Abstract
Activation of guanine nucleotide-binding protein (G protein)-coupled receptors is believed to involve conformational change that exposes a domain for G protein coupling at the cytosolic surface of the helical confluence, although the mechanisms for achieving this are not well understood. This conformational change can be achieved by docking a diverse variety of agonist ligands, known to occur by interacting with different regions of these receptors. In the current study, we focus on the importance of a specific basic residue (Lys187) within the second extracellular loop of the receptor for the peptide hormone, cholecystokinin. Alanine-replacement and charge-reversal mutagenesis of this residue showed that it had no effect on the binding of natural peptide and non-peptidyl ligands of this receptor, but markedly interfered with agonist-stimulated signaling. It was demonstrated that this negative effect on biological activity could be eliminated with the truncation of the first thirty residues of the amino-terminal tail of this receptor. Complementary charge-reversal mutagenesis of each of the five conserved acidic residues within this region of the receptor in the presence of the charge-reversed Lys187 revealed that only the Asp5 mutant fully reversed the negative functional impact of the Lys187 charge reversal. Thus, we have demonstrated that a basic residue within the second extracellular loop of the cholecystokinin receptor interacts with a specific acidic residue within the amino terminus of this receptor. This residue-residue interaction is nicely accommodated within a new molecular model of the agonist-occupied cholecystokinin receptor.
Keywords: G protein-coupled receptor, cholecystokinin receptor, cholecystokinin, receptor activation, charge-reversal mutagenesis
An understanding of the molecular basis of agonist ligand docking and activation of receptors can provide insights that could be important in the development and refinement of receptor-active drugs. Analysis of structure-activity relationships among closely related receptors and their ligands can be very useful. This approach, termed correlated mutational analysis, has been quite successful in identifying functionally important receptor residues (1, 2). Charged residues identified in this approach are among the potentially most interesting residues since they can be involved in charge-charge interactions. In this work, we have focused on a specific charged residue identified in such analysis that resides in the ectodomain of the cholecystokinin (CCK) receptor.
The type A cholecystokinin (CCK) receptor is a physiologically important guanine nucleotide-binding protein (G protein)-coupled receptor in the rhodopsin-β adrenergic receptor family that regulates pancreatic exocrine secretion, gallbladder contraction, gastrointestinal motility, and contributes to post-cibal satiety. We have focused on the importance of Lys187 within the second extracellular loop of this receptor that is highly conserved among the type A CCK receptors in all species cloned to date, while being absent in the closely related type B CCK receptors. Despite sharing approximately 55 percent structural homology, the type A and B CCK receptors have distinct structural specificities for agonist ligands (3, 4).
There are two functionally important acidic residues within the pharmacophoric region of CCK that interacts with the type A CCK receptor, Tyr-sulfate in position 27 and Asp in position 32 (5–7). Charge-pairing of the Tyr-sulfate with Arg197 in the second extracellular loop of the receptor has already been established, based on receptor mutagenesis and photoaffinity labeling studies (5, 8, 9). Molecular modeling has indicated not only the importance of Arg197 in the specific interaction with the Tyr-sulfate-27, but also an interaction with Phe109 in the first extracellular loop and a second interaction with Tyr339 in the third extracellular loop of this receptor to stabilize the receptor conformation (5). In fact, charge modification of Arg197 essentially completely abolished natural agonist binding and biological activity, and induced a global conformational disturbance of the receptor, resulting in a receptor that was more susceptible to proteolysis and degradation. However, no clear partner for the Asp residue in position 32 of CCK has yet been defined.
In the current work, we have focused on residue Lys187 in the second extracellular loop of receptor as a potential partner for CCK ligand residue Asp32. Receptor residue Lys187 was mutated to a neutral alanine or to a charge-reversed aspartic acid residue, with each mutant receptor construct functionally characterized. Surprisingly, there was dissociation of effects on ligand binding and on biological activity. Mutation of receptor residue Lys187 had a negative impact on the biological activity of both peptide and non-peptidyl agonists without affecting their binding. This shifted attention from possible interaction of Lys187 with Asp32 within the CCK ligand to its possible interaction elsewhere within the receptor. A series of studies was performed in attempt to identify a receptor region and, ultimately, a residue within that region that might interact with this basic residue. Focus was redirected to a region in the distal amino terminus of the receptor and to an acidic residue within that region. An interdomain residue-residue interaction in external regions of a G protein-coupled receptor has not previously been postulated to be important in achieving a biologically active conformation of these molecules. This interaction was also quite useful in developing a molecular model of the CCK-occupied CCK receptor that include s the previously unconstrained amino-terminal domain of the receptor.
MATERIALS AND METHODS
Materials
Synthetic CCK-8 was purchased from Peninsula Laboratories (Belmont, CA). Phenylmethylsulfonyl fluoride was from Sigma (St. Louis, MO); soybean trypsin inhibitor was from Worthington Biochemicals (Lakewood, NJ); Fura-2 acetoxymethyl esterwas from Molecular Probes (Eugene, Oregon). The benzodiazepine CCK antagonist, L-364,718, was kindly provided by Dr. R. Freidinger of Merck, Sharp, and Dohme Laboratories (West Point, PA), while a 1,5-benzodiazepine CCK agonist, GlaxoSmithKline compound #1d described in Darrow et al. (10), was provided by Elizabeth Sugg. 3H-L-364,718 was purchased from New Life Science Products (Boston, MA). The non-peptidyl CCK antagonist, SR-27897, was from Sanofi Recherche (Montpellier, France); the radiolabeled form, 3H-SR-27897, was from Amersham (Les Ulis, France). The well characterized CCK-like radioiodinatable analogue, D-Tyr- Gly-[(Nle28,31)CCK-26–33], and its fluorescent analogue, Alexa488-Gly-[(Nle28,31)CCK-26–33] (Alexa-CCK), were synthesized as we have described previously (11, 12). The non-fluorescent peptide was radioiodinated oxidatively using the solid-phase oxidant, iodobeads, and purified to yield a specific radioactivity of 2000 Ci/mmol using reversed-phase HPLC. Other reagents were analytical grade.
Receptor Mutagenesis and Expression
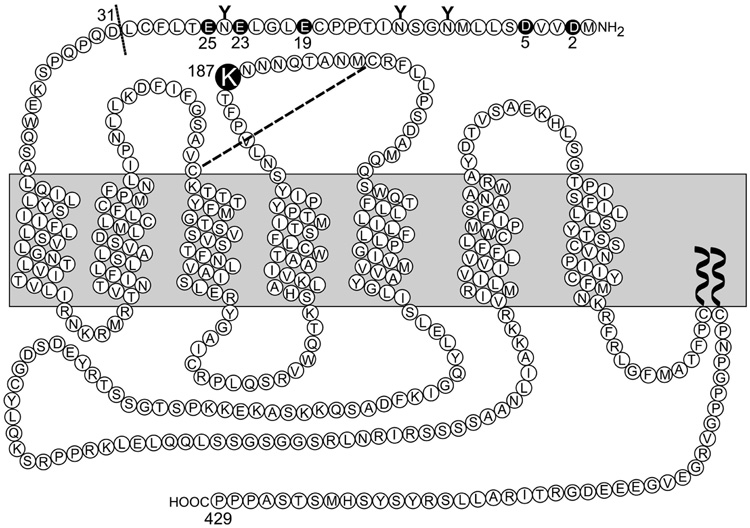
A series of CCK receptor constructs were prepared (illustrated in Figure 1). In some of these, the CCK receptor residue Lys187 was mutated to a non-charged Ala residue and a charge-reversed Asp residue by oligonucleotide-directed mutagenesis using the rat type A CCK receptor cDNA as template (13). In another series of mutations, CCK receptor constructs were truncated at position 31 (eliminating the first 30 residues of the amino terminus, including five acidic residues) to study the relevance of the amino-terminal segment or the charged residues in this region with the charge-reversed residue at position 187. In addition, five CCK receptor mutants were generated that mutated each of the acidic residues within the first 30 residues of the amino terminus (Asp2, Asp5, Glu19, Glu23 and Glu25) to a Lys residue (D2K(K187D), D5K(K187D), E19K(K187D), E23K(K187D) and E25K(K187D)), each containing the Lys to Asp charge-reversal mutation in position 187. Additionally, another CCK receptor construct was prepared in which the Asp5 to Lys mutation was inserted into the wild type receptor (D5K). All receptor constructs were prepared using an oligonucleotide-directed approach with the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA). Each of the mutant CCK receptor constructs was subcloned into the pcDNA3 eukaryotic expression vector (Invitrogen, Carlesbad, CA). All mutations had their identities proven by direct dideoxy-nucleotide chain termination DNA sequencing (14).
FIGURE 1.
Schematic diagram of the type A CCK receptor and the sites of modification in various key receptor constructs used in this project.
The Chinese hamster ovary (CHO) cell line that stably expresses the wild type CCK receptor (CHO-CCKR) and has been previously described and characterized was used as source of wild type receptor (13). Clonal CHO cell lines stably expressing the mutant receptors were established in a similar manner to the CHO-CCKR cell line. In brief, non-CCK receptor-bearing CHO-K1 cells were transfected with mutant receptor cDNAs in the pcDNA3 expression vector using lipofectin, and neomycin-resistant cells were selected by incubation with G418 (1 mg/ml). Clonal populations of surviving cells were then selected by a series of limiting dilutions, followed by screening assay of binding to CCK. Selected cell lines expressing an appropriate receptor density were cultured and used as source of mutant receptors in the binding and biological activity assays. The above-described five amino-terminal CCK receptor K187D mutants and the D5K mutant were expressed transiently in COS cells (American Type Culture Collection,Manassas, VA) after transfection using a modification of theDEAE-dextran method (15).
Cell Culture and Receptor Preparation
CHO cell lines were cultured as monolayers in flasks containing Ham’s F-12 medium supplemented with 5% Fetal Clone-2 (Hyclone Laboratories, Logan, UT) in a humidified environment containing 5% CO2, and were passaged twice a week. COS cells were maintained in Dulbecco'smodified Eagle's medium supplemented with 5% Fetal Clone-2, and were harvested 72 h after transfection. For cell membrane preparation, all cells were lifted mechanically, suspended in 0.3 M sucrose containing 0.01% soybean trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride, and sonicated in a Sonifier cell disrupter (Plainview, NY) at setting 7 for 10 s. The concentration of sucrose in the homogenate was adjusted to 1.3 M with 2.0 M sucrose, and the homogenate was overlayered with 0.3 M sucrose prior to centrifugation at 225,000 × g for 1 h. Membranes at the sucrose interface were then collected and diluted with iced-cold water. This was followed by centrifugation at 225,000 × g for 30 min to pellet the membranes. Membranes were then resuspended in Krebs-Ringers/HEPES (KRH) medium containing 25 mM HEPES, pH 7.4, 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, 1.2 mM MgSO4, 0.01% soybean trypsin inhibitor, 1 mM phenylmethylsulfonyl fluoride for storage at −80 °C until ready for use.
Binding and Biological Activity Assays
Binding activities of CCK receptor mutants were assessed with enriched plasma membranes from the receptor-bearing CHO cell lines in standard competition-binding assays, using conditions that have been previously established (13). 125I-D-Tyr-Gly-[(Nle28,31)CCK-26–33] (125I-CCK), 3H-L-364,718, and 3H-SR-27897 were used as radioligands. Membranes (5–10 µg proteins) were incubated with a constant amount of the CCK radioligand (5–10 pM for 125I-CCK, and 0.4 nM for 3H-L-364,718 and 3H-SR-27897) and increasing concentrations of non-radioactive ligand (0–1 µM) in KRH medium containing 0.2% bovine serum albumin for 1 h at room temperature to achieve steady-state binding. Rapid separation of bound from free radioligand was performed with a Skatron cell harvester (Molecular Devices, Sunnyvale, CA), using receptor-binding filtermats. Bound radioactivity was quantified with a γ-spectrometer or a liquid scintillation system (Beckman LS 6000SC). Non-specific binding was determined in the presence of 1 µM unlabeled CCK ligands and represented less than 10–15% of total binding. Data were graphed using Prism 3.0 (GraphPad software, San Diego, CA) and were analyzed using the nonlinear least-squares curve-fitting program of Munson and Rodbard, LIGAND (16).
The biological activities of the CCK receptor mutants were studied using assays for stimulation of intracellular calcium (13). Cells were lifted with non-enzymatic cell dissociation medium and were loaded with 5 µM Fura-2 acetoxymethyl ester in serum-free culture medium at 37 °C for 20 min, followed by washing with KRH medium. In each assay, approximately 2 million cells (for receptor-bearing CHO cells) or 1 million cells (for receptor-expressing COS cells) were stimulated with varied concentrations of CCK or the CCK non-peptidyl agonist, GlaxoSmithKline 1,5-benzodiazepine #1d (10), at 37 °C, with fluorescence quantified in a Perkin-Elmer LS50B luminescence spectrometer (Norway, CT). Excitation was performed at 340 and 380 nm and emissions were determined at 520 nm, with calcium concentrations calculated from the ratios as described by Grynkiewicz et al. (17). The peak intracellular calcium transients were utilized to determine the concentration-dependence of the biological responses. Basal levels of calcium were measured in the absence of agonist stimulation, whereas maximum levels were determined as the peak intracellular calcium concentrations measured in the presence of a 1 µM CCK or 1,5-benzodiazepine #1d. Stimulated values were calculated as percentages of the range of calcium concentrations between basal and maximal levels. Data were analyzed using Microsoft Excel and graphed using Prism 3.0 (GraphPad software, San Diego, CA).
Morphologic Assays of Receptor Surface Expression and Internalization
Examination of receptor biosynthesis and trafficking to the surface of CHO cells was performed using the fluorescent CCK analogue, Alexa-CCK, as we have previously described (18). This is a full agonist that binds with high affinity and specifically to the CCK receptor (11). A morphologic assay was utilized for this series of studies (18). In brief, CHO cells stably expressing the wild type and the K187A and K187D mutant CCK receptors grown on coverslips were washed with PBS containing 0.1 mM MgCl2 and 0.08 mM CaCl2 and labeled with 50 nM Alexa-CCK for 1 h at 4 °C. In a control competition experiment, 1 µM non-fluorescent CCK was included in the labeling reaction. The coverslips were then rinsed in cold PBS, fixed in 2% paraformaldehyde, washed, mounted on slides and examined with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss, Oberkochen, Germany) equipped for epifluorescence. The Alexa fluorescence was observed using FITC filter set (excitation, 480/40 nm; emission, 515 nm, long pass) with a dichroic mirror (505 nm, long pass) (Chroma Technology Corp., Rockingham, VT). The images were collected with an Axiocam MRC camera with identical exposure times using Axiovision 3.1 software.
The ability of CCK to internalize after binding to its cell surface receptor is a well characterized property of the wild type receptor (18). Here, we followed the previously-described technique to examine how the position 187 mutant CCK receptors behaved in this assay. Fluorescent labeling of the cells were performed as described above. After labeling, the coverslips were then rinsed in cold PBS and incubated in PBS at 37 °C for 0, 2, 5, 10 and 30 min before being fixed, mounted on slides and examined.
Molecular modeling
We have previously reported and sequentially refined a molecular model of the complex of CCK agonist peptides occupying the type A CCK receptor (5, 11, 19). These models utilized an amino-terminally-truncated form of the receptor, since no meaningful constraints for the conformation of this region of the intact receptor existed. In the current work, we have added the amino terminus of this receptor to the previously-reported structure (11), focusing efforts to examine whether the insights from the current project could be meaningfully accommodated. All molecular modeling was performed using the Internal Coordinate Mechanics (ICM) software package that employs stochastic global energy optimization procedures (20), running on a Pentium IV Duo Core processor at 3.0 GHz frequency. The previously-reported receptor structure (11) was first optimized to comply with ideal geometry of residues based on ICM calculations. The first forty residues of the amino-terminal segment of the receptor were then attached to establish continuity of the peptide backbone, using the conformation established by NMR analysis of this peptide (PDB Code: 1D6G, (21). A Monte Carlo simulation was then attempted at 300 K for 2 h to refine the conformation of this amino-terminal segment in this model to accommodate a distance constraint of 3.5Å between residues Asp5 and Lys187 established in the current work, as well as the disulfide bond reported to exist between Cys18 and Cys29 (22). The distance constraint was imposed in the form of quadratic restraint energy with a strength of 10 kcal/molÅ2. However, the position of the third extracellular loop in the earlier model interfered with the formation of the salt bridge between Asp5 and Lys187. Therefore, we allowed that loop to relax external to the helical bundle before starting another Monte Carlo simulation at 300 K for 4 h, with 10,000 random sampling followed by energy minimization. The lowest energy conformation fully accommodated both constraints. The CCK peptide was then successfully docked to this model with further Monte Carlo simulation at 300 K for 48 h, with 50,000 steps of random sampling of the backbone and side-chain dihedral angles of the amino-terminal segment and the third extracellular loop using the ICM rotamer library. The lowest energy conformation fully accommodated all previously-reported photoaffinity labeling constraints between residues Phe33, Tyr27, Gly29 and Tyr24 of the CCK peptide and residues Trp39, Arg197, Val342 and Glu345 of the receptor, respectively, as well as published structure-activity considerations, such as the ability to modify or extend the peptide amino terminus without disrupting function (5, 11, 19, 23, 24). In all Monte Carlo simulations, the backbone and side-chain atoms of the transmembrane helices and of the first and second extracellular loops were tethered to the coordinates in our previously-reported model (11) using a harmonic restraint.
RESULTS
Binding Activities of the Lys187 Site Mutants
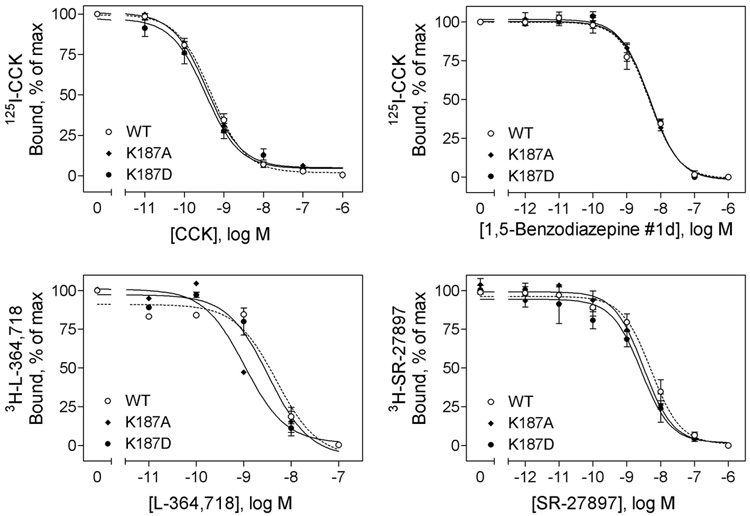
To examine the role of receptor residue 187 in ligand binding, this residue was mutated to a neutral Ala and a charge-reversed acidic Asp, and binding to receptor-bearing membranes was examined (Figure 2 and Table 1). CCK binding characteristics to K187A and K187D receptor mutants were not significantly different from those to wild type receptor. This suggests that residue Lys187 is not directly important for CCK binding to this receptor. Characteristics of binding of a structurally distinct CCK receptor agonist, GlaxoSmithKline 1,5-benzodiazapine #1d, and two antagonists, L-364,718 and SR-27897, to the position 187 mutants were also similar to their binding characteristics to wild type receptor (Figure 2 and Table 1).
FIGURE 2.
Effects of mutation of CCK receptor residue Lys187 on binding of peptide and non-peptidyl ligands. Top left, curves for CCK to compete for binding of the 125I-CCK radioligand to membranes from CHO cells expressing the wild type, K187A, and K187D CCK receptor constructs. Top right, curves for non-peptidyl agonist, 1,5-benzodiazepine #1d, to compete for binding of the 125I-CCK radioligand to these cell membranes. Bottom left, curves for non-peptidyl antagonist, L-364,718, to compete for binding of the 3H-L-364,718 radioligand to these cell membranes. Bottom right, curves for non-peptidyl antagonist, SR-27897, to compete for binding of the 3H-SR-27897 radioligand to these cell membranes. Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Table 1 includes Ki and Bmax values for these series of studies. Mutation of CCK receptor residue Lys187 to either a neutral charge (Ala) or a reversed-charge (Asp) residue had no significant effects on ligand binding of either peptidyl agonist CCK or non-peptidyl agonist, 1,5-benzodiazepine #1d or antagonists, L-364,718 and SR-27897.
Table 1.
Characterization of CCK Receptor Binding and Activationa
| Binding Characteristics |
Intracellular Calcium Response |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agonists |
Antagonists |
Agonists |
||||||||||
| Constructs | CCK |
1,5-Benzodiazepine #1d |
L-364,718 |
SR-27897 |
CCK |
1,5-Benzodiazepine #1d |
||||||
| Ki | Bmax | Ki | Bmax | Ki | Bmax | Ki | Bmax | EC50 | Emax | EC50 | Emax | |
| nM | pmol/mg protein | nM | pmol/mg protein | nM | pmol/mg protein | nM | pmol/mg protein | nM | nM | nM | nM | |
| WT | 0.5 ± 0.1 | 0.7 ± 0.1 | 4.6 ± 0.3 | 10.3 ± 0.2 | 4.7 ± 1.2 | 14.1 ± 1.4 | 5.3 ± 1.0 | 22.9 ± 3.9 | 0.03 ± 0.01 | 236 ± 30 | 6.1 ± 0.4 | 213 ± 26 |
| K187A | 0.4 ± 0.1 | 0.6 ± 0.2 | 4.7 ± 0.3 | 9.0 ± 1.1 | 1.1 ± 0.1 | 12.0 ± 2.5 | 3.1 ± 0.6 | 20.4 ± 3.0 | 0.1 ± 0.1 | 232 ± 39 | 122 ± 30 | 198 ± 37 |
| K187D | 0.4 ± 0.1 | 0.5 ± 0.1 | 4.7 ± 0.7 | 9.9 ± 1.1 | 3.2 ± 0.5 | 12.5 ± 1.7 | 2.7 ± 0.4 | 18.8 ± 0.7 | 7.0 ± 1.0 | 120 ± 29 | N.D. | N.D. |
| WT)31–429 | 0.7 ± 0.1 | 5.7 ± 1.5 | 4.3 ± 0.5 | 4.7 ± 1.3 | 0.13 ± 0.03 | 228 ± 32 | 7.6 ± 1.8 | 191 ± 39 | ||||
| K187D)31–429 | 0.9 ± 0.1 | 1.4 ± 0.4 | 7.7 ± 2.2 | 1.4 ± 0.4 | 0.16 ± 0.04 | 240 ± 46 | 25.4 ± 4.0 | 175 ± 40 | ||||
| D5K | 1.3 ± 0.2 | 10.0 ± 1.9 | 4.3 ± 0.9 | 4.6 ± 1.5 | 0.29 ± 0.06 | 100 ± 24 | 9.0 ± 2.0 | 105 ± 27 | ||||
| D5K(K187D) | 1.6 ± 0.2 | 7.9 ± 2.0 | 4.5 ± 0.9 | 1.4 ± 0.2 | 0.03 ± 0.01 | 102 ± 28 | 28.8 ± 5.6 | 96 ± 19 | ||||
Shown are Ki and Bmax values for ligand binding to membranes prepared from cells expressing each of the noted CCK receptor constructs. Shown also are EC50 and Emax values (delta changes from basal values, nM) for ligand-stimulated intracellular calcium responses in these cells. All values represent the means ± S.E.M. of data from at least three independent experiments. N.D., not detected.
Biological Activities of the Lys187 Site M utants
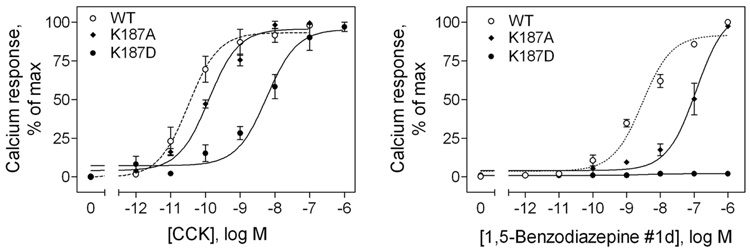
We next examined the effect of the modification of this residue (Lys187) on biological activity (Figure 3 and Table 1). Of particular interest, modification of this residue had substantial impact on intracellular calcium signaling. The CCK-induced intracellular calcium response decreased a small amount when the charge of this residue was changed from a basic lysine residue to a neutral alanine residue, and it was decreased dramatically when its charge was reversed to an acidic aspartic acid residue. The potency of the CCK-induced intracellular calcium response was decreased approximately 4-fold in the K187A mutant and 233-fold in the K187D mutant receptor-bearing CHO cells when compared to that in the wild type receptor.
FIGURE 3.
Effects of mutation of the CCK receptor residue Lys187 on biological activity of peptide and non-peptidyl ligands. Shown are intracellular calcium responses in CHO cells expressing the wild type, K187A, and K187D CCK receptor constructs to increasing concentrations of CCK (left) and non-peptidyl ligand, 1,5-benzodiazepine #1d (right). Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Basal level of intracellular calcium was similar for all the constructs (157 ± 26 nM), and maximal levels of stimulation by CCK reached 393 ± 33 (WT), 389 ± 47 (K187A) and 277 ± 33 (K187D) nM and by 1,5-benzodiazepine #1d reached 370 ± 29 (WT) and 355 ± 40 (K187A) nM. EC50 and Emax (delta change from basal) values are shown in Table 1. The CCK-stimulated intracellular calcium response was decreased about 4-fold by neutral charge K187A and 233-fold by reversed-charge K187D mutations. The 1,5-benzodiazepine #1d-stimulated calcium response was decreased about 20-fold by neutral charge K187A mutation, while the reversed-charge K187D substitution completely abolished the response.
The GlaxoSmithKline 1,5-benzodiazapine #1d agonist induced a calcium response in the wild type and the K187A receptor-bearing CHO cells, albeit with lower potency. However, no biological activity was detected for this compound in the charge-reversed K187D mutant receptor (Figure 3).
Biosynthesis and Cell Surface Expression of CCK Receptor Constructs
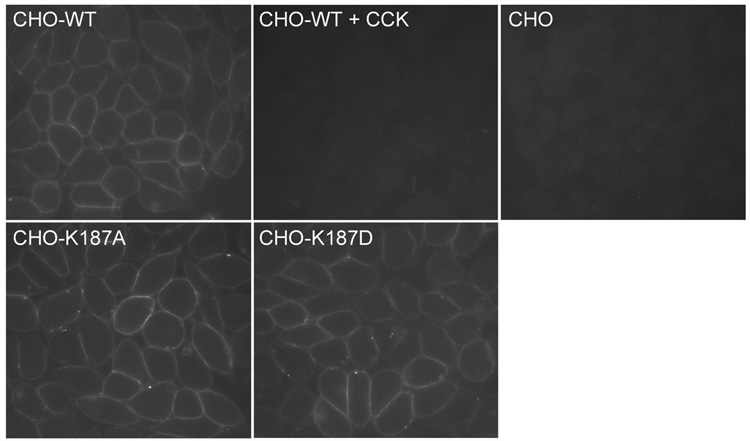
Morphologic assays were performed to assess the cell surface expression of the CCK receptor constructs, following the methods previously reported (18) and described in Methods (Figure 4). As previously described (18), wild type CCK receptor trafficked normally to the surface of CHO cells, where the fluorescent CCK analogue binds to the receptor in a high affinity and saturablemanner. T he non-transfected parental CHO cell line did not express any CCK-binding proteins on its surface that could be identified in this assay. Like wild type CCK receptor, both of the K187A and K187D mutant CCK receptor constructs trafficked normally to the surface of the receptor-expressing CHO cells during biosynthesis, achieving cell surface densities similar to that of the wild type receptor. Abnormal biosynthetic trafficking was, therefore, not an explanation for the reduced signaling in response to agonist ligands observed for these CCK receptor constructs.
FIGURE 4.
Morphological evidence for normal cell surface expression of the Lys187 mutant CCK receptor constructs. Shown are representative examples of CHO cells expressing the wild type CCK receptor in the absence (CHO-WT) and presence of competing CCK (CHO-WT + CCK), as well as K187A (CHO-K187A) and K187D (CHO-K187D) CCK receptor mutants, and non-receptor-bearing parental CHO cells labeled with Alexa-CCK. There was no significant surface labeling of either the parental CHO cells or the receptor-bearing CHO-WT cells in the presence of competing non-fluorescent CCK. All images were acquired under similar settings and were representative of at least three independent experiments.
Internalization of CCK Receptor Constructs
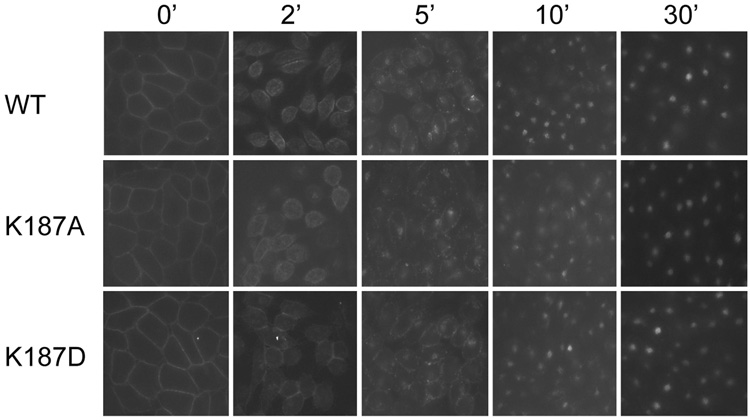
One of the properties well recognized for wild type CCK receptor is its internalization after occupation with agonist CCK ligands (18). Figure 5 demonstrates this in a morphologic fluorescence assay. As shown, like the wild type CCK receptor, both K187A and K187D receptor mutants were clearly labeled on the cell surfaces by Alexa-CCK at the 0 min time point. After incubation at 37 °C for 2 min, the fluorescence displayed a more punctate pattern near the plasmalemma. By 5 min, the fluorescence was predominantly in small cytoplasmic vesicles with some in the perinuclear region. After 10 min, most fluorescencewas present in the perinuclear region. These data suggest that both the K187A and K187D CCK receptor mutants behaved normally in their internalization properties after CCK binding.
FIGURE 5.
Time course of internalization of the Lys187 mutant CCK receptor constructs in CHO cells. CHO cells stably expressing the wild type (WT), K187A, and K187D CCK receptor constructs were preincubated for 1 h at 4 °C with 50 nM Alexa-CCK, and were subsequently washed and warmed to 37 °C for 0, 2, 5, 10 and 30 min before being fixed. Images are representative of three independent experiments. Mutation of CCK receptor residue Lys187 to either a neutral charge (Ala) or a reversed-charge (Asp) residue had no significant effects on the extent or time-course of CCK receptor internalization.
Restoration of the Biological Activity of the Charge-reversed Mutant Receptor by Amino-terminal Truncation and Mutation of the Receptor
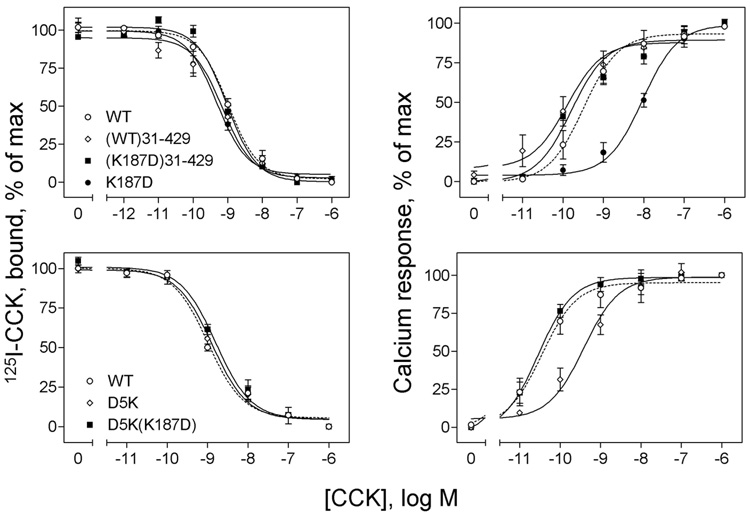
Since mutation of Lys187 did not interfere with CCK binding and since our current model of the CCK-receptor complex could allow approximation of this region of the second extracellular loop with some part of the amino-terminal tail of the receptor (19), we proceeded to evaluate the relationship between receptor residue 187 and the receptor tail. We postulated that Lys187 might normally be involved in a charge-charge interaction with one of the charged residues within the receptor amino terminus. To explore this, we attempted to restore the markedly reduced biological response observed for the charge-reversed K187D mutant (233-fold less than control) by truncating the receptor amino terminus. Indeed, truncation of the receptor at position 31 fully restored the biological response to CCK, while having no effect on ligand binding (Figure 6 and Table 1).
FIGURE 6.
Effects of truncation and site mutation of the amino terminus of the K187D mutant CCK receptor construct on their peptidyl ligand binding and biological activity. Left, curves for CCK to compete for binding of the 125I-CCK radioligand to membranes from CHO cells expressing the truncated WT ((WT)31–429) and K187D ((K187D)31–429) CCK receptor constructs (top) and membranes from COS cells expressing the site mutants, D5K and D5K(K187D) (bottom). As controls, data from WT and K187D are also shown. Ki and Bmax values are shown in Table 1. Right, curves of intracellular calcium responses in these cells to increasing concentrations of CCK. Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Basal levels of intracellular calcium in CHO cells were similar for all the constructs (147 ± 28 nM), and maximal levels achieved after stimulation with CCK reached 380 ± 40 (WT), 375 ± 36 ((WT)31–429) and 387 ± 49 ((K187D)31–429) nM. There were no significant differences in basal and maximal intracellular calcium levels for any of the CCK receptor mutants expressed in COS cells. In those cells, basal levels of intracellular calcium were 166 ± 34 nM, and maximal levels achieved after stimulation with CCK were 268 ± 43 nM. EC50 and Emax (delta change from basal) values are shown in Table 1. These data suggest that neither truncation nor site mutation of the amino terminus of the K187D mutant CCK receptor had any effect on peptidyl ligand binding, while both completely restored the intracellular calcium responses to CCK.
In an attempt to localize the residue or residues that might be involved in such a charge-charge interaction with this region of the receptor, we proceeded to mutate each of its component five acidic residues to a charge-reversed Lys residue in the presence of K187D and to express them in COS cells (data in Figure 6 and Table 1). As expected, reversed-charge mutation of each of these residues had no effect on CCK binding. Of note, mutation of Asp5 to Lys fully restored the biological response to CCK in the K187D construct. It is of further interest that this same mutation (D5K) in the setting of the wild type receptor resulted in reduced potency of CCK action. Mutation of Asp2, Glu23 and Glu25 in the K187D construct partially restored biological responses to CCK, but these constructs were substantially less effective than the K187D receptor construct with mutation of Asp5. Mutation of Glu19 had no positive effects on CCK action in the setting of K187D. (EC50 values in nM: D5K(K187D), 0.03 ± 0.01; E25K(K187D), 0.3 ± 0.1; E23K(K187D), 0.9 ± 0.2; D2K(K187D), 1.2 ± 0.2; E19K(K187D), 7.1 ± 1.7). Each of these constructs had similar efficacy, stimulating similar Emax values to each other.
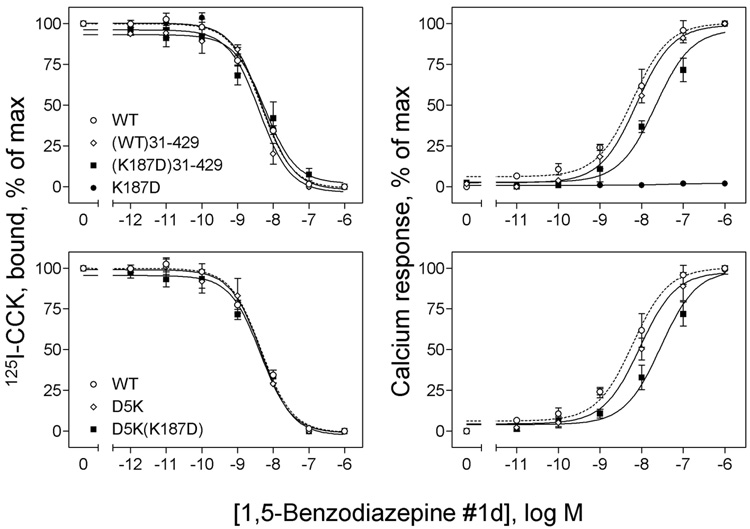
As described above, the non-peptidyl CCK receptor agonist, GlaxoSmithKline 1,5-benzodiazepine #1d, was unable to elicit any intracellular calcium response in the charge-reversed K187D mutant CCK receptor (Figure 3). We know that this molecule binds to the CCK receptor in a distinct location to the natural peptide agonist, CCK (25). It was, therefore, notable that truncation and site-directed mutagenesis had analogous effects on the biological action of this agonist as they did on the action of CCK. As shown in Figure 7 and Table 1, amino-terminal truncation ((K187D)31–429) and single site charge-reversal mutation (D5K(K187D)) fully restored the calcium responses to this agonist, although with a lower potency than wild type receptor (EC50 values in nM: WT, 6.1 ± 0.4; (K87D)31–429, 25.4 ±4.0; D5K(K187D), 28.8 ±5.6). Both constructs bound the non-peptidyl agonist normally.
FIGURE 7.
Effects of truncation and site mutation of the amino terminus of the Lys187 mutant CCK receptor constructs on their non-peptidyl ligand binding and biological activity. Shown in left panels are curves for 1,5-benzodiazepine #1d to compete for binding of the 125I-CCK radioligand to membranes from CHO cells expressing the truncated WT ((WT)31–429) and K187D ((K187D)31–429) CCK receptor constructs (top) and membranes from COS cells expressing the site mutants, D5K and D5K(K187D) (bottom). As controls, data from WT and K187D are also shown. Ki and Bmax values are shown in Table 1. Shown in right panels are curves of intracellular calcium responses in these cells to increasing concentrations of 1,5-benzodiazepine #1d. Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Basal levels of intracellular calcium in CHO cells were similar for all the constructs (143 ± 21 nM), and maximal levels achieved after stimulation with 1,5-benzodiazepine #1d were 363 ± 49 (WT), 333 ± 29 ((WT)31–429) and 320 ± 33 ((K187D)31–429) nM. No significant differences in basal or maximal intracellular calcium levels were observed for any of the CCK receptor mutants expressed in COS cells. Basal levels of intracellular calcium were 160 ± 40 nM, and maximal levels achieved after stimulation with 1,5-benzodiazepine #1d were 262 ± 47 nM. These data demonstrate that amino-terminal truncation and site mutation of the K187D mutant CCK receptor are both able to restore intracellular calcium responses to 1,5-benzodiazepine #1d. Both constructs bound normally.
Molecular modeling
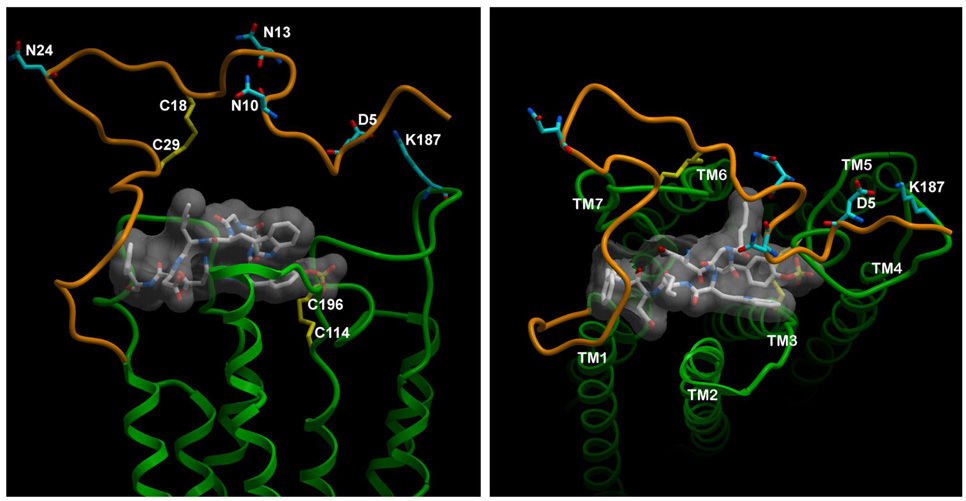
Shown in Figure 8 is a representation of the lowest energy model of CCK peptide bound to the homology model of the intact type A CCK receptor. This molecular model accommodates all of the previously reported photoaffinity labeling constraints (residue-residue approximations) (5, 11, 19, 23, 24), disulfide bonds (22), and structure-activity considerations (12), as well as the currently proposed approximation of receptor residues Asp5 and Lys187.
FIGURE 8.
Molecular model of CCK bound to the type A CCK receptor. Shown are lateral (left) and top (right) views of the lowest energy molecular model of CCK bound to the CCK receptor. The receptor amino-terminal domain is illustrated in brown, while the rest of the receptor is green. The peptide ligand, CCK-8, is shown as a stick model with transparent surface skin in white. Key residues of interest have been highlighted. These include receptor residues Asp5 and Lys187 that are involved in charge-pairing, Cys pairs that are involved in disulfide bonds (Cys18-Cys29 and Cys114-Cys196), and three sites of N-linked glycosylation (Asn10, Asn13, and Asn24). The TM helices have been identified in the top view.
DISCUSSION
The superfamily of G protein-coupled receptors includes individual receptors that respond to natural agonist ligands with extraordinarily diverse structures, ranging from small odorants and biogenic amines, to peptides, and large glycoproteins, and even viral particles. For each of these types of ligands, the receptors have evolved unique structural themes for ligand recognition and activation. Much of this diversity exists within the amino-terminal tail and external loop domains of these receptors. The more highly conserved sequences within the transmembrane segments of these receptors that contribute to their typical helical confluence provides a relatively stable architectural base that shifts conformation with activation, thereby exposing the cytosolic face of the receptor that couples with its G protein partner and initiates the intracellular signaling cascade typical of that receptor. Indeed, most of the receptor residues that have been shown to play important roles in signaling are present within these architecturally important and conserved regions or in the intracellular face of receptors. It was, therefore, surprising in the current series of studies that we identified an extracellular loop residue that could be mutated to have had no effect on ligand binding, while profoundly affecting receptor signaling. It was even more unexpected and novel that this effect could be proposed to occur via intramolecular, interdomain charge-charge interaction with a residue within the receptor amino terminus.
Like many receptors in this superfamily, the CCK receptor can bind and be activated by structurally-diverse ligands. These can include small peptides and benzodiazepines (10, 25). Mutagenesis of Lys187, even to the point of reversing its charge, had no significant effect on the binding of any of these ligands. Each of these receptor constructs was shown to traffic normally to the cell surface during biosynthesis. Of particular interest, each of these receptor constructs also underwent a conformational change in response to CCK binding that resulted in normal agonist-stimulated internalization. The charge-reversal, however, had a marked effect on the initiation of signaling by either peptide or benzodiazepine agonists. The molecular mechanism of this observation was not readily apparent, given our current understanding of the structure of this receptor and of the conformational changes involved in receptor activation.
Based on the absence of negative functional impact of Lys187 mutagenesis on CCK binding and based on our current working models of CCK and benzodiazepine ligands bound to this receptor (19, 25), we predicted that Lys187, rather than having any direct contact with the docked ligands, might come in contact with the amino-terminal tail region of the receptor. Of interest, this is a region of the CCK receptor that has previously been felt to have no function, based on studies in which it has been truncated without having any negative effect on CCK binding (19, 26). However, we were able to demonstrate a clear function in the current report. This region of the CCK receptor contains five acidic residues that are highly conserved across all type A CCK receptors from various species cloned to date. It is possible that one of these acidic residues normally pairs with the basic Lys187 residue. Replacement of such an acidic residue with a charge-reversed basic residue could thereby introduce a non-natural repulsive interaction that could interfere with achieving the active conformation of such a receptor construct.
Mutagenesis of Lys187 resulted in reduced or absent biological responses to agonists that continued to bind to the mutant receptor quite normally. The profound negative effect of reversing the charge of the residue in the 187 position (233-fold reduction in potency of CCK responsiveness) was essentially eliminated by truncating the amino terminus of this receptor. Such truncation likely eliminated the partner residue proposed to interact with Lys187. Replacing Lys187 with a neutral Ala residue had a relatively small negative effect on biological activity, while reversing the charge with an Asp residue had a more profound negative effect. This suggests that the acidic Asp replacement for the Lys187 probably not only eliminated an important charge-charge attraction present normally, but may also have added an abnormal charge-charge repulsion. By eliminating the target of this postulated repulsion, the negative effect of the position 187 mutation was eliminated.
There are five candidate charged residues within the first thirty residues of the CCK receptor amino terminus that might be involved in the proposed charge-charge interaction. All of these residues are highly conserved acidic residues. We, therefore, went on to mutate each of these residues to a basic Lys residue in the presence of the Lys187 to Asp mutation. Of note, mutation of Asp5 to Lys fully restored the biological response to CCK in the setting of the K187D construct, while mutation of Asp2, Glu23, and Glu25 each partially restored biological responses to CCK, and mutation of Glu19 had no effect. The D5K mutation in the wild type CCK receptor had an inhibitory effect on the biological response to CCK. This successful complementary charge-reversal mutagenesis between positions 5 and 187 was interpreted to support normal charge-charge interaction between these residues.
This interaction was also nicely accommodated by spatial approximation between these residues in the molecular model generated in this study. This provides a clear explanation for the mechanism of the functional importance of Lys187. It is likely that Asp2 is situated closely enough in three-dimensional space to the normal partner for charge-pairing to Asp5 to also partially compensate for the normal interaction, while Glu19 is further removed and unable to provide constructive pairing. Of interest, mutation of Glu23 and Glu25 also had a positive effect. Given where they are situated in the proposed molecular model, the explanation may be either that there is another different partner for their charge-pairing or the loop structure in which they reside is not optimally placed in this model and can move toward the second loop region. Spatial constraints like these have previously been unavailable for meaningful modeling of the flexible loop and tail regions of this receptor.
Thus, in this report, we have identified a basic residue within the second extracellular loop of the CCK receptor that likely interacts with a specific acidic residue within the amino terminus of this receptor to help achieve its fully active conformation. This provides a tool to dissociate normal high affinity agonist binding from the induction of biological activity. It also points toward the functional importance of the amino-terminal tail of this receptor that was not previously recognized.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (DK32878) and the Fiterman Foundation. The authors acknowledge the technical assistance of Renee M. Happs, Laura A. Bruins, Eileen L. Holicky, Elizabeth M. Hadac, and Delia I. Pinon, the secretarial assistance from Evelyn M. Posthumus, and the helpful discussions with Dr. Kaleeckal G. Harikumar.
Footnotes
This work was supported by grants from the National Institutes of Health (DK32878) and the Fiterman Foundation.
Abbreviations: Alexa-CCK, Alexa488-Gly-[(Nle28,31)CCK-26-33]; CCK, cholecystokinin; CHO, Chinese hamster ovary; G protein, guanine nucleotide-binding protein; KRH, Krebs-Ringers/HEPES.
Contributor Information
Maoqing Dong, Cancer Center and Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Scottsdale, AZ 85259.
Xi-Qin Ding, Cancer Center and Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Scottsdale, AZ 85259.
Scott E. Thomas, Cancer Center and Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Scottsdale, AZ 85259
Fan Gao, Cancer Center and Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Scottsdale, AZ 85259.
Polo C.-H. Lam, Department of Molecular Biology, Scripps Research Institute and Molsoft LLC, La Jolla, CA 92037
Ruben Abagyan, Department of Molecular Biology, Scripps Research Institute and Molsoft LLC, La Jolla, CA 92037.
Laurence J. Miller, Cancer Center and Department of Molecular Pharmacology and Experimental Therapeutics, Mayo Clinic, Scottsdale, AZ 85259
REFERENCES
- 1.Oliveira L, Paiva PB, Paiva AC, Vriend G. Sequence analysis reveals how G protein-coupled receptors transduce the signal to the G protein. Proteins. 2003;52:553–560. doi: 10.1002/prot.10489. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira L, Paiva AC, Vriend G. Correlated mutation analyses on very large sequence families. Chembiochem. 2002;3:1010–1017. doi: 10.1002/1439-7633(20021004)3:10<1010::AID-CBIC1010>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz F, Pratt DS, Wu MJ, Kolakowski LF, Jr, Beinborn M, Kopin AS. Identification of cholecystokinin-B/gastrin receptor domains that confer high gastrin affinity: utilization of a novel Xenopus laevis cholecystokinin receptor. Mol Pharmacol. 1996;50:436–441. [PubMed] [Google Scholar]
- 4.Noble F, Wank SA, Crawley JN, Bradwejn J, Seroogy KB, Hamon M, Roques BP. International Union of Pharmacology. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- 5.Ding XQ, Pinon DI, Furse KE, Lybrand TP, Miller LJ. Refinement of the conformation of a critical region of charge-charge interaction between cholecystokinin and its receptor. Mol Pharmacol. 2002;61:1041–1052. doi: 10.1124/mol.61.5.1041. [DOI] [PubMed] [Google Scholar]
- 6.Huang SC, Yu DH, Wank SA, Mantey S, Gardner JD, Jensen RT. Importance of sulfation of gastrin or cholecystokinin (CCK) on affinity for gastrin and CCK receptors. Peptides. 1989;10:785–789. doi: 10.1016/0196-9781(89)90114-9. [DOI] [PubMed] [Google Scholar]
- 7.Pan GZ, Martinez J, Bodanszky M, Jensen RT, Gardner JD. The importance of the amino acid in position 32 of cholecystokinin in determining its interaction with cholecystokinin receptors on pancreatic acini. Biochim Biophys Acta. 1981;678:352–357. doi: 10.1016/0304-4165(81)90114-8. [DOI] [PubMed] [Google Scholar]
- 8.Gigoux V, Maigret B, Escrieut C, Silvente-Poirot S, Bouisson M, Fehrentz JA, Moroder L, Gully D, Martinez J, Vaysse N, Fourmy AD. Arginine 197 of the cholecystokinin-A receptor binding site interacts with the sulfate of the peptide agonist cholecystokinin. Protein Sci. 1999;8:2347–2354. doi: 10.1110/ps.8.11.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arlander SJ, Dong M, Ding XQ, Pinon DI, Miller LJ. Key differences in molecular complexes of the cholecystokinin receptor with structurally related peptide agonist, partial agonist, and antagonist. Mol Pharmacol. 2004;66:545–552. doi: 10.1124/mol.104.001396. [DOI] [PubMed] [Google Scholar]
- 10.Darrow JW, Hadac EM, Miller LJ, Sugg EE. Structurally similar small molecule photoaffinity CCK-A agonists and antagonists as novel tools for directly probing 7TM receptors-ligand interactions. Bioorg Med Chem Lett. 1998;8:3127–3132. doi: 10.1016/s0960-894x(98)00548-4. [DOI] [PubMed] [Google Scholar]
- 11.Harikumar KG, Pinon DI, Wessels WS, Dawson ES, Lybrand TP, Prendergast FG, Miller LJ. Measurement of intermolecular distances for the natural agonist Peptide docked at the cholecystokinin receptor expressed in situ using fluorescence resonance energy transfer. Mol Pharmacol. 2004;65:28–35. doi: 10.1124/mol.65.1.28. [DOI] [PubMed] [Google Scholar]
- 12.Powers SP, Pinon DI, Miller LJ. Use of N,O-bis-Fmoc-D-Tyr-ONSu for introduction of an oxidative iodination site into cholecystokinin family peptides. Int J Pept Protein Res. 1988;31:429–434. doi: 10.1111/j.1399-3011.1988.tb00899.x. [DOI] [PubMed] [Google Scholar]
- 13.Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas. 1996;13:130–139. doi: 10.1097/00006676-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtmann MH, Ganguli S, Hadac EM, Dolu V, Miller LJ. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J Biol Chem. 1996;271:14944–14949. doi: 10.1074/jbc.271.25.14944. [DOI] [PubMed] [Google Scholar]
- 16.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 18.Roettger BF, Rentsch RU, Pinon D, Holicky E, Hadac E, Larkin JM, Miller LJ. Dual pathways of internalization of the cholecystokinin receptor. J Cell Biol. 1995;128:1029–1041. doi: 10.1083/jcb.128.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding XQ, Dolu V, Hadac EM, Holicky EL, Pinon DI, Lybrand TP, Miller LJ. Refinement of the structure of the ligand-occupied cholecystokinin receptor using a photolabile amino-terminal probe. J Biol Chem. 2001;276:4236–4244. doi: 10.1074/jbc.M003798200. [DOI] [PubMed] [Google Scholar]
- 20.Abagyan R, Totrov M, Kuznetsov D. Icm - a new method for protein modeling and design - applications to docking and structure prediction from the distorted native confirmation. J Comp Chem. 1994;15:488–506. [Google Scholar]
- 21.Pellegrini M, Mierke DF. Molecular complex of cholecystokinin-8 and N-terminus of the cholecystokinin A receptor by NMR spectroscopy. Biochemistry. 1999;38:14775–14783. doi: 10.1021/bi991272l. [DOI] [PubMed] [Google Scholar]
- 22.Ding XQ, Dolu V, Hadac EM, Schuetz M, Miller LJ. Disulfide bond structure and accessibility of cysteines in the ectodomain of the cholecystokinin receptor: specific mono-reactive receptor constructs examine charge-sensitivity of loop regions. Receptors Channels. 2003;9:83–91. [PubMed] [Google Scholar]
- 23.Hadac EM, Pinon DI, Ji Z, Holicky EL, Henne RM, Lybrand TP, Miller LJ. Direct identification of a second distinct site of contact between cholecystokinin and its receptor. J Biol Chem. 1998;273:12988–12993. doi: 10.1074/jbc.273.21.12988. [DOI] [PubMed] [Google Scholar]
- 24.Ji Z, Hadac EM, Henne RM, Patel SA, Lybrand TP, Miller LJ. Direct identification of a distinct site of interaction between the carboxyl-terminal residue of cholecystokinin and the type A cholecystokinin receptor using photoaffinity labeling. J Biol Chem. 1997;272:24393–24401. doi: 10.1074/jbc.272.39.24393. [DOI] [PubMed] [Google Scholar]
- 25.Hadac EM, Dawson ES, Darrow JW, Sugg EE, Lybrand TP, Miller LJ. Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. J Med Chem. 2006;49:850–863. doi: 10.1021/jm049072h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy K, Escrieut C, Dufresne M, Clerc P, Vaysse N, Fourmy D. Identification of a region of the N-terminal of the human CCKA receptor essential for the high affinity interaction with agonist CCK. Biochem Biophys Res Commun. 1995;213:845–852. doi: 10.1006/bbrc.1995.2206. [DOI] [PubMed] [Google Scholar]