Abstract
The plant growth-promoting rhizobacterium Enterobacter cloacae UW5 synthesizes the plant growth hormone indole-3-acetic acid (IAA) via the indole-3-pyruvate pathway utilizing the enzyme indole-3-pyruvate decarboxylase that is encoded by ipdC. In this bacterium, ipdC expression and IAA production occur in stationary phase and are induced by an exogenous source of tryptophan, conditions that are present in the rhizosphere. The aim of this study was to identify the regulatory protein that controls the expression of ipdC. We identified a sequence in the promoter region of ipdC that is highly similar to the recognition sequence for the Escherichia coli regulatory protein TyrR that regulates genes involved in aromatic amino acid transport and metabolism. Using a tyrR insertional mutant, we demonstrate that TyrR is required for IAA production and for induction of ipdC transcription. TyrR directly induces ipdC expression, as was determined by real-time quantitative reverse transcription-PCR, by ipdC promoter-driven reporter gene activity, and by electrophoretic mobility shift assays. Expression increases in response to tryptophan, phenylalanine, and tyrosine. This suggests that, in addition to its function in plant growth promotion, indolepyruvate decarboxylase may be important for aromatic amino acid uptake and/or metabolism.
Auxins are an important class of phytohormones that are essential for many aspects of plant growth and development, including organogenesis; tropic responses; cellular processes such as cell expansion, division, and differentiation; and gene regulation (1, 9, 21, 25, 60). The predominant natural auxin is indole-3-acetic acid (IAA) (8, 21, 60). In addition to synthesis in plant tissues, many plant-associated bacteria also produce and secrete IAA that can influence the health of host plants. Production of IAA by some phytopathogenic bacteria causes plant diseases such as gall tumor formation by Agrobacterium tumefaciens, Erwinia herbicola pv. gypsophilae, and Pseudomonas syringae pv. savastanoi and necrotic lesions caused by Pseudomonas syringae pv. syringae (15, 16, 35, 38, 42, 63). Loss of the ability to synthesize IAA, through mutagenesis, reduces the virulence of these pathogens (2, 16).
Paradoxically, IAA produced by plant growth-promoting rhizobacteria (PGPR) has been found to enhance host root system development. Plant roots colonized with the PGPR species Azospirillum brasilense Sp6, Enterobacter cloacae UW5, and Pseudomonas putida GR12-2 displayed increases in root hair formation, the number and length of lateral roots, and/or primary root length that were dependent on bacterial IAA production. Mutants that were unable to synthesize IAA did not increase root proliferation (7, 23, 45, 58, 65). Well-developed root systems are important for natural nutrient uptake and for anchoring plants in soil. The differences in the effect of IAA produced by these two groups of bacteria may be due to differences in the levels of IAA production in planta or other contributing factors (46, 57, 65).
A number of IAA biosynthetic pathways have been identified in bacteria, most requiring tryptophan as a precursor. Synthesis via the intermediates indole-3-acetamide or indole-3-pyruvate is widespread among IAA-producing bacteria. Most phytopathogens, such as A. tumefaciens and P. syringae pv. savastonoi, use the indole-3-acetamide pathway to synthesize IAA (32, 54), while the indole-3-pyruvate pathway is found in many PGPR species, including A. brasilense and E. cloacae, and in the nonpathogenic epiphytic bacterium Erwinia herbicola 299R (11, 17, 30, 45, 73). In the latter pathway, the precursor tryptophan is converted to indole-3-pyruvate by tryptophan transaminase, and indole-3-pyruvate is then converted to indole-3-acetaldehyde by indole-3-pyruvate decarboxylase (IPDC). IAA is produced after oxidation of indole-3-acetaldehyde by indole-3-acetaldehyde oxidase. The key enzyme in this pathway, IPDC, is encoded by ipdC, and elimination of ipdC abolishes IAA biosynthesis in E. cloacae UW5 and greatly reduces IAA production in A. brasilense and E. herbicola 299R (11, 14, 17, 45, 51).
Currently, regulation of IAA synthesis in bacteria is not completely understood at the molecular level, although it is clear that synthesis responds to environmental cues. Acidic pH and anaerobic conditions, often encountered in the rhizosphere, increase ipdC expression in A. brasilense Sp245 (43, 61), while in E. herbicola 299R, osmotic stress and low water availability induce ipdC expression (10). High levels of IAA accumulate in culture media only after entrance into the stationary phase in A. brasilense Sp7 and Sp245 and in E. cloacae UW5 (14, 44, 61). Consistent with this, the highest expression levels of ipdC were observed in stationary phase in A. brasilense Sp7 and E. cloacae UW5, and it is known that the stationary-phase sigma factor RpoS upregulates ipdC expression in E. cloacae UW5 and E. herbicola 299R (12, 14, 44). IAA and other auxins induced expression of ipdC in A. brasilense Sp245, and an auxin-responsive element similar to that found in the promoters of some auxin-regulated plant genes was found in the ipdC promoter region (34, 61).
Production of detectable quantities of IAA usually requires an exogenous source of tryptophan which, in the rhizosphere, is present in host root exudates (13, 14, 27, 43, 44, 45, 64, 72, 73). The addition of tryptophan to culture media induced ipdC expression in E. cloacae UW5 and in A. brasilense strain Sp7 (44, 53, 73), although the regulatory proteins that control tryptophan-mediated ipdC expression were not identified in these studies. The upregulation of ipdC by an exogenous inducer that is present in root exudates suggests that the host plant may influence production of IAA in PGPR.
In the present study we have identified the regulatory protein that controls ipdC expression in response to exogenous tryptophan in E. cloacae UW5. The presence of a sequence in the upstream regulatory region of ipdC that is highly similar to the recognition site of the transcriptional regulator TyrR that responds to aromatic amino acids and the responsiveness of ipdC to tryptophan suggested that TyrR may regulate ipdC expression. We confirm here that TyrR is required for activation of ipdC expression and for IAA production.
MATERIALS AND METHODS
Bacterial strains, culture conditions and plasmids.
Bacterial strains and plasmids used and created in the present study are presented in Table 1. Note that E. cloacae UW5 was previously misidentified as Pseudomonas putida (44) but has recently been confirmed to be E. cloacae by 16S rRNA gene sequence analysis (GenBank accession no. EU136677). All bacteria were routinely grown in Luria-Bertani (LB) medium (Fisher Scientific) and M9 glucose minimal salts medium (Difco) at 30°C for E. cloacae or 37°C for Escherichia coli in a shaking incubator at 250 rpm. Bacteria grown in the presence of aromatic amino acids were grown in M9 glucose minimal salts medium supplemented with 200 μg of l-tryptophan, l-phenylalanine, or l-tyrosine/ml. The antibiotic concentrations routinely used were 100 μg of ampicillin/ml, 50 μg of kanamycin/ml, 25 μg of gentamicin/ml, and 5 μg of tetracycline/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Featuresa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| S17-1 (λpir) | Cloning host; λpir recA thi pro hsdR M+ RP4:2-Tc:Mu:Km Tn7TprStrr | 55 |
| JM109 | Cloning host; endA1 recA1 gyrA96 thiA hsdR17 relA1 supE44 Δ(lac-proAB) [F′ traD36 proAB+laqIqlacZ ΔM15] | 71 |
| M15 | Protein expression host; E. coli K-12 Strr F− ΔlacZ (pREP4) | Qiagen |
| E. cloacae | ||
| UW5 | Wild-type strain | 45 |
| J3 | UW5 ipdC::Kmr | 45 |
| J35 | UW5 tyrR::Tcr | This study |
| J51 | J35 ipdC::uidA; Tcr Gmr | This study |
| J55 | UW5 ipdC::uidA; Gmr | This study |
| Plasmids | ||
| pJP2 | Source of Tcr cassette | 50 |
| pGEM-T Easy | Cloning vector; Ampr | Promega |
| pG16 | 124-bp 16S rRNA fragment (amplified with 16S 1369F, and 1492R) in pGEM-T Easy; Ampr | This study |
| pGIC | 120-bp ipdC fragment (amplified with IPRT1F and -1R) in pGEM-T Easy; Ampr | This study |
| pJQ200SK | Low-copy suicide vector; GmrsacB | 52 |
| pJQ200TM | 1,177-bp tyrR fragment with 2.1-kb Tcr cassette insertion cloned into pJQ200SK; Gmr TcrsacB | This study |
| pJQS | pJQ200SK with sacB deletion; Gmr | This study |
| pJQSIPG | ipdC::uidA in pJQS; Gmr | This study |
| pREP4 | Expresses lac repressor in trans; Kmr | Qiagen |
| pQE30 | Protein expression plasmid; N-terminal His6 tag for protein purification; Ampr | Qiagen |
| pQEtyrR | 1,542-bp tyrR coding region in pQE30; Ampr | This study |
Tcr, tetracycline resistance; Ampr, ampicillin resistance; Strr, streptomycin resistance; Kmr, kanamycin resistance; Tpr, trimethoprim resistance; Gmr, gentamicin resistance.
Sequencing and analysis of tyrR.
Degenerate primers (T1F and T1R) were designed based on tyrR sequences from E. coli strains 536, K-12 substrains MG1655 and UT189, Salmonella enterica subsp. enterica serovar Paratyphi A strain ATCC 9150, S. enterica serovar Typhi strain CT18, S. enterica serovar Typhimurium LT2, Shigella boydii Sb227, Shigella flexneri 5 strain 8401, and Shigella sonnei Ss046 (GenBank accession nos. CP000247, U00096, CP000243, AL627270, CP000026, AE008774, CP000036, CP000266, and CP000038) and were used to generate a 1,493-bp product using E. cloacae UW5 genomic DNA as a template. After this fragment was cloned into pGEM-T Easy (Promega), T7 and SP6 primers were used to acquire a partial sequence, and a primer walking strategy was used to obtain the complete sequence of the fragment. The 3′-terminal end of tyrR was not included in the amplicon produced using T1F and T1R primers; therefore, reverse primer tpx1R was designed to a conserved region flanking the 3′ end of tyrR (within the tpx gene), identified using the above-mentioned sequences and Enterobacter sp. strain 638 (GenBank accession no. CP000653), to obtain the complete 1,542-bp tyrR coding sequence. Sequencing was performed by Robarts Research Institute Sequencing Facility (London, Ontario, Canada) that is equipped with an Applied Biosystems 3730 analyzer. Homology searches were performed by using the Basic Local Alignment Search Tool (BLAST) against the GenBank database (3).
Construction of a tyrR insertional mutant.
The activity of tyrR in E. cloacae UW5 was abolished by insertion of a tetracycline resistance cassette into the coding sequence, creating E. cloacae J35. The 2.1-kb tetracycline resistance cassette was amplified by using tet-KpnI F and R primers and pJP2 as the template for PCR and then subcloned into pGEM-T Easy. A 1,177-bp tyrR fragment, amplified using the primers T1F and T4R, was also subcloned into pGEM-T Easy. The tetracycline resistance cassette was excised from pGEM-T Easy and inserted into a native KpnI site in tyrR (708 bp downstream from translation start codon) as a KpnI fragment. The interrupted tyrR gene fragment was excised from pGEM-T Easy and cloned into the NotI site in the suicide plasmid pJQ200SK, creating pJQ200TM. pJQ200TM was transformed into calcium chloride-competent E. coli S17-1 (λpir) cells and subsequently introduced into E. cloacae UW5 by conjugation. Double recombinants were identified by tetracycline resistance and gentamicin sensitivity. The site of insertion in the genome was verified by PCR amplification using the primers T1F and T1R (note that the T1R sequence is not present in pJQ200TM). The PCR amplicon generated from the genome of the tyrR insertional mutant (E. cloacae J35) was 2.1 kb larger than that from the wild-type strain, confirming the replacement of wild-type tyrR with the mutant tyrR gene fragment.
Quantification of IAA production.
E. cloacae UW5 (wild-type), E. cloacae J35 (tyrR), and E. cloacae J3 (ipdC) were grown overnight in LB broth with appropriate antibiotics, pelleted, and washed twice with saline (0.85% NaCl). Cultures were diluted 1,000-fold into 3 ml of M9 glucose minimal medium with or without 200 μg of tryptophan/ml. After further incubation for 44 h, Salkowski's method (24) was used to quantitate IAA production as follows. Bacterial cells were removed from the culture medium by centrifugation, and then 800 μl of Salkowski's reagent (150 ml of concentrated H2SO4, 250 ml of double-distilled H2O, and 7.5 ml of 0.5 M FeCl3·6H2O) was mixed with 200 μl of culture supernatant, followed by incubation at room temperature for 20 min. The absorbance at 535 nm was read with a Novaspec II spectrophotometer using M9 glucose minimal medium with or without tryptophan as references. The concentration of IAA in each culture medium was determined by comparison to a standard curve generated from known concentrations of IAA. The absorbance at 600 nm was also recorded at hourly intervals up to 24 h and then at 34 and 44 h to generate growth curves.
RNA extraction and real-time quantitative reverse transcriptase PCR (qRT-PCR).
RNA was extracted from E. cloacae UW5 and E. cloacae J35 cells grown in M9 glucose minimal medium with or without tryptophan as described above. Log-phase cultures (3 ml) were collected at 8 h, and stationary-phase cultures (1.5 ml) were collected at 44 h. Cells were pelleted and treated with 1 ml of RNAprotect (Qiagen) as recommended by the supplier. Pelleted cells were resuspended in 700 μl of homogenization buffer (0.1 M NaCl, 2% sodium dodecyl sulfate, 50 mM Tris-HCl [pH 9.0], 10 mM EDTA) and incubated at 80°C to lyse the cells (up to 30 min). Nucleic acids were extracted with 700 μl of phenol-chloroform-isoamyl alcohol (124:25:1; pH 4.3). After separation of the organic phase by centrifugation, 500 μl of the aqueous layer was retrieved and subjected to overnight precipitation at −20°C with 1 M LiCl and 62.5% ethanol. After centrifugation for 20 min at 13,000 rpm, the nucleic acid pellet was treated with up to 40 U of DNase I (Invitrogen) for 1.5 h at 37°C in a total volume of 200 μl to remove residual DNA. RNA was extracted with 200 μl of phenol-chloroform-isoamyl alcohol (124:25:1; pH 4.3) and pelleted as mentioned above. The final RNA pellet was resuspended in diethyl pyrocarbonate-treated double-distilled H2O and quantified spectrophotometrically at 260 nm (using a Beckman Coulter DU series 700 spectrophotometer).
cDNA was generated using 1 μg of RNA; the gene-specific reverse primers ICRT1R and 16S 1492R for ipdC and 16S cDNA, respectively; and SuperScript III reverse transcriptase (Invitrogen) according to the supplier's recommendations. Quantitative PCR was performed with Invitrogen's Platinum SYBR green qPCR SuperMix-UDG using cDNA prepared from 100 ng of RNA. The primers ICRT1F and ICRT1R were used to amplify a 120-bp fragment from the ipdC cDNA sequence, and the primers 16S 1369F and 1492R were used to amplify a 124-bp fragment from the 16S cDNA sequence for normalization. The cycling conditions were as follows: 2 min at 50°C; 2 min at 95°C; and 45 cycles of 95°C for 15 s, 60°C (ipdC) or 50°C (16S) for 30 s, and 72°C for 10 s. Real-time quantification of amplicons was performed by using a Rotor-Gene 6000 thermal cycler (Corbett Life Science, Sydney, Australia). cDNA preparations from each independent triplicate RNA extraction were measured in duplicate, and 100 ng of each DNase I-treated RNA preparation, prior to cDNA preparation, was used as a template for quantitative PCR to verify the lack of DNA contamination in each extraction. Amplification products were subjected to melting-curve analysis to confirm the specificity of the amplicons. Standard curves, prepared in duplicate from known copy numbers (to 109 copies) of plasmids pGIC and pG16 for ipdC and 16S rRNA, respectively, were used to extrapolate absolute levels of expression.
Quantification of ipdC promoter-driven reporter gene expression.
ipdC promoter-driven expression was quantified by using β-glucuronidase, encoded by uidA. The 1.8-kb uidA gene was amplified by PCR using U1F-PacI and U1R primers and pJP2 as a template. A 520-bp fragment containing the ipdC promoter sequence was amplified by using the primers IP1F and IP1R-PacI. Both PCR fragments were digested with PacI, purified (PCR purification kit; Qiagen), and ligated together using T4 DNA ligase. To increase the yield of the reporter gene construct, splicing-by-overlapping-extension (SOE) PCR was performed on the ligated product using IP1F and U1R primers. The 2.3-kb ipdC promoter-uidA SOE PCR products were gel extracted (QIAquick gel extraction kit; Qiagen) and subcloned into pGEM-T Easy. The ipdC promoter-uidA construct was excised from pGEM-T Easy and inserted into suicide plasmid pJQS as an ApaI and PstI fragment, creating pJQSIPG. Plasmid pJQS was created by sequential double digestion with AclI and BspMI to remove sacB from pJQ200SK, filling in the sticky ends produced by the restriction enzymes using Klenow polymerase, and then the plasmid was recircularized by blunt end ligation. pJQSIPG was transformed into calcium chloride-competent E. coli S17-1 (λpir) and then transferred to E. cloacae UW5 and E. cloacae J35 by conjugation. Transconjugants were screened for gentamicin resistance, an indication of plasmid integration into the genome. PCR amplification using the primers IP2F (binds to a region upstream of the ipdC promoter sequence not present in pJQSIPG) and U2R (binds within uidA) yielded a 733-bp fragment that confirmed the site of recombination.
E. cloacae J55 and E. cloacae J51, carrying the ipdC-uidA fusion in E. cloacae UW5 and J35, respectively, were grown in LB broth with antibiotics overnight, pelleted, and washed twice with saline (0.85% NaCl). Cells were diluted 1,000-fold into 3 ml of M9 minimal medium with or without 200 μg of tryptophan, phenylalanine, or tyrosine/ml. β-Glucuronidase activity was assayed in stationary-phase cells after 44 h of growth by using the method reported by Cowie et al. (18). Briefly, 80 μl of reaction buffer (50 mM sodium phosphate [pH 7], 50 mM dithiothreitol, 1 mM EDTA, 0.0125% sodium dodecyl sulfate) containing 0.44 mg of p-nitrophenyl β-d-glucuronide (PNPG)/ml was mixed with 20 μl of cell culture in 96-well microtiter plates (CellStar; Greiner Bio One, Frickenhausen, Germany), followed by incubation at room temperature for approximately 1 h. After the addition of 100 μl of 1 M Na2CO3 to stop the enzymatic reaction, the absorbance at 405 nm was read in a SpectroMax M5 spectrophotometer (Molecular Devices). The specific activity (in Miller units) was calculated as (1,000 × OD405)/(time × OD600 × volume of culture in reaction in ml). Wild-type E. cloacae UW5 and uninoculated medium were included as controls to assess background levels of absorbance at 405 nm.
TyrR purification and electrophoretic mobility shift assays (EMSAs).
The T1F-BamHI and T5R-PstI primers were used to amplify the entire tyrR coding sequence, and the resulting PCR fragment was cloned as a BamHI-PstI fragment into pQE30, which harbors the sequence for an N-terminal His6 tag (Qiagen). An N-terminal His6 tag was chosen to avoid impacting the function of TyrR's DNA-binding motif, which is located in the C-terminal domain (26, 67). The resulting plasmid, pQEtyrR, was transformed into calcium chloride-competent E. coli M15(pREP4). Expression of His6-TyrR was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the protein was purified by using Ni-NTA (Qiagen). Instructions provided by the supplier were followed for purification, with the exception of a modified wash protocol to eliminate nonspecific protein binding to the Ni-NTA. Wash buffer 1 (WB1) consisted of 50 mM NaH2PO4, 300 mM NaCl, and 50 mM imidazole (pH 8.0); WB2 was as described for WB1 but with the addition of 10% glycerol; WB3 was as described for WB2 but with an increase in NaCl to 1 M; and WB4 was as described for WB3 but with 100 mM imidazole. Each wash buffer was applied twice to the column. Elution of His6-TyrR was performed according to the supplier's protocol. Bradford reagent (Bio-Rad) was used to determine the concentration of the purified protein (31).
The ability of TyrR to bind to the ipdC promoter region was tested by using EMSAs. Three oligonucleotides were used, all derived from sequences found in the ipdC promoter: IP3, CAGCCTTTTTTGTAAAGCATTCTTTCCATGCCCTTCTT; IP3M, CAGCCTTTTTTaTAAAGCATTCTTTCtATGCCCTTCTT; and IP5, GAAAAATCAGTGTATACGTTTACATTTACATGAAAAAAAA. The locations of these sequences in the ipdC promoter region are depicted in Fig. 1A. Underlined in IP3 and IP3M is the putative TyrR box. Bases known to be critical in the interaction between TyrR boxes and TyrR in E. coli (26, 39) were substituted in IP3M (lowercase nucleotides in the sequences above and shown below the wild-type sequence in Fig. 1A). IP5 is a sequence that is found upstream of IP3 in the E. cloacae UW5 genome that possesses a sequence similar to the TyrR box consensus sequence; however, the length of the sequence is not optimal for TyrR interaction (underlined in the IP5 sequence above) (26, 39, 49). Single-stranded oligonucleotides were synthesized with a digoxigenin (DIG) moiety at the 5′ end (Sigma Proligo, Toronto, Ontario, Canada) and annealed to their complementary unlabeled oligonucleotides (IP3rc, IP3Mrc, and IP5rc) (Sigma Genosys, Toronto, Ontario, Canada) to generate double-stranded fragments for the EMSAs.
FIG. 1.
(A) Nucleotide sequence of the region upstream of ipdC in E. cloacae UW5 (GenBank accession no. AF285632). The putative TyrR box is indicated in boldface and underlined, the translational start site is underlined, the 5′ partial coding sequence is capitalized, and putative ribosome-binding sites are highlighted in boldface and underlined. The location of IP5, IP3, and IP3M used in EMSAs are overlined. Nucleotide substitutions and locations of mutations present in IP3M are shown below the wild-type IP3 sequence. (B) Alignment of ipdC promoter sequences from E. cloacae UW5 (Ec UW5), Enterobacter sp. strain 638 (E 638), S. enterica subsp. enterica serovar Paratyphi A (Sp A), and serovar Typhimurium LT2 (Sp LT2) (GenBank accession nos. AF285632, CP000653, CP000026, and AE008808, respectively). The consensus sequence for the TyrR binding site in E. coli (cons) is shown. Shaded in gray are the conserved putative TyrR boxes in the ipdC promoter sequences.
To assess TyrR binding, purified His6-TyrR (90 nM), 1 μg of poly(dI-dC), 0.1 mM ATP, and a 0.1 mM concentration of either l-tryptophan, l-phenylalanine, or l-tyrosine were combined with 4 μl of 5× binding buffer (20% glycerol, 250 mM NaCl, 50 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 2.5 mM dithiothreitol, 2.5 mM EDTA) to a final volume of 19 μl, followed by incubation for 5 min at room temperature. Then, 1 μl (3 ng) of DIG-labeled oligonucleotide IP3, IP3M, or IP5 was added, followed by incubation for 30 min at room temperature. Next, 5 μl of 5× gel loading buffer (0.25× Tris-borate-EDTA, 60% glycerol, 0.02% bromophenol blue) was added to stop the reaction. A competition assay was also performed to ensure that TyrR binding to the IP3 promoter sequence was sequence specific. The reaction mixtures were similar to those described above except that 1.5, 3, or 6 ng of unlabeled double-stranded IP3 was first added to each reaction, followed by incubation for 5 min at room temperature. Lastly, 1 μl of 5′ DIG IP3 was added, followed by incubation for 30 min at room temperature.
Binding reaction mixtures were run on 6% nondenaturing gels (6% acrylamide-bisacrylamide [37.5:1]; 2.5% glycerol; 0.15% ammonium persulfate; 0.1 mM ATP; 0.1 mM l-tryptophan, l-phenylalanine, or l-tyrosine; 0.5× Tris-borate-EDTA; 0.1% TEMED [N,N,N′,N′-tetramethylethylenediamine]) at 50 V for approximately 2.5 h. A Trans-Blot SD Semi-Dry electrophoretic transfer cell (Bio-Rad) was used to transfer the DIG-labeled oligonucleotides to positively charged nylon membranes (Roche), and these were visualized by using anti-DIG-alkaline phosphatase antibodies and NBT/BCIP according to the manufacturer's instructions (Roche).
RESULTS
The ipdC promoter contains a putative TyrR box.
The promoter region of ipdC in E. cloacae UW5 contains a sequence that is highly similar to the TyrR recognition sequence in E. coli (TGTAAA-N6-TTTACA), known as the TyrR box, shown in Fig. 1A (22, 28, 49). This box is centered 91 bp upstream of the ipdC start codon (Fig. 1A). Sequences matching the TyrR box are also found in the region upstream of genes annotated as ipdC in the genomic sequences of several closely related bacteria, Enterobacter sp. strain 638, S. enterica subsp. enterica serovar Paratyphi A, and S. enterica serovar Typhimurium LT2, although the sequence in this region is otherwise not well conserved (Fig. 1B). Conservation of these nucleotides upstream of the ipdC coding region suggests that they may be important for the regulation of ipdC expression and that TyrR may be required for ipdC transcription.
Sequence analysis of tyrR.
The tyrR coding sequence (GenBank accession no. EU570974) in E. cloacae UW5 is 1,542 bp and is predicted to encode a 513-amino-acid protein with a molecular mass of 57.5 kDa. The nucleotide sequence shares identity with tyrR of other enteric bacteria, including Enterobacter sp. strain 638 (81%), Citrobacter koseri (80%), serovar Typhimurium (79%), S. enterica subsp. enterica serovar Paratyphi A (79%) and B (78%), Klebsiella pneumoniae subsp. pneumoniae (78%), Citrobacter braakii (78%), E. coli (78%), Enterobacter sakazakii (76%), and the Shigella species S. flexneri, S. dysenteriae, S. sonnei, and S. boydii (77%). TyrR is a regulatory protein that interacts with aromatic amino acid effector molecules and is both an activator and a repressor of gene expression (19, 22, 47, 49). The structure of TyrR is divided into three domains. The N-terminal domain of TyrR in E. coli was shown to be vital for activation of gene expression and is known to interact with the α-subunit of RNA polymerase. This domain is also required for dimerization and contains an ATP-independent binding site for aromatic amino acids (20, 22, 37, 48, 49, 66, 70). An ATP binding site and an ATP-dependent tyrosine binding site are located in the central domain and are required for tyrosine-dependent hexamerization that results in gene repression (22, 33, 67). The C-terminal domain contains a helix-turn-helix motif responsible for DNA binding (26, 67). Analysis of the E. cloacae UW5 TyrR amino acid sequence using InterProScan (http://www.ebi.ac.uk/InterProScan/; performed May 2008) confirmed the presence of an N-terminal amino acid binding domain, an ATP binding and hydrolyzing AAA-ATPase core in the central domain, and a DNA-binding motif in the C-terminal domain. Interestingly, the central domain also contains a putative RpoN (σ54) domain, although, to date, TyrR has only been shown to interact with RpoD (σ70) (22, 33, 48). The central domain shares homology with RpoN interacting regulatory proteins NtrC (activates expression of nitrogen-responsive genes) and a TyrR homologue, PhhR (regulates phenol degradation), in pseudomonads (6, 22, 40, 41, 49, 56).
TyrR is required for IAA biosynthesis.
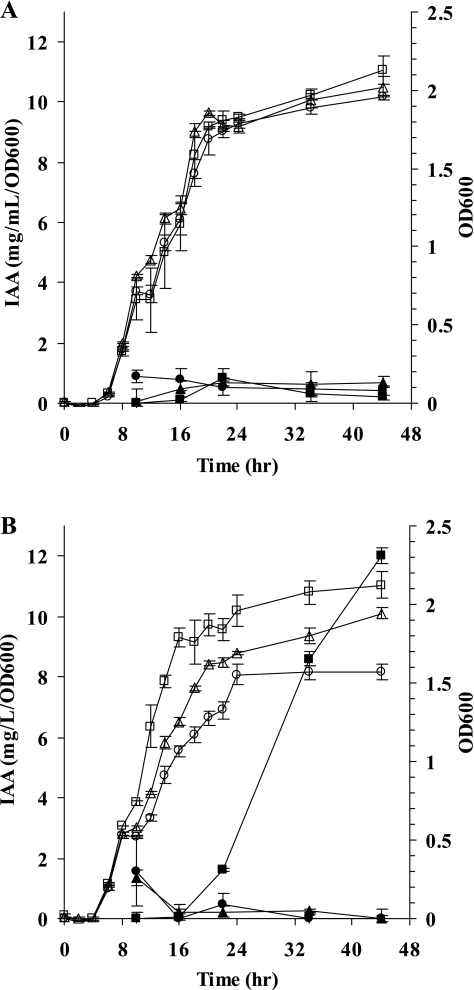
To test the hypothesis that TyrR regulates IAA production, a tyrR insertional mutant, E. cloacae J35, was generated. Accumulation of IAA in the culture medium of wild-type E. cloacae UW5 occurred only in the presence of tryptophan after entrance into stationary phase as previously described (Fig. 2) (44). In contrast, neither tyrR mutant E. cloacae J35 nor ipdC mutant E. cloacae J3 produced detectable levels of IAA in the presence or absence of tryptophan over a period of 44 h that encompasses both logarithmic and stationary phases of growth (Fig. 2). Although E. cloacae strains J35 and J3 had longer generation times than the wild-type strain when grown in M9 glucose minimal medium with 200 μg of l-tryptophan/ml (434, 315, and 286 min for E. cloacae strains J35, J3, and UW5, respectively), all three strains entered stationary phase within 24 h. These results indicate that TyrR and tryptophan are required for IAA production.
FIG. 2.
Growth (open symbols) and IAA produced (closed symbols) by E. cloacae UW5 (squares), E. cloacae J35 (circles), and E. cloacae J3 (triangles) in the absence (A) or presence (B) of 200 μg of l-tryptophan/ml over 44 h. The data points represent the average from three independent colonies of each strain. Error bars indicate the standard errors of the mean.
TyrR induces ipdC expression.
The requirement for TyrR for IAA production and the presence of a sequence matching the TyrR box consensus sequence in the ipdC promoter region suggested that TyrR may regulate transcription of ipdC that encodes a key enzyme in the IAA biosynthetic pathway (44). Quantification of ipdC transcript levels by real-time qRT-PCR revealed that ipdC transcript levels were highest in wild-type E. cloacae UW5 cells grown to stationary phase in media supplemented with tryptophan (Table 3), as was predicted from the high levels of IAA produced under these conditions. In contrast, in the absence of TyrR, in mutant E. cloacae J35, ipdC was expressed only at low levels (ca. 1 to 1.5 transcripts/million transcripts of 16S rRNA) in both log and stationary phases even in tryptophan-supplemented cultures. Similarly low levels of ipdC expression were also seen in log-phase wild-type cultures grown in the absence of tryptophan, conditions under which IAA is not normally produced. Although IAA was not produced by wild-type cultures in log-phase grown with tryptophan or in stationary phase in the absence of exogenous tryptophan, transcription of ipdC was apparent under both conditions, albeit at lower levels than the transcript levels produced by stationary-phase cultures supplemented with tryptophan (Table 3). These results confirm that activation of ipdC transcription is TyrR dependent. They also suggest that even when conditions are appropriate for IAA production, that is, when exogenous tryptophan is present, TyrR is produced (tyrR mRNA levels are relatively constant throughout the E. cloacae UW5 growth cycle; data not shown), and ipdC is expressed, cells must be in stationary phase before IAA is synthesized. It is possible that regulation of IAA production involves other factors in addition to TyrR.
TABLE 3.
Levels of ipdC transcription in wild-type E. cloacae UW5 and tyrR mutant E. cloacae J35 as determined by real-time qRT-PCR analysis
| Growth phasea | Trpb | No. of ipdC transcripts/million transcripts of 16S rRNA ± SEc
|
|
|---|---|---|---|
| UW5 | J35 | ||
| Log | - | 1.6 ± 0.5 | 1.1 ± 0.1 |
| + | 9.3 ± 0.6 | 1.0 ± 0.1 | |
| Stationary | - | 7.2 ± 1.8 | 1.0 ± 0.2 |
| + | 23.2 ± 4.4 | 1.5 ± 0.1 | |
RNA was extracted from log- and stationary-phase cells at 8 h (average OD600 = 0.5) and 44 h (average OD600 = 2.0), respectively, postinoculation.
Cultures were grown in M9 glucose without (-) or with (+) 200 μg of l-tryptophan (Trp)/ml.
The values shown are the means of at least three independent replicates.
Expression of ipdC is upregulated by aromatic amino acids.
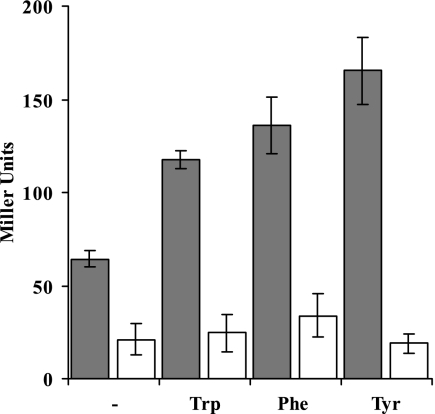
To further investigate TyrR-dependent ipdC activation, ipdC promoter-driven reporter gene assays were performed. Measurement of β-glucuronidase activity in ipdC::uidA transcriptional fusions confirmed the results from real-time qRT-PCR analysis that upregulation of ipdC expression in stationary phase is TyrR dependent and that high levels of expression required an exogenous source of tryptophan (Fig. 3). Because TyrR is known to regulate gene expression in response to all three aromatic amino acids in E. coli (48, 49), ipdC promoter activity was also quantified in E. cloacae UW5 in response to phenylalanine and tyrosine. Both amino acids increased TyrR-dependent ipdC expression to levels comparable to those expressed in response to tryptophan (Fig. 3). The finding that ipdC expression increased in response to all three aromatic amino acids suggests that this gene may be important in aromatic amino acid metabolism in this bacterium.
FIG. 3.
ipdC promoter driven β-glucuronidase activity in E. cloacae J55 (░⃞) and E. cloacae J51 (□) in stationary phase. Bacteria were grown for 44 h in M9 glucose minimal medium with 200 μg of l-tryptophan, l-phenylalanine, or l-tyrosine/ml or in the absence of exogenously supplied aromatic amino acids. The data shown are the averages of three independent replicates, and error bars represent the standard errors of the mean.
TyrR binds to the ipdC promoter.
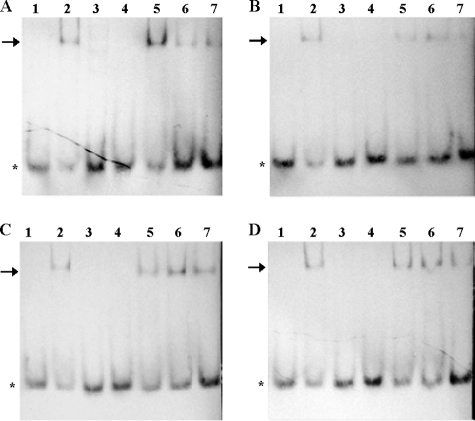
To differentiate between ipdC activation as a consequence of a direct interaction between TyrR and the ipdC promoter, or regulation at a point upstream of ipdC activation, EMSAs were used to assess the ability of TyrR to bind to the putative TyrR box found in the ipdC upstream sequence (see Fig. 1A for the promoter sequences used). Purified His6-TyrR bound to oligonucleotide fragment IP3, containing a putative TyrR box, in the presence of tryptophan, phenylalanine, or tyrosine (lane 2 in Fig. 4B to D). As was predicted from the nucleotide sequence, the TyrR box in the ipdC promoter is a strong box since TyrR also bound to IP3 in the absence of aromatic amino acids (Fig. 4A, lane 2). This corroborates our observations from expression studies that showed that while aromatic amino acids supplied exogenously increased ipdC expression, they were not absolutely required for ipdC promoter activity. This is consistent with binding studies in E. coli that showed that TyrR can bind to strong boxes, sequences that are highly similar to the consensus sequence (TGTAAA-N6-TTTACA), without binding aromatic amino acids. Binding of aromatic amino acids to TyrR increases its affinity for DNA binding, especially at weak TyrR boxes (4, 49). Binding of His6-TyrR to IP3 was shown to be sequence specific since the addition of TyrR did not cause a shift when incubated with fragment IP3M containing the promoter sequence with a mutated TyrR box (Fig. 4, lanes 3). In this fragment, two nucleotides that are essential for TyrR binding in E. coli, G near the 5′ end of the TyrR box and C near the 3′ end (26, 39), were replaced with A and T, respectively (Fig. 1A). The specificity of the interaction was further supported by competition assays performed with 0.5:1, 1:1, and 2:1 molar ratios of unlabeled IP3 to 5′-DIG-labeled IP3; as the proportion of unlabeled IP3 increased, the amount of 5′-DIG-labeled IP3 bound to TyrR decreased (Fig. 4A to D, lanes 5 to 7). A sequence similar to the TyrR box consensus sequence but with a nonoptimal length for TyrR interaction (IP5) was also assessed but did not bind to TyrR (Fig. 4A to D, lane 4). These results confirm that TyrR directly activates expression from the ipdC promoter in E. cloacae UW5.
FIG. 4.
Binding of purified TyrR to the ipdC promoter in the presence of aromatic amino acids as determined by EMSAs. The conditions tested in these assays were as follows: no aromatic amino acids (A), 0.1 mM l-tryptophan (B), 0.1 mM l-phenylalanine (C), and 0.1 mM l-tyrosine (D). Lanes 1 to 4 contained 90 nM His6-TyrR with 3 ng of 5′ -DIG-labeled ipdC promoter sequence as follows: lane 1, IP3; lane 2, IP3 plus TyrR; lane 3, IP3M plus TyrR; and lane 4, IP5 plus TyrR. Competition assays were conducted with 90 nM His6-TyrR and 3 ng of 5′-DIG-labeled IP3 and various amounts of unlabeled IP3 as follows: lane 5, 1.5 ng; lane 6, 3 ng; and lane 7, 6 ng. The asterisks and arrows show the location of free and TyrR-bound 5′DIG-labeled IP3, respectively.
DISCUSSION
IAA production is widespread among PGPR and its positive effects on plant growth have been well documented (for reviews, see references 36, 46, 57, and 62). Bacterial IAA has been shown to enhance the development of the host plant root system (7, 23, 45, 58, 65), which increases the surface area through which soil nutrients are absorbed and helps to establish young seedlings firmly in soil. Thus, IAA-producing PGPR contribute to increased yields of healthy crops and are of interest as a replacement for chemical fertilizers in agricultural applications. Optimal exploitation of the beneficial effects of PGPR can be accomplished only if the factors that induce IAA-producing pathways are fully understood. This is especially important because IAA is also a virulence factor in plant pathogens, and higher levels of IAA production in planta have been proposed to explain the differential effect on plants (46, 57, 65). Several studies have shown that environmental signals present in the rhizosphere increase bacterial IAA production; however, the mechanisms by which these signals are transduced in bacteria are not well understood.
In many PGPR, IAA is synthesized only when tryptophan is supplied in the culture medium (13, 14, 43, 44, 64, 73). This may be due to increased availability of the biosynthetic precursor tryptophan and/or to a requirement for tryptophan for induction of the genes in the IAA biosynthetic pathway. It has been shown that the expression of ipdC encoding indole-3-pyruvate decarboxylase, a key enzyme in the IAA biosynthetic pathway of some PGPRs, including E. cloacae UW5 and A. brasilense Sp7, is upregulated by tryptophan (44, 53, 73). In the rhizosphere, tryptophan is present in root exudates and from dead microorganisms and plant tissue (27, 72), suggesting that a signal for upregulation of the indole-3-pyruvate pathway and, therefore, IAA production, in PGPR is present in the rhizosphere. Indeed, ipdC-driven reporter gene expression studies have shown that expression of ipdC in E. herbicola 299R is plant inducible. Induction of ipdC increased by a factor of 32 on bean and tobacco leaves, and 1,000-fold on pear flowers compared to induction in culture medium (10, 12).
We show here that the transcription factor TyrR directly and positively controls ipdC expression and IAA production in the PGPR E. cloacae UW5 and that TyrR-dependent expression increases in response to exogenous tryptophan. A sequence with only a single base mismatch to the consensus sequence for the TyrR box (TGTAAA-N6-TTTACA) in E. coli (49) was identified in the promoter region of ipdC in this bacterium and in other closely related bacteria, even though the promoter sequences were otherwise quite dissimilar. Loss of IAA production and lower levels of ipdC expression following disruption of TyrR function through insertional mutagenesis in mutant strain E. cloacae J35 confirmed the requirement for TyrR. The high degree of nucleotide sequence identity to the consensus sequence for the TyrR binding site suggests a strong TyrR protein-promoter DNA interaction. This is supported by the ability of purified TyrR to bind to the ipdC promoter fragment containing the TyrR box in vitro, and by the induction of ipdC expression, in the absence of an effector molecule. The observed transcription of ipdC in the absence of tryptophan supplements to the culture medium may be mediated by binding of endogenous aromatic amino acid cofactors to TyrR. Although TyrR can bind to strong boxes in the absence of cofactors, the addition of aromatic amino acids strengthens the interaction between TyrR and its recognition sequence (4, 49). The increased affinity of TyrR for the promoter results in increased transcription, as was observed here by an increase in ipdC transcript abundance measured by real-time qRT-PCR and by an increase in ipdC promoter-driven β-glucuronidase activity after addition of the TyrR cofactor tryptophan. The single base mismatch, at nucleotide position 15 in the TyrR box, is at a noncritical position for TyrR binding, whereas mutations introduced into positions previously determined to be essential (5, 26, 49) abolished TyrR binding to the ipdC promoter.
Much of what we know about regulation by TyrR has been determined in E. coli; however, E. cloacae is closely related to E. coli and is also a member of the Enterobacteriaceae, and therefore regulation may be similar. TyrR has a rather complex mode of regulation. All three aromatic amino acids can bind to TyrR and act as cofactors for either repression or activation of the TyrR regulon. Often a single promoter can be both induced and repressed by TyrR, and each of the aromatic amino acids can differentially regulate expression as is exemplified by the tyrP promoter (69). Genes known to be repressed by TyrR in E. coli include tyrR itself, aroF, aroL, tyrP, aroP, tyrB, and aroG; all are involved with aromatic amino acid synthesis and transport (22, 37, 48, 49). Of the four E. coli genes that are positively regulated by TyrR, three, namely, aroP, mtr, and tyrP, encode aromatic amino acid transporters (49). A fourth positively regulated member of the E. coli TyrR regulon, folA, catalyzes the reduction of dihydrofolate to tetrahydrofolate an important intermediate required for synthesis of folate from chorismate in E. coli (68). Previously, TyrR was thought to regulate only promoters that interact with the housekeeping sigma factor, RpoD (22, 33, 48). The addition of ipdC, which is induced by the stationary-phase sigma factor RpoS (12, 44), to the TyrR regulon reveals that TyrR can also regulate RpoS-responsive genes.
A broader function for IPDC in aromatic amino acid transport or metabolism is suggested by the positive TyrR-dependent response of the ipdC promoter to all three aromatic amino acids. The induction of ipdC by aromatic amino acids has also been shown in A. brasilense Sp7 (53). Consistent with this, Spaepen et al. (59) propose that the A. brasilense indolepyruvate decarboxylase is a phenylpyruvate decarboxylase based on the higher rate of catalysis with the substrate phenylpyruvate, derived from phenylalanine, compared to indolepyruvate, although Koga et al. (29) had previously determined that phenylpyruvate is not a substrate for indolepyruvate decarboxylase in E. cloacae. The increased transcription of ipdC in the presence of tyrosine observed here probably reflects the increased strength of promoter binding by TyrR, which binds as a hexamer when bound to tyrosine (49).
The ipdC gene encoding indole-3-pyruvate decarboxylase is a newly recognized member of the TyrR regulon. Consistent with other members of this regulon, ipdC is involved in the metabolism of tryptophan, and possibly other aromatic compounds. Soil bacteria are attracted to nutrients such as amino acids in root exudates that not only supply carbon, nitrogen, and energy but also influence bacterial gene expression. Exogenous aromatic amino acids induce indolepyruvate decarboxylase expression, via TyrR, that leads to production and secretion of IAA, and perhaps other compounds, that benefit the host plant.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| 16S 1492R | GGWTACCTTGTTACGACTT |
| 16S 1369F | CGGTGAATACGTTCYCGG |
| ICRT1F | TCGAACTCAGCAAACAGCAC |
| ICRT1R | AGGTTTGCAACGTTCTCCAG |
| IP1F | GCCATGGCAGGAAATCTTC |
| IP1R-PacI | GCTCGCTTAATTAAGACGGTCCAGCAGGTAATG |
| IP2F | ACATATTCCAGCCCCATACG |
| IP3 | CAGCCTTTTTTGTAAAGCATTCTTTCCATGCCCTTCTT |
| IP3rc | AAGAAGGGCATGGAAAGAATGCTTTACAAAAAAGGCTG |
| IP3M | CAGCCTTTTTTATAAAGCATTCTTTCTATGCCCTTCTT |
| IP3Mrc | AAGAAGGGCATAGAAAGAATGCTTTATAAAAAAGGCTG |
| IP5 | GAAAAATCAGTGTATACGTTTACATTTACATGAAAAAAAA |
| IP5rc | TTTTTTTTCATGTAAATGTAAACGTATACACTGATTTTTC |
| SP6 | GATTTAGGTGACACTATAG |
| T1F | ATGCGTYTGGAAGTCTTTTGTG |
| T1F-BamHI | TGGTACGGATCCATGCGTYTGGAAGTCTTTTGTG |
| T1R | GCAATCGCGGTRTGYGAWAC |
| T4R | CCTGCTCGTCGGCAAAGC |
| T5R-PstI | AGTCTACTGCAGTTCGTCACCCTTCTTCTGATT |
| T7 | TAATACGACTCACTATAGGG |
| tet-KpnI F | GCTAGGTACCTCTTCACGTTCTGCCTTGCG |
| tet-KpnI R | GTTAGGTACCACGCTAGGGCAGGGCATG |
| tpx1R | TCACCMMCGARCCGGATTAC |
| U1F-PacI | GTCCGCTTAATTAATAGGTGAGTGAGAGGAAACAGCTATGGTCCGTCCTGTAGAAACCCCAACa |
| U1R | TCATTGTTTGCCTCCCTG |
| U2R | TTCCACAGTTTTCGCGATCC |
Stop codons in all three reading frames and a ribosome-binding site are indicated in boldface for U1F-PacI.
Acknowledgments
This study was supported by grants to C.L.P. and a scholarship to R.J.R. from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Published ahead of print on 29 August 2008.
REFERENCES
- 1.Abel, S., and A. Theologis. 1996. Early genes and auxin action. Plant Physiol. 1119-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyoshi, D. E., R. O. Morris, R. Hinz, B. S. Mischke, T. Kosuge, D. J. Garfinkel, M. P. Gordon, and E. W. Nester. 1983. Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc. Natl. Acad. Sci. USA 80407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 253389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews, A. E., B. Lawley, and A. J. Pittard. 1991. Mutational analysis of repression and activation of the tyrP gene in Escherichia coli. J. Bacteriol. 1735068-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews, A. E., B. Dickson, B. Lawley, C. Cobbett, and A. J. Pittard. 1991. Importance of the position of TyrR boxes for repression and activation of the tyrP and aroF genes in Escherichia coli. J. Bacteriol. 1735079-5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin, S., and R. Dixon. 1992. The prokaryotic enhancer binding protein NTRC has an ATPase activity which is phosphorylation and DNA dependent. EMBO J. 112219-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbieri, P., T. Zanelli, E. Galli, and G. Zanetti. 1986. Wheat inoculation with Azospirillum brasilense Sp6 and some mutants altered in nitrogen fixation and indole-3-acetic acid production. FEMS Microbiol. Lett. 3687-90. [Google Scholar]
- 8.Bartel, B. 1997. Auxin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 4851-66. [DOI] [PubMed] [Google Scholar]
- 9.Benjamins, R., and B. Scheres. 2008. Auxin: the looping star in plant development. Annu. Rev. Plant Biol. 59443-465. [DOI] [PubMed] [Google Scholar]
- 10.Brandl, M. T., and S. E. Lindow. 1997. Environmental signals modulate the expression of an indole-3-acetic acid biosynthetic gene in Erwinia herbicola. Mol. Plant-Microbe Interact. 10499-505. [DOI] [PubMed] [Google Scholar]
- 11.Brandl, M. T., and S. E. Lindow. 1996. Cloning and characterization of a locus encoding an indolepyruvate decarboxylase involved in indole-3-acetic acid synthesis in Erwinia herbicola. Appl. Environ. Microbiol. 624121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandl, M. T., B. Quinones, and S. E. Lindow. 2001. Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc. Natl. Acad. Sci. USA 983454-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandl, M. T., E. M. Clark, and S. E. Lindow. 1996. Characterization of the indole-3-acetic acid (IAA) biosynthetic pathway in an epiphytic strain of Erwinia herbicola and IAA production in vitro. Can. J. Microbiol. 42586-592. [Google Scholar]
- 14.Carreno-Lopez, R., N. Campos-Reales, C. Elmerich, and B. E. Baca. 2000. Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in Azospirillum brasilense. Mol. Gen. Genet. 264521-530. [DOI] [PubMed] [Google Scholar]
- 15.Clark, E., S. Manulis, Y. Ophir, I. Barash, and Y. Gafni. 1993. Cloning and characterization of iaaM and iaaH from Erwinia herbicola pathovar gypsophilae. Mol. Plant Pathol. 83234-240. [Google Scholar]
- 16.Comai, L., and T. Kosuge. 1982. Cloning characterization of iaaM, a virulence determinant of Pseudomonas savastanoi. J. Bacteriol. 14940-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costacurta, A., V. Keijers, and J. Vanderleyden. 1994. Molecular cloning and sequence analysis of an Azospirillum brasilense indole-3-pyruvate decarboxylase gene. Mol. Gen. Genet. 243463-472. [DOI] [PubMed] [Google Scholar]
- 18.Cowie, A., J. Cheng, C. D. Sibley, Y. Fong, R. Zaheer, C. L. Patten, R. M. Morton, G. B. Golding, and T. M. Finan. 2006. An integrated approach to functional genomics: construction of a novel reporter gene fusion library for Sinorhizobium meliloti. Appl. Environ. Microbiol. 727156-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui, J., and R. L. Somerville. 1993. Mutational uncoupling of the transcriptional activation function of the TyrR protein of Escherichia coli K-12 from the repression function. J. Bacteriol. 175303-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui, J., L. Ni, and R. L. Somerville. 1993. ATPase activity of TyrR, a transcriptional regulatory protein for sigma 70 RNA polymerase. J. Biol. Chem. 26813023-13025. [PubMed] [Google Scholar]
- 21.Delker, C., A. Raschke, and M. Quint. 2008. Auxin dynamics: the dazzling complexity of a small molecule's message. Planta 227929-941. [DOI] [PubMed] [Google Scholar]
- 22.Dixon, M. P., R. N. Pau, G. J. Howlett, D. E. Dunstan, W. H. Sawyer, and B. E. Davidson. 2002. The central domain of Escherichia coli TyrR is responsible for hexamerization associated with tyrosine-mediated repression of gene expression. J. Biol. Chem. 27723186-23192. [DOI] [PubMed] [Google Scholar]
- 23.Dobbelaere, S., A. Croonenborghs, A. Thys, A. Vande Broek, and J. Vanderleyden. 1999. Phytostimulatory effect of Azospirillum brasilense wild type and mutant strains altered in IAA production on wheat. Plant Soil 212155-164. [Google Scholar]
- 24.Gordon, S. A., and R. P. Weber. 1951. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagen, G., and T. Guilfoyle. 2002. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49373-385. [PubMed] [Google Scholar]
- 26.Hwang, J. S., J. Yang, and A. J. Pittard. 1997. Critical base pairs and amino acid residues for protein-DNA interaction between the TyrR protein and tyrP operator of Escherichia coli. J. Bacteriol. 1791051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaeger, C. H., III, S. E. Lindow, W. Miller, E. Clark, and M. K. Firestone. 1999. Mapping of sugar and amino acid availability in soil around roots with bacterial sensors of sucrose and tryptophan. Appl. Environ. Microbiol. 652685-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama, T., H. Suzuki, K. Yamamoto, and H. Kumagai. 1999. Transcriptional regulation of tyrosine phenol-lyase gene mediated through TyrR and cAMP receptor protein. Biosci. Biotechnol. Biochem. 631823-1827. [DOI] [PubMed] [Google Scholar]
- 29.Koga, J., T. Adachi, and H. Hidaka. 1992. Purification and characterization of indolepyruvate decarboxylase. A novel enzyme for indole-3-acetic acid biosynthesis in Enterobacter cloacae. J. Biol. Chem. 26715823-15828. [PubMed] [Google Scholar]
- 30.Koga, J., T. Adachi, and H. Hidaka. 1991. Molecular cloning of the gene for indolepyruvate decarboxylase from Enterobacter cloacae. Mol. Gen. Genet. 22610-16. [DOI] [PubMed] [Google Scholar]
- 31.Kruger, N. J. 1994. The Bradford method for protein quantitation. Methods Mol. Biol. 329-15. [DOI] [PubMed] [Google Scholar]
- 32.Kuo, T., and T. Kosuge. 1970. Role of amino transferase and indole-3-pyruvic acid in the synthesis of indole-3-acetic acid in Pseudomonas savastanoi. J. Gen. Appl. Microbiol. 16191-204. [Google Scholar]
- 33.Kwok, T., J. Yang, A. J. Pittard, T. J. Wilson, and B. E. Davidson. 1995. Analysis of an Escherichia coli mutant TyrR protein with impaired capacity for tyrosine-mediated repression, but still able to activate at sigma 70 promoters. Mol. Microbiol. 17471-481. [DOI] [PubMed] [Google Scholar]
- 34.Lambrecht, M., A. Vande Broek, F. Dosselaere, and J. Vanderleyden. 1999. The ipdC promoter auxin-responsive element of Azospirillum brasilense, a prokaryotic ancestral form of the plant AuxRE? Mol. Microbiol. 32889-891. [DOI] [PubMed] [Google Scholar]
- 35.Liu, S. T., K. L. Perry, C. L. Schardl, and C. I. Kado. 1982. Agrobacterium Ti plasmid indoleacetic acid gene is required for crown gall oncogenesis. Proc. Natl. Acad. Sci. USA 792812-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lucy, M., E. Reed, and B. R. Glick. 2004. Applications of free living plant growth-promoting rhizobacteria. Antonie van Leeuwenhoek 861-25. [DOI] [PubMed] [Google Scholar]
- 37.MacPherson, K. H., P. D. Carr, D. Verger, T. Kwok, B. E. Davidson, and D. L. Ollis. 1999. Crystallization of the N-terminal domain of the Escherichia coli regulatory protein TyrR. Acta Crystallogr. D Biol. Crystallogr. 551923-1924. [DOI] [PubMed] [Google Scholar]
- 38.Mazzola, M., and F. F. White. 1994. A mutation in the indole-3-acetic acid biosynthesis pathway of Pseudomonas syringae pv. syringae affects growth in Phaseolus vulgaris and syringomycin production. J. Bacteriol. 1761374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mironov, A. A., E. V. Koonin, M. A. Roytberg, and M. S. Gelfand. 1999. Computer analysis of transcription regulatory patterns in completely sequenced bacterial genomes. Nucleic Acids Res. 272981-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morett, E., and L. Segovia. 1993. The sigma 54 bacterial enhancer-binding protein family: mechanism of action and phylogenetic relationship of their functional domains. J. Bacteriol. 1756067-6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng, L. C., C. L. Poh, and V. Shingler. 1995. Aromatic effector activation of the NtrC-like transcriptional regulator PhhR limits the catabolic potential of the (methyl)phenol degradative pathway it controls. J. Bacteriol. 1771485-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Offringa, I. A., L. S. Melchers, A. J. Regensburg-Tuink, P. Costantino, R. A. Schilperoort, and P. J. Hooykaas. 1986. Complementation of Agrobacterium tumefaciens tumor-inducing aux mutants by genes from the T(R)-region of the Ri plasmid of Agrobacterium rhizogenes. Proc. Natl. Acad. Sci. USA 836935-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ona, O., J. Van Impe, E. Prinsen, and J. Vanderleyden. 2005. Growth and indole-3-acetic acid biosynthesis of Azospirillum brasilense Sp245 is environmentally controlled. FEMS Microbiol. Lett. 246125-132. [DOI] [PubMed] [Google Scholar]
- 44.Patten, C. L., and B. R. Glick. 2002. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can. J. Microbiol. 48635-642. [DOI] [PubMed] [Google Scholar]
- 45.Patten, C. L., and B. R. Glick. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 683795-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patten, C. L., and B. R. Glick. 1996. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42207-220. [DOI] [PubMed] [Google Scholar]
- 47.Pittard, A. J., and B. E. Davidson. 1991. TyrR protein of Escherichia coli and its role as repressor and activator. Mol. Microbiol. 51585-1592. [DOI] [PubMed] [Google Scholar]
- 48.Pittard, J. 1996. The various strategies within the TyrR regulation of Escherichia coli to modulate gene expression. Genes Cells 1717-725. [DOI] [PubMed] [Google Scholar]
- 49.Pittard, J., H. Camakaris, and J. Yang. 2005. The TyrR regulon. Mol. Microbiol. 5516-26. [DOI] [PubMed] [Google Scholar]
- 50.Prell, J., B. Boesten, P. Poole, and U. B. Priefer. 2002. The Rhizobium leguminosarum bv. viciae VF39 gamma-aminobutyrate (GABA) aminotransferase gene (gabT) is induced by GABA and highly expressed in bacteroids. Microbiology 148615-623. [DOI] [PubMed] [Google Scholar]
- 51.Prinsen, E., A. Costacurta, K. Michiels, J. Vanderleyden, and H. Van Onckelen. 1993. Azospirillum brasilense indole-3-acetic acid biosynthesis: evidence for a non-tryptophan-dependent pathway. Mol. Plant-Microbe Interact. 6609-615. [Google Scholar]
- 52.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 12715-21. [DOI] [PubMed] [Google Scholar]
- 53.Rothballer, M., M. Schmid, A. Fekete, and A. Hartmann. 2005. Comparative in situ analysis of ipdC-gfpmut3 promoter fusions of Azospirillum brasilense strains Sp7 and Sp245. Environ. Microbiol. 71839-1846. [DOI] [PubMed] [Google Scholar]
- 54.Schroder, G., S. Waffenschmidt, E. W. Weiler, and J. Schroder. 1984. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur. J. Biochem. 138387-391. [DOI] [PubMed] [Google Scholar]
- 55.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 56.Song, J., and R. A. Jensen. 1996. PhhR, a divergently transcribed activator of the phenylalanine hydroxylase gene cluster of Pseudomonas aeruginosa. Mol. Microbiol. 22497-507. [DOI] [PubMed] [Google Scholar]
- 57.Spaepen, S., J. Vanderleyden, and R. Remans. 2007. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 31425-448. [DOI] [PubMed] [Google Scholar]
- 58.Spaepen, S., S. Dobbelaere, A. Croonenborghs, and J. Vanderleyden. 2008. Effects of Azospirillum brasilense indole-3-acetic acid production on inoculated wheat plants. Plant Soil doi: 10.1007/s11104-008-9560-1. [DOI]
- 59.Spaepen, S., W. Versees, D. Gocke, M. Pohl, J. Steyaert, and J. Vanderleyden. 2007. Characterization of phenylpyruvate decarboxylase, involved in auxin production of Azospirillum brasilense. J. Bacteriol. 1897626-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teale, W. D., I. A. Paponov, and K. Palme. 2006. Auxin in action: signalling, transport, and the control of plant growth and development. Nat. Rev. Mol. Cell. Biol. 7847-859. [DOI] [PubMed] [Google Scholar]
- 61.Vande Broek, A., P. Gysegom, O. Ona, N. Hendrickx, E. Prinsen, J. Van Impe, and J. Vanderleyden. 2005. Transcriptional analysis of the Azospirillum brasilense indole-3-pyruvate decarboxylase gene and identification of a cis-acting sequence involved in auxin responsive expression. Mol. Plant-Microbe Interact. 18311-323. [DOI] [PubMed] [Google Scholar]
- 62.Vessey, J. K. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255571-586. [Google Scholar]
- 63.White, F. F., and S. F. Ziegler. 1991. Cloning of the genes for indoleacetic acid synthesis from Pseudomonas syringae pv. syringae. Mol. Plant-Microbe Interact. 4207-210. [Google Scholar]
- 64.Xie, B., K. Xu, H. X. Zhao, and S. F. Chen. 2005. Isolation of transposon mutants from Azospirillum brasilense Yu62 and characterization of genes involved in indole-3-acetic acid biosynthesis. FEMS Microbiol. Lett. 24857-63. [DOI] [PubMed] [Google Scholar]
- 65.Xie, H., J. J. Pasternak, and B. R. Glick. 1996. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr. Microbiol. 3267-71. [Google Scholar]
- 66.Yang, J., H. Camakaris, and A. J. Pittard. 1996. Further genetic analysis of the activation function of the TyrR regulatory protein of Escherichia coli. J. Bacteriol. 1781120-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang, J., S. Ganesan, J. Sarsero, and A. J. Pittard. 1993. A genetic analysis of various functions of the TyrR protein of Escherichia coli. J. Bacteriol. 1751767-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang, J., Y. Ogawa, H. Camakaris, T. Shimada, A. Ishihama, and A. J. Pittard. 2007. folA, a new member of the TyrR regulon in Escherichia coli K-12. J. Bacteriol. 1896080-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, J., J. S. Hwang, H. Camakaris, W. Irawaty, A. Ishihama, and J. Pittard. 2004. Mode of action of the TyrR protein: repression and activation of the tyrP promoter of Escherichia coli. Mol. Microbiol. 52243-256. [DOI] [PubMed] [Google Scholar]
- 70.Yang, J., K. Murakami, H. Camakaris, N. Fujita, A. Ishihama, and A. J. Pittard. 1997. Amino acid residues in the alpha-subunit C-terminal domain of Escherichia coli RNA polymerase involved in activation of transcription from the mtr promoter. J. Bacteriol. 1796187-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33103-119. [DOI] [PubMed] [Google Scholar]
- 72.Yaryura, P. M., M. Leon, O. S. Correa, N. L. Kerber, N. L. Pucheu, and A. F. Garcia. 2008. Assessment of the role of chemotaxis and biofilm formation as requirements for colonization of roots and seeds of soybean plants by Bacillus amyloliquefaciens BNM339. Curr. Microbiol. 56625-632. [DOI] [PubMed] [Google Scholar]
- 73.Zimmer, W., M. Wesche, and L. Timmermans. 1998. Identification and isolation of the indole-3-pyruvate decarboxylase gene from Azospirillum brasilense Sp7: sequencing and functional analysis of the gene locus. Curr. Microbiol. 36327-331. [DOI] [PubMed] [Google Scholar]