (25-27, 37, 43, 44).
Previously, our laboratory further investigated the mutacin I phenotype of the luxS mutant and identified an uncharacterized transcription regulator, which we referred to as irvA, as a mediator of the mutacin I deficiency (25). Following the deletion of luxS, this gene was found to be strongly expressed, along with the concomitant loss of mutacin I production. A luxS irvA double-deletion strain regained the ability to produce mutacin I, whereas an engineered constitutive irvA expression strain was mutacin I deficient, even in a wild-type luxS background. Thus, it was concluded that irvA was an intermediate component of the pathway responsible for the repression of mutacin I in a luxS mutant background. In addition, our laboratory and others have found that the stress-responsive gene gbpC is also induced in the luxS background (25, 37). Since gbpC expression has been found to be highly responsive to numerous environmental stresses (6, 34, 35), we had speculated that the luxS mutation may similarly trigger various stress pathways in the cell, which may account for both gbpC and irvA induction (25). Likewise, irvA induction has also been found to be associated with several other genetic mutations known to have multiple stress- and virulence-related phenotypes (40). Moreover, irvA has thus far been detected in numerous strains of S. mutans (1, 25, 42); therefore, it may be a fundamental component of the basic machinery utilized to modulate multiple virulence-associated functions required for persistence.
In the current study, we report the identification of a putative repressor which is required for preventing irvA expression. This gene, which we refer to as irvR, is also absolutely required for the development of genetic competence and the proper regulation of dextran-dependent aggregation (DDAG). Furthermore, both of these phenotypes are critically dependent upon the presence of irvA as well. Thus, irvR and irvA may form a regulatory pair that is responsible for controlling important stress responses and virulence factors and may be mediators of a variety of phenotypes found in various mutant strains of S. mutans.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids and their relevant characteristics are listed in Table 1. Escherichia coli cells were grown in Luria-Bertani (LB; Difco) medium at 37°C. E. coli strains carrying plasmids were selected with 100 μg ml−1 ampicillin (Fluka), 100 μg ml−1 kanamycin (EMD), or 150 μg ml−1 spectinomycin (Sigma). All S. mutans strains were grown anaerobically (85% N2, 10% CO2, and 5% H2) at 37°C. S. mutans strains were cultivated in either brain-heart infusion (BHI) or Todd-Hewitt medium (Difco). For the selection of antibiotic-resistant colonies, BHI plates were supplemented with 800 μg ml−1 kanamycin, 15 μg ml−1 tetracycline (Sigma), or 900 μg ml−1 spectinomycin.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain/plasmid | Relevant characteristicsa | Reference/source |
|---|---|---|
| Strains | ||
| E. coli JM109 | e14−(McrA−) recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 Δ(lac-proAB) [F′ traD36proAB lacIqZΔM15] | Cloning strain |
| S. mutans | ||
| UA159 | Wild-type S. mutans | 1 |
| GN01R | UA159 ΔirvR Kmr | This study |
| GN01Rs | UA159 ΔirvR Kmr; pDL278 | This study |
| GN01Rc | UA159 ΔirvR Kmr; pDL278-irvR | This study |
| GN01RA | UA159 ΔirvA ΔirvR Kmr | This study |
| LZ02C | UA159 gbpC::pFW5 Spr | This study |
| GN01RC | UA159 ΔirvRgbpC::pFW5 Kmr Spr | This study |
| Plasmids | ||
| pBluescript | Cloning vector; Apr | Stratagene |
| pCR2.1 | Cloning vector; Apr Kmr | Invitrogen |
| pSC-A | Cloning vector; Apr | Stratagene |
| pFW5 | Suicide vector; Spr | 32 |
| pLZ02C | pFW5 gbpC internal fragment; Spr | This work |
| pGN01R | pFW5 allelic replacement of irvR; Spr | This work |
| pDL278 | E. coli-Streptococcus shuttle vector; Spr | 11 |
| pGN01Rc | pDL278 irvR+ Spr | This work |
| pGNaa3 | pSC-A aphAIII+ Apr Kmr | This work |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Spr, spectinomycin resistance.
Construction of mutants.
To study the role of irvR in S. mutans, we constructed an irvR deletion mutant via double-crossover homologous recombination. To generate the construct, two fragments corresponding to approximately 1 kb of the upstream and downstream sequences of irvR were generated by PCR, using Pfu polymerase with the primer pairs irvR Up F/irvR Up R, and irvR Dn F/irvR Dn R (Table 2). Each of the primers incorporated restriction enzyme sites, and the PCR amplicons were subsequently cleaved with the appropriate restriction enzymes and cloned into pFW5. The kanamycin resistance gene aphAIII was amplified by PCR from the plasmid pTV1-OK (16) by using the primers Kan F and Kan R (Table 2) and cloned into pSC-A (Stratagene) to create pGNaa3. The kanamycin resistance gene was excised from pGNaa3 with EcoRI and ligated in between the irvR upstream and downstream fragments to create the plasmid pGN01R. The plasmid was confirmed via restriction digestion and linearized for transformation into S. mutans UA159. For complementation analysis, the complete irvR open reading frame and 250 bp of the upstream intergenic region was amplified by PCR with irvR-c F and irvR-c R (Table 2). The addition of 5′-phosphates to the primers with T4 polynucleotide kinase (NEB) allowed the subsequent ligation of the PCR product to the HincII site of pDL278 to create pGN01Rc. The irvA irvR double deletion was created by using a strategy similar to that for the irvR deletion mutant except that the fragment upstream of irvA was obtained from a previously cloned fragment in pCR2.1 (Invitrogen) used for the deletion of irvA (25). This fragment was digested by using restriction sites compatible to the kanamycin resistance cassette and ligated along with the irvR downstream fragment as described previously (Table 2). The gbpC mutant was constructed by single-crossover insertion inactivation using an internal 0.5-kb fragment of gbpC amplified by PCR with the primer pairs gbpC F and gbpC R (Table 2). The PCR product was subcloned into the pCR2.1 vector (Invitrogen), digested with BamHI and SalI, and cloned into the suicide vector pFW5. The resulting plasmid, pLZ02C, was confirmed via restriction digestion and transformed into S. mutans UA159. The gbpC irvR double-mutant strain GN01RC was obtained by transforming linearized pGN01R into the gbpC mutant strain. All mutant derivatives of UA159 were confirmed by PCR.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) | Purpose |
|---|---|---|
| Kan F | AGGTGATAGGTAAGATTATACCG | irvR; irvR irvA deletions |
| Kan R | CCCTATCTAGCGAACTTTTAGA | irvR; irvR irvA deletions |
| irvR Up F | CGCTGCAGTTTCCTTGTGTGTGACTTTC | irvR deletion |
| irvR Up R | GCGAATTCAGCCTTCATTGCTTCTAAATC | irvR deletion |
| irvR Dn F | GCGAATTCATCGGAACGGTCATTACCTAA | irvR; irvR irvA deletions |
| irvR Dn R | CCACTAGTCCTGTAATTTTTCATAAGGAAGAGG | irvR; irvR irvA deletions |
| irvR-c F | CATATGAAATACTTTTAATT | irvR complementation |
| irvR-c R | CCGCTCGAGTTAGGTAATGACCGTTCC | irvR complementation |
| gbpC F | GTCGACATCTGGGCGTGTTGAAAAAG | gbpC insertion |
| gbpC R | GGATCCAATGGCATTATTGCCGCTTA | gbpC insertion |
| irvA RT F | CCCTCAACACACTCTGCTAAGCT | irvA RT-PCR |
| irvA RT R | CCAAATCATTGGCCAGTTGAA | irvA RT-PCR |
| comEA RT F | AGGAACAATCCCTTCAGGTAACC | comEA RT-PCR |
| comEA RT R | CAGTCGTCTGCGTCTTCTTCTG | comEA RT-PCR |
| dprA RT F | GCGGTTTAGCGCGTGGTAT | dprA RT-PCR |
| dprA RT R | GCTCCACCGCTTTTAAGACTTG | dprA RT-PCR |
| comY RT F | CTTTTTTCTGGACGTCACGATTT | comYA RT-PCR |
| comY RT R | TCGCCCCTTGATTTCATTTAA | comYA RT-PCR |
| gbpC RT F | AATTCTGATACTGTTGCAGCACCTA | gbpC RT-PCR |
| gbpC RT R | TTCTGTTGCAGCCGGTTCT | gbpC RT-PCR |
| 16S RT F | ATCACTAGTAGATGGACCTG | RT-PCR normalization |
| 16S RT R | TGTATCGTCGCCTTGGTAAG | RT-PCR normalization |
RNA extraction and quantitative real-time RT-PCR.
S. mutans UA159 and its derivatives were cultivated overnight at 37°C. The overnight cultures were diluted 1:30 in BHI with 0.4% bovine serum albumin in a total volume of 30 ml. The cells were allowed to grow to an optical density at 600 nm of ∼0.3 and collected by centrifugation. The pellets were resuspended in 700 μl Tris-EDTA buffer (pH 8.0) and transferred to a 2-ml screw-cap tube containing 500 μl 0.1-mm silica beads (Biospec). Six hundred microliters of Trizol (Sigma) was added to the tube, vortexed, and submitted to three consecutive 30-s homogenization cycles with a FastPrep-24 system (MP Biomedicals) set at a speed of 6.0 M/s. After homogenization, 200 μl chloroform (Sigma) was added, and the solution was centrifuged for 10 min at full speed. The supernatant was extracted three times with 450 μl acidic phenol (Sigma) and 200 μl chloroform. RNA was precipitated with isopropyl alcohol and washed with 70% ethanol. After drying, the RNA pellet was resuspended in 87 μl RNase-free water-10 μl 10× DNase buffer-3 μl RNase-free DNase (Ambion). The mixture was incubated at 37°C for 45 min. After incubation, samples were further purified with an RNeasy spin column (Qiagen) and eluted in 30 μl RNase-free water. Five hundred nanograms of total RNA was used for cDNA synthesis using SuperScript II (Invitrogen) according to the manufacturer's protocol. For real-time reverse transcription-PCR (RT-PCR), oligonucleotide primers were designed with Primer Express 3.0 software (Applied Biosystems), which selects primers optimized for “delta-delta threshold cycle” (ΔΔCT) method analysis. Real-time PCR was performed using an Applied Biosystems 7300 system, and the reaction mixtures were prepared using Applied Biosystems Sybr green PCR master mix. Changes in levels of gene expression were calculated automatically with the Applied Biosystems 7300 system software using the ΔΔCT method, which is briefly described as follows: ΔCT = CT(target) − CT(housekeeping gene); ΔΔCT = ΔCT1 − ΔCT2; the levels of change are calculated as 2−ΔΔCT. The 16S rRNA gene was used as the housekeeping gene reference, and all cDNA synthesis reactions included a replicate reaction without added reverse transcriptase to assess genomic DNA contamination. The primers used for real-time RT-PCR are listed in Table 2.
Transformation assay.
Genetic competence was determined by a transformation efficiency assay with genomic DNA and the E. coli-Streptococcus shuttle vector pDL278. Cells were grown as mentioned above to an optical density at 600 nm of ∼0.3. Genomic DNA (10 μg ml−1) or plasmid DNA (1 μg ml−1) was added to each reaction mixture, and the cultures were incubated for an additional 2 h. After the incubation, the cultures were briefly sonicated (Misonix) to disperse the cells and plated on antibiotic-containing BHI agar plates, as well as on nonselective BHI plates. Successful transformation was scored based on antibiotic resistance, and the total viable cell population was determined by counting the number of colonies growing on nonselective plates. The transformation efficiency was determined by calculating the ratio of transformants to total viable cells.
Analysis of DDAG.
The DDAG assay was performed using the BTR-G medium described by Sato et al. (34), as well as with BHI. In brief, S. mutans colonies were picked from BHI plates and incubated anaerobically in BHI broth overnight at 37°C. The cells were then diluted (1:100) into 3 ml fresh BHI or BTR-G broth. The cultures were incubated for an additional 24 h and then divided into two 1-ml portions with or without 100 μg ml−1 dextran T2000 from Leuconostoc spp. Each pair of tubes was swirled briefly, and aggregation was observed as obvious clumping and cell precipitation. Generally, DDAG was obvious within 1 to 2 min of swirling the cultures.
RESULTS
irvR is required to repress irvA transcription.
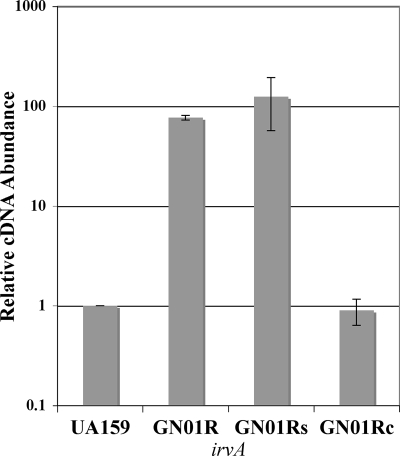
Since the results of previous studies suggested that irvA itself appeared to be strongly regulated at the transcriptional level, we aimed to identify and characterize its upstream regulator(s). Previously, we found through a BLASTP analysis of the putative sequence of IrvA that it matched strongly to a variety of Cro repressors from various gram-positive bacteriophages (25). Thus, we speculated that perhaps IrvA was itself controlled by a “CI-like” repressor, as is also the case with numerous temperate bacteriophages. Surprisingly, the most promising candidate was located directly adjacent to irvA in the genome (NCBI annotation, SMU.1398; Oralgen annotation, SMu1275). Its predicted amino acid sequence matched well with a variety of gram-positive bacteriophage CI repressors, with the highest homology to the streptococcal phage EJ-1 (37% identity; 50% similar overall). Based upon its putative role as a transcription repressor, we surmised that this gene would be required to maintain the low, basal-level expression of irvA normally seen during typical growth conditions. Therefore, we deleted SMU.1398 and measured the expression of irvA by real-time RT-PCR to determine whether this mutation could cause a derepression of irvA. As shown by the results in Fig. 1, the expression of irvA in the SMU.1398 deletion background was increased 76-fold over the level in the wild type and could be fully complemented by providing a wild-type copy of SMU.1398 in trans. As expected, the empty shuttle vector used for complementation had no effect upon irvA expression in the SMU.1398 mutant (Fig. 1). Thereafter, SMU.1398 was designated irvR, a repressor of irvA.
FIG. 1.
Expression of irvA in various irvR (SMU.1398) backgrounds. The relative expression levels of irvA were determined by real-time RT-PCR and compared between the wild-type strain UA159 (arbitrarily assigned as 1), the irvR mutant (GN01R), the irvR mutant strain harboring an empty shuttle vector (GN01Rs), and the complemented mutant strain (GN01Rc). Data are shown as the averages of the results of three independent experiments. All real-time RT-PCR values were normalized according to the abundance of 16S rRNA in each sample. Error bars show standard deviations.
irvR is essential for the development of genetic competence.
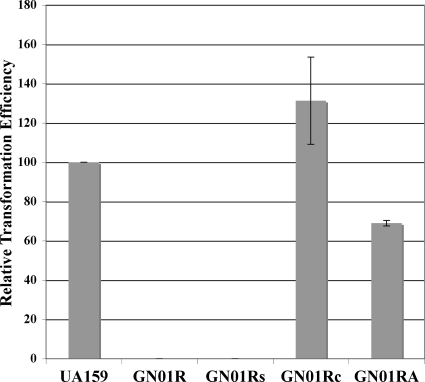
Previously, we had determined that a luxS mutant has a reduced capacity for genetic competence (27). Likewise, we had observed a similar result in several other irvA-inducing mutants as well (unpublished observations). Therefore, we were curious to determine whether irvR could also affect genetic competence, since it seemed to be a major regulator of irvA expression. Interestingly, the irvR mutant strain was found to be severely deficient in its genetic competence ability, as we were unable to transform this strain after repeated attempts (Fig. 2). We also scaled up the transformation assay by 10-fold in order to sample a larger number of cells, but we were still unable to detect any transformants. Thus, the transformation efficiency of the irvR mutant was <4 × 10−10. In addition, we assayed competence over a range of optical densities, as well as in the presence of added synthetic competence-stimulating peptide, and found that the irvR mutant remained untransformable (data not shown). However, despite the various failed attempts to transform the irvR mutant, we were able to fully restore the transformation defect with a trans-complementation of irvR (Fig. 2). Furthermore, since the results of our previous studies suggested that irvA expression seemed to be correlated with reduced genetic competence, we created a double mutant of both irvA and irvR to determine whether the competence defect of the irvR mutant could be suppressed. As shown by the results in Fig. 2, this did appear to be the case, which suggests that both irvR and irvA function in the same pathway responsible for regulating genetic competence.
FIG. 2.
Results of transformation efficiency assays in irvR mutant strains. The transformation efficiency values are presented relative to the wild-type value (6.0 × 10−7), which was arbitrarily assigned as 100% and compared to the values for the irvR mutant (GN01R), the mutant strain harboring an empty shuttle vector (GN01Rs), the complemented mutant strain (GN01Rc), and the irvR irvA double mutant (GN01RA). Data are presented as the averages of the results of three independent experiments performed in triplicate. Error bars show standard deviations.
irvR affects the expression of late competence genes via irvA.
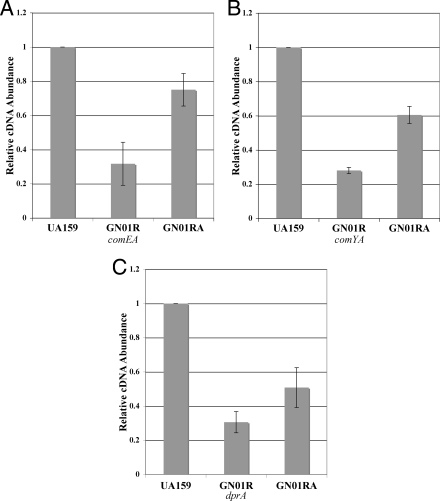
The regulation of genetic competence occurs through a well-characterized cascade of transcriptional regulation, ultimately resulting in the expression of numerous late competence genes required for the uptake and recombination of transforming DNA. Given that both irvR and irvA seemed to have a major role in competence development, we were curious to determine whether the competence defect seen in the irvR mutant strain would also correlate with the altered expression of any of the known competence genes. Therefore, we tested the expression of the well-characterized competence gene regulators comC, comED, and comX, as well as genes required during late competence, such as comYA, comEA, comEB, comFA, cinA, coiA, dprA, endA, mecA, and recA. Of these, the expression levels of three late competence genes essential for natural transformability (comEA, comYA, and dprA) were significantly reduced in the irvR background (P < 0.001) and rescued by an irvR irvA double mutation (P < 0.01) (Fig. 3). Surprisingly, no effect was seen upon the expression of the late competence gene regulators comC, comED, and comX (data not shown). Thus, the irvA-dependent pathway appears to circumvent the upstream components of the competence cascade to affect these genes through an alternate mechanism.
FIG. 3.
Results of real-time RT-PCR analysis of late competence genes. The expression levels of late competence genes are presented relative to that of the wild type (UA159), which was arbitrarily assigned a value of 1. (A) Expression level of comEA in the irvR mutant strain GN01R compared to the levels in the wild-type strain UA159 (P < 0.001) and the irvR irvA double-mutant strain GN01RA (P < 0.01). (B) Expression level of comYA in strain GN01R compared to the levels in the wild type (P < 0.0001) and GN01RA (P < 0.0001). (C) Expression level of dprA in strain GN01R compared to the levels in the wild type (P < 0.0001) and GN01RA (P < 0.01) All data are presented as the averages of the results of five independent experiments. Statistical analysis was performed by using the two-tailed Student's t test. Error bars show standard deviations.
Mutation of irvR induces DDAG via gbpC.
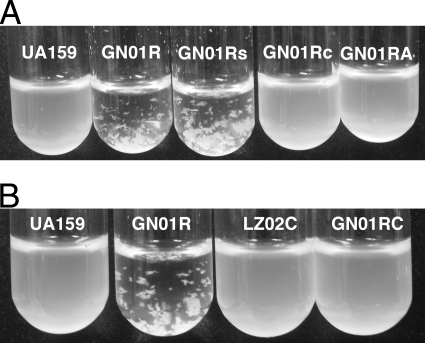
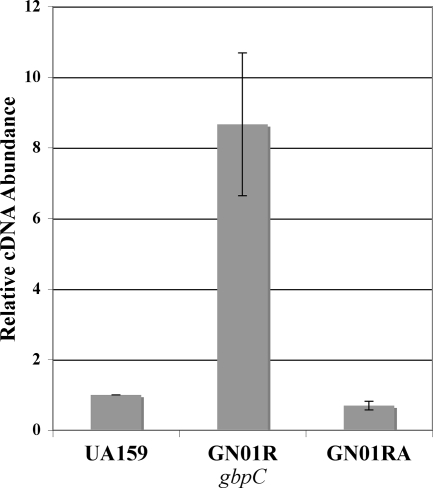
As mentioned previously, in our prior studies of a luxS mutant, we had observed increased expression of gbpC (25), an essential mediator of DDAG (34, 35). Conspicuously, we had noted increased gbpC expression in several other irvA-inducing mutations as well (unpublished observations). Therefore, we reasoned that perhaps the irvR mutant might exhibit phenotypes related to DDAG. Upon the addition of 100 μg ml−1 dextran T2000, we found that the irvR mutant strain displayed rapid aggregation, with the cells visibly clumped and fully precipitated within 1 to 2 min (Fig. 4A). Furthermore, this phenotype appeared independent of the growth medium, as it occurred similarly with both the published medium for DDAG assays (BTR-G) (35) and BHI medium. Interestingly, this response also did not require pretreatment with any of the environmental stress conditions normally used to induce DDAG, such as the addition of a sub-MIC concentration of tetracycline or heat shock (6, 35). As expected, no aggregation was observed in the absence of dextran (data not shown). Similar to the competence phenotype, full complementation was observed with the introduction of a wild-type copy of irvR on a shuttle plasmid, and a double mutant of irvR and irvA was also able to suppress the irvR phenotype (Fig. 4A). This suggests that both irvR and irvA are components of the pathway responsible for the DDAG phenotype. In order to further characterize this pathway, we examined the role of gbpC as well. Consistent with previous suggestions of gbpC as a mediator of DDAG, a double mutation of gbpC and irvR potently suppressed the DDAG phenotype of the irvR mutant (Fig. 4B). As a further confirmation of this result, we also compared the expression of gbpC in the wild-type, irvR mutant, and irvR irvA double-mutant strains. In the irvR background, gbpC expression was significantly increased over its level in both the wild type (P = 0.022) and the irvR irvA double mutant (P = 0.018). In addition, the irvR irvA double mutant had gbpC expression nearly identical to that of the wild type (Fig. 5). These results suggest that both irvA and gbpC are essential mediators of the DDAG phenotype in the irvR mutant strain and that irvA is apparently upstream of gbpC in this pathway.
FIG. 4.
Results of DDAG assays in various irvR backgrounds. (A) Dextran T2000 was added to the wild-type strain UA159, the irvR mutant (GN01R), the irvR mutant strain harboring an empty shuttle vector (GN01Rs), the complemented irvR mutant (GN01Rc), and the irvR irvA double mutant (GN01RA). (B) The same experiment was performed using the wild-type strain UA159, the irvR mutant (GN01R), the gbpC mutant (LZ02C), and the gbpC irvR double mutant (GN01RC). Shown here are representative results seen after approximately 1 to 2 min of gentle agitation. These experiments were performed three times with similar results.
FIG. 5.
Results of real-time RT-PCR analysis of gbpC. The expression level of gbpC in the wild type (arbitrarily assigned as 1) is compared to the levels in the irvR (GN01R) and irvR irvA (GN01RA) mutant backgrounds. The data are the averages of the results of three independent experiments. Statistical analysis was performed by using the two-tailed Student's t test. Error bars show standard deviations.
DISCUSSION
In the current study, we describe a novel regulator, irvR, which comprises an essential portion of the upstream regulatory pathway required for the repression of irvA, a recently characterized transcription regulator induced by a variety of genetic mutations (25, 40). Based upon our previous experience with phenotypes associated with irvA induction, we tested both genetic competence and DDAG in the irvR background. Competence was found to be severely inhibited, whereas DDAG appeared to be hyperactive. Both of these phenotypes could be complemented efficiently by the introduction of a wild-type copy of irvR on a shuttle plasmid or by creating a double mutant of both irvR and irvA. These data implicate irvR and irvA as forming a regulatory pair that functions to mediate a variety of phenotypes. Based upon the genetic data, it appears that the function of irvR is largely devoted to preventing irvA transcription, whereas IrvA appears to be the major mediator of the associated phenotypes.
Both the competence and DDAG phenotypes were associated with the altered expression of effector genes. However, given the severity of the competence phenotype, it was somewhat surprising that only a subset of competence genes/operons exhibited reduced expression. Additionally, each of the differentially expressed genes/operons in the irvR mutant has been shown to be absolutely required for genetic competence, but none appeared to be affected to an extent that might be expected for a competence-negative phenotype. There are several possible explanations for this result. First, it may be simply that the combined effect of a reduction in transcription for all of these genes is sufficient to fully disrupt genetic competence. For example, both comEA and comYA are located in operons that contain a total of at least 11 genes between them, and almost all of their individual operon components play an essential role in natural transformation (5, 27, 31). Furthermore, in the irvR irvA double-mutant strain, late competence gene expression was restored to about 60% of the wild-type level on average (Fig. 3), which seems to correlate reasonably well with the transformation efficiency seen in this strain (Fig. 2). This result may be expected if reduced competence gene transcription was the source of the transformation deficiency. Alternatively, there may be an as-yet-unrecognized level of regulation of the competence system. For example, this could occur at the level of protein-protein interaction. Indeed, the comY operon gene products are thought to form a complex with each other, as well as with additional competence proteins (10). Disruption of this complex will result in a total loss of transformability (12, 13, 27). Therefore, it may be possible that in the irvR background one or more of these interactions are disrupted. Similarly, any loss in the ability to bind exogenous DNA or to recombine transforming DNA would also result in a loss of transformability. Further studies are necessary to fully reconcile the connection between irvA and competence. However, the transcription data do suggest that the reduction in gene expression of these late competence genes is irvA dependent. Studies are currently under way to determine the scope of the IrvA regulon, which should eventually determine whether IrvA affects these genes through direct regulation of their promoters. If this is indeed the case, it may explain how these late competence genes could exhibit reduced expression without any noticeable changes in the level of expression of comC, comED, or comX. However, there are some indications that S. mutans regulates the late competence genes in a manner that differs from the Streptococcus pneumoniae paradigm (19). Therefore, an alternate possibility is that IrvA regulates an uncharacterized component of the competence cascade. Further analysis of the IrvA regulon should help to determine whether this is the case, as well as identify any additional components of the pathway between irvA and gbpC.
The data presented in this study suggest that irvR and irvA are closely associated and probably function as a regulatory pair. This is consistent with the homology both putative gene products have with the CI and Cro proteins from numerous bacteriophages. If IrvR and IrvA also function similarly to CI and Cro, we would predict that stress signals influence IrvR transcription factor activity, which would subsequently modulate the expression status of irvA. The stress hypothesis is also consistent with our observation that increased gbpC expression seems to correlate with irvA expression in various genetic backgrounds (unpublished observations). As previously mentioned, gbpC induction and DDAG in S. mutans have been shown to occur in the presence of various environmental stress conditions (6, 35). Thus, an interesting possibility is that irvA-inducing genetic mutations create stress signals in the cell and/or simulate an intracellular stress state similar to that encountered during unfavorable growth conditions. Furthermore, irvR may be one of the downstream targets of these stress-regulated pathways. Studies are currently under way to examine whether there is any evidence to support this hypothesis.
Acknowledgments
We thank the laboratory of R. Burne for the E. coli-S. mutans shuttle plasmid pDL278.
This work was supported by an NCRR COBRE grant, P20-RR018741-05, to J.M. and an NIDCR grant, DE014757-05, to F.Q.
Footnotes
Published ahead of print on 29 August 2008.
REFERENCES
- 1.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 9914434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banas, J. A. 2004. Virulence properties of Streptococcus mutans. Front. Biosci. 91267-1277. [DOI] [PubMed] [Google Scholar]
- 3.Barsamian-Wunsch, P., J. H. Park, M. R. Watson, N. Tinanoff, and G. E. Minah. 2004. Microbiological screening for cariogenic bacteria in children 9 to 36 months of age. Pediatr. Dent. 26231-239. [PubMed] [Google Scholar]
- 4.Beighton, D. 2005. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 33248-255. [DOI] [PubMed] [Google Scholar]
- 5.Berge, M., M. Moscoso, M. Prudhomme, B. Martin, and J. P. Claverys. 2002. Uptake of transforming DNA in gram-positive bacteria: a view from Streptococcus pneumoniae. Mol. Microbiol. 45411-421. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, I., L. Drake, and S. Biswas. 2007. Regulation of gbpC expression in Streptococcus mutans. J. Bacteriol. 1896521-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, I., L. Drake, D. Erkina, and S. Biswas. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J. Bacteriol. 19068-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowden, G. H. 1990. Microbiology of root surface caries in humans. J. Dent. Res. 691205-1210. [DOI] [PubMed] [Google Scholar]
- 9.Brailsford, S. R., B. Shah, D. Simons, S. Gilbert, D. Clark, I. Ines, S. E. Adams, C. Allison, and D. Beighton. 2001. The predominant aciduric microflora of root-caries lesions. J. Dent. Res. 801828-1833. [DOI] [PubMed] [Google Scholar]
- 10.Chen, I., R. Provvedi, and D. Dubnau. 2006. A macromolecular complex formed by a pilin-like protein in competent Bacillus subtilis. J. Biol. Chem. 28121720-21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Y. Y., and D. J. LeBlanc. 1992. Genetic analysis of scrA and scrB from Streptococcus sobrinus 6715. Infect. Immun. 603739-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, Y. S., F. Breidt, and D. Dubnau. 1998. Cell surface localization and processing of the ComG proteins, required for DNA binding during transformation of Bacillus subtilis. Mol. Microbiol. 29905-913. [DOI] [PubMed] [Google Scholar]
- 13.Chung, Y. S., and D. Dubnau. 1998. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J. Bacteriol. 18041-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claverys, J. P., M. Prudhomme, and B. Martin. 2006. Induction of competence regulons as a general response to stress in gram-positive bacteria. Annu. Rev. Microbiol. 60451-475. [DOI] [PubMed] [Google Scholar]
- 15.Frees, D., K. Savijoki, P. Varmanen, and H. Ingmer. 2007. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, gram-positive bacteria. Mol. Microbiol. 631285-1295. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez, J. A., P. J. Crowley, D. P. Brown, J. D. Hillman, P. Youngman, and A. S. Bleiweis. 1996. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J. Bacteriol. 1784166-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasona, A., P. J. Crowley, C. M. Levesque, R. W. Mair, D. G. Cvitkovitch, A. S. Bleiweis, and L. J. Brady. 2005. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle pathway or YidC2. Proc. Natl. Acad. Sci. USA 10217466-17471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaraman, G. C., J. E. Penders, and R. A. Burne. 1997. Transcriptional analysis of the Streptococcus mutans hrcA, grpE and dnaK genes and regulation of expression in response to heat shock and environmental acidification. Mol. Microbiol. 25329-341. [DOI] [PubMed] [Google Scholar]
- 19.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Mol. Microbiol. 57392-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 1877193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuramitsu, H. K. 2003. Molecular genetic analysis of the virulence of oral bacterial pathogens: an historical perspective. Crit. Rev. Oral Biol. Med. 14331-344. [DOI] [PubMed] [Google Scholar]
- 22.Lemos, J. A., Y. Y. Chen, and R. A. Burne. 2001. Genetic and physiologic analysis of the groE operon and role of the HrcA repressor in stress gene regulation and acid tolerance in Streptococcus mutans. J. Bacteriol. 1836074-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Len, A. C., D. W. Harty, and N. A. Jacques. 2004. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology 1501339-1351. [DOI] [PubMed] [Google Scholar]
- 24.Levesque, C. M., R. W. Mair, J. A. Perry, P. C. Lau, Y. H. Li, and D. G. Cvitkovitch. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merritt, J., J. Kreth, W. Shi, and F. Qi. 2005. LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component. Mol. Microbiol. 57960-969. [DOI] [PubMed] [Google Scholar]
- 26.Merritt, J., F. Qi, S. D. Goodman, M. H. Anderson, and W. Shi. 2003. Mutation of luxS affects biofilm formation in Streptococcus mutans. Infect. Immun. 711972-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merritt, J., F. Qi, and W. Shi. 2005. A unique nine-gene comY operon in Streptococcus mutans. Microbiology 151157-166. [DOI] [PubMed] [Google Scholar]
- 28.Munson, M. A., A. Banerjee, T. F. Watson, and W. G. Wade. 2004. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 423023-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napimoga, M. H., J. F. Hofling, M. I. Klein, R. U. Kamiya, and R. B. Goncalves. 2005. Transmission, diversity and virulence factors of Streptococcus mutans genotypes. J. Oral Sci. 4759-64. [DOI] [PubMed] [Google Scholar]
- 30.Nobre dos Santos, M., L. Melo dos Santos, S. B. Francisco, and J. A. Cury. 2002. Relationship among dental plaque composition, daily sugar exposure and caries in the primary dentition. Caries Res. 36347-352. [DOI] [PubMed] [Google Scholar]
- 31.Pestova, E. V., and D. A. Morrison. 1998. Isolation and characterization of three Streptococcus pneumoniae transformation-specific loci by use of a lacZ reporter insertion vector. J. Bacteriol. 1802701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Podbielski, A., B. Spellerberg, M. Woischnik, B. Pohl, and R. Lutticken. 1996. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS). Gene 177137-147. [DOI] [PubMed] [Google Scholar]
- 33.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 724895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato, Y., Y. Yamamoto, and H. Kizaki. 1997. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 65668-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, Y., Y. Yamamoto, and H. Kizaki. 2000. Xylitol-induced elevated expression of the gbpC gene in a population of Streptococcus mutans cells. Eur. J. Oral Sci. 108538-545. [DOI] [PubMed] [Google Scholar]
- 36.Senadheera, M. D., B. Guggenheim, G. A. Spatafora, Y. C. Huang, J. Choi, D. C. Hung, J. S. Treglown, S. D. Goodman, R. P. Ellen, and D. G. Cvitkovitch. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 1874064-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sztajer, H., A. Lemme, R. Vilchez, S. Schulz, R. Geffers, C. Y. Yip, C. M. Levesque, D. G. Cvitkovitch, and I. Wagner-Dobler. 2008. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. J. Bacteriol. 190401-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thenisch, N. L., L. M. Bachmann, T. Imfeld, T. Leisebach Minder, and J. Steurer. 2006. Are mutans streptococci detected in preschool children a reliable predictive factor for dental caries risk? A systematic review. Caries Res. 40366-374. [DOI] [PubMed] [Google Scholar]
- 39.Tsang, P., J. Merritt, T. Nguyen, W. Shi, and F. Qi. 2005. Identification of genes associated with mutacin I production in Streptococcus mutans using random insertional mutagenesis. Microbiology 1513947-3955. [DOI] [PubMed] [Google Scholar]
- 40.Tsang, P., J. Merritt, W. Shi, and F. Qi. 2006. IrvA-dependent and IrvA-independent pathways for mutacin gene regulation in Streptococcus mutans. FEMS Microbiol. Lett. 261231-234. [DOI] [PubMed] [Google Scholar]
- 41.van Houte, J. 1993. Microbiological predictors of caries risk. Adv. Dent. Res. 787-96. [DOI] [PubMed] [Google Scholar]
- 42.Waterhouse, J. C., D. C. Swan, and R. R. Russell. 2007. Comparative genome hybridization of Streptococcus mutans strains. Oral Microbiol. Immunol. 22103-110. [DOI] [PubMed] [Google Scholar]
- 43.Wen, Z. T., and R. A. Burne. 2004. LuxS-mediated signaling in Streptococcus mutans is involved in regulation of acid and oxidative stress tolerance and biofilm formation. J. Bacteriol. 1862682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida, A., T. Ansai, T. Takehara, and H. K. Kuramitsu. 2005. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 712372-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambon, J. J., and S. A. Kasprzak. 1995. The microbiology and histopathology of human root caries. Am. J. Dent. 8323-328. [PubMed] [Google Scholar]