Abstract
Subjective, physiological and electroencephalographic (EEG) profiles were studied in cocaine dependent study participants in response to cocaine cue exposure or a dose of smoked cocaine. Both stimuli increased subjective ratings of cocaine high and craving, enhanced negative affect, and boosted plasma ACTH and skin conductance levels. However, cocaine dose produced a greater increase in high and a more prolonged increase in plasma ACTH, while cocaine cue produced a decline in skin temperature. Both stimuli produced increases in absolute theta, alpha and beta EEG power over the prefrontal cortex. However, interhemispheric EEG coherence over the prefrontal cortex decreased during cocaine cue exposure but increased following cocaine dose. Moreover, correlation analysis of subjective, physiological and EEG responding to cocaine cue and dose revealed distinct profiles. Delta and theta activity were associated with negative affect during cocaine cue exposure, but were associated with cocaine craving and reward following cocaine dosing. In both conditions, alpha activity was marker for anxiousness but not high. These data demonstrate similar subjective, physiological responding in clinical laboratory states of cocaine craving and reward. However, differences in EEG response profiles, and their relationship to function, indicate distinct neurophysiological mediators of cocaine craving and reward within the prefrontal cortex.
Keywords: cocaine, cues, ACTH, electroencephalography, craving, reward
1. Introduction
Understanding the neurobiological processes responsible for the motivation to use cocaine remains an important challenge in addiction research. Studies modeling the subjective and physiological effects of cocaine craving and reinforcement have been a key element in this endeavor, and are rapidly reaching a point of convergence. Currently, clinical lab studies show evidence for both cocaine craving and cocaine-like high in human stimulant abusers whom either receive an acute dose of cocaine or are exposed to cocaine-related environmental cues (Jaffe et al., 1989; Ehrman et al., 1992; Donny et al., 2006). Physiological data collected during each of these two paradigms also indicate a generalized arousal that is similar across both conditions. However, it remains unclear if these response profiles do in fact reflect similar neurobehavioral phenomena.
Studies employing a naturalistic model of cocaine administration, examining the effects of smoked cocaine, have found that cocaine acutely enhances a participant's ratings of “desire” or “wanting” to use cocaine (Ward et al., 1997; Foltin and Fischman, 1991; Hart et al., 2004). Additional acute effects of smoked cocaine include increases in heart rate and blood pressure, plasma cortisol levels, and enhanced subjective ratings including “high”, “good drug effect”, “stimulated” and “anxious” (Ward et al., 1997; Foltin and Fischman, 1991; Evans et al., 1999; Foltin and Haney, 2000). Studies on the effects of intravenously (i.v.) injected cocaine have reported similar subjective and physiological responses in cocaine dependent participants (Jaffe et al., 1989; Breiter et al., 1997; Foltin and Fischman, 1992, 1998; Donny et al., 2006). Cue-induced cocaine craving, readily elicited in cocaine dependent participants in a clinical laboratory setting, is characterized by an increase in subjective ratings of “urge” or “desire” to use cocaine, anxiety, enhanced plasma HVA and cortisol levels, and a rise in skin conductance accompanied by a decrease in skin temperature (O'Brien et al., 1990; Ehrman et al., 1992; Berger et al., 1996; Reid et al., 1998, 1999; Robbins et al., 1999). Many of these studies also reported an increase in self-reported cocaine high following cue exposure (O'Brien et al., 1990; Reid et al., 1998, 1999; Robbins et al., 1999). It should be noted, however, that ratings for cocaine high are consistently lower following cue exposure relative to cocaine dosing, that cues can also elicit subjective cocaine withdrawal-like effects, and that pharmacological modulation of cocaine craving and high is not always similar across both measures (Jaffe et al., 1989; Ehrman et al., 1992; Reid et al., 1998, 1999, 2003; Haney et al., 1999; Kouri et a., 2001; Foltin et al., 2003).
Recent fMRI and PET neuroimaging studies have begun to shed light on the similarities, and dissimilarities, of the neurobiological substrates of cocaine craving and high. Cocaine cue exposure has been demonstrated to increase blood oxygen level dependent (BOLD) activation in prefrontal cortex, anterior and posterior cingulate and parietal lobes (Maas et al., 1998; Garavan et al., 2000; Kilts et al., 2001), as well as regional blood flow measured by PET (18F-fluorodeoxyglucose) in the same regions plus the orbitofrontal and temporal cortex, amygdala and caudate (Grant et al., 1996; Childress et al., 1999; Bonson et al., 2002) in cocaine dependent individuals. In many of these studies regional brain activation was positively correlated with ratings of cocaine craving (Grant et al., 1996; Childress et al., 1999; Kilts et al., 2001; Bonson et al., 2002). Clinical studies on the acute effects of cocaine (0.6 mg/kg) infusion (Breiter et al., 1997) found short lasting BOLD activation in the ventral tegmental area, basal forebrain, caudate, anterior cingulate and prefrontal cortex that was positively correlated with ratings of drug high and longer lasting BOLD activation in the nucleus accumbens/subcallosal cortex, parahippocampal gyrus and prefrontal cortex that was positively correlated with ratings of cocaine craving. A more recent study employing a cocaine self-administration procedure (20 mg/70 kg, i.v.) reported a similar pattern of brain activation, and extended the prior findings by demonstrating opposing positive and negative correlations of the BOLD signal with ratings of cocaine craving and high, respectively (Risinger et al., 2005). These neuroimaging findings demonstrate extensive overlap among brain regions that are activated by cocaine craving and acute cocaine. However, their correlations with ratings of cocaine craving and high were not always in the same direction, or localized to the same brain region.
We have been investigating the quantitative EEG (qEEG) profiles of cocaine dependent study participants using neurometric analytical methods to topographically map the spectral power of each primary EEG bandwidth over the cortical surface (Prichep et al., 1996a,b, 1999; Reid et al. 2003, 2006). Similar to the MRI and PET studies, we found evidence for qEEG activation over the prefrontal cortex during cocaine cue exposure (Reid et al., 2003) and following smoked cocaine (Reid et al., 2006). In order to further investigate this apparent relationship of cocaine craving and cocaine high, we compared effects of cocaine cue exposure and smoked cocaine (50 mg, base formula) on qEEG profiles, subjective ratings, and physiological responding in a within subjects design. It was hypothesized that the relationship of qEEG to subjective and physiological responding would be different in each condition. Parts of these data were presented in an earlier report comparing the qEEG effects of cocaine and placebo administration (Reid et al., 2006).
2. Methods
This study was approved by the New York University School of Medicine Institutional Review Board and performed under FDA approved IND (# 60,631). Study participant recruitment, screening and testing took place at the New York University School of Medicine General Clinical Research Center (NYU GCRC) outpatient facilities and the New York University Medical Center, Tisch Hospital Inpatient Psychiatry Clinic. All participants provided informed written consent.
2.1. Participants
Study participants were crack cocaine dependent according to DSM-IV criteria from the SCID, with active habits verified by a urine drug screen positive for cocaine in the prior two weeks, and were not seeking or enrolled treatment for cocaine abuse. Individuals dependent on alcohol or other drugs of abuse (except nicotine), using prescribed psychotropic medications, or diagnosed with an axis I psychiatric disorder (other than addiction), were excluded. During the informed consent process, all participants were informed that in each dose session they had a 50:50 chance of receiving the cocaine dose. A total of 13 participants were tested (Table 1).
Table 1. Demographics and Drug Use History.
Means and standard deviation (SD) are reported.
| Demographics | Total | |
|---|---|---|
| Race | African American | 9 |
| Caucasian | 3 | |
| Hispanic | 1 | |
| Sex | Male | 11 |
| Female | 2 | |
| Mean (SD) | ||
| Age | 38.6 (11.0) | |
| Years of Education | 13.9 (4.0) | |
| Self Reported Drug and Alcohol Use | ||
| Number of days of drug use in last 30 days | ||
| Cocaine | 12.4 (3.5) | |
| Alcohol | 6.3 (1.8) | |
| Cannabis | 5.3 (10.1) | |
| Amphetamine | 0.0 (0.0) | |
| Number of days since last cocaine use | 3.1 (0.9) | |
| Money spent in last 7 days ($) | ||
| Alcohol | 10 (26) | |
| Cocaine | 110 (31) | |
| Years of Lifetime Use | ||
| Cocaine | 13 (4) | |
| Alcohol | 17 (14) | |
| Cannabis | 11 (13) | |
| Amphetamine | 0 (0) | |
| ASI Drug Composite Score | 0.155 (0.101) | |
| BDI | 8.7 (2.5) | |
Participants meeting study eligibility criteria were admitted into the Psychiatry Inpatient Clinic where they remained hospitalized as inpatients for a total of 48 hours; approximately 24 hr prior to testing and 16 hours following testing. During the inpatient hospitalization participants were allowed to smoke cigarettes 2-3 times a day under staff escort and standard hospital meals were provided. On the morning of testing the participants had a low-fat breakfast, followed by a cigarette break (for those that were smokers) approximately 1 hr before beginning test procedures.
2.2. Test Procedures
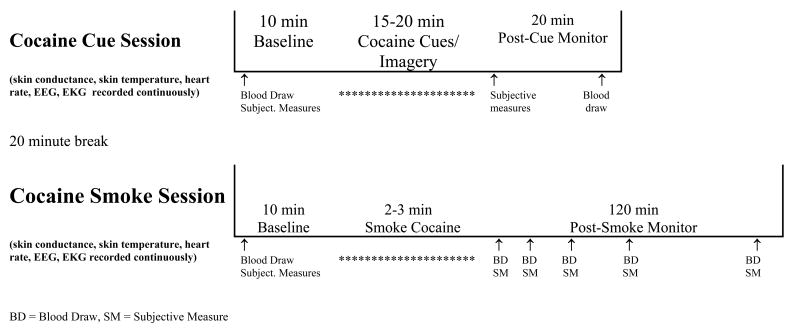
The cue exposure and cocaine smoking procedures took approximately 6 hours, including set-up and removal of intravenous (i.v.) catheters, physiological monitoring equipment and the EEG electrodes. Testing employed a within session, crossover study design, in which each participant underwent cocaine cue exposure followed by administration of a smoked dose of cocaine (50 mg, cocaine base formula) followed three hours later by cocaine cues and then a smoked dose of placebo (or vice versa). The order of dosing was randomly selected on a 1:1 ratio, using block sizes of 4, and was administered in a single blind fashion. Cocaine cue exposure testing was designed based on our previous cocaine cue reactivity studies (Reid et al., 2003). Cocaine smoking sessions were designed based on previous cocaine self-administration studies of Foltin and colleagues (Foltin and Haney, 2000). Cocaine cue sessions always preceded the cocaine dose sessions (see Figure 1). Placebo smoking session, and parts of the cocaine session, data are reported elsewhere (Reid et al., 2006).
Figure 1. Study Design.
Study design for cocaine cue exposure testing and assessment followed by cocaine dose testing and assessment.
2.2.1. Cocaine Cue Exposure Testing
Upon the beginning of testing a standard automated blood pressure cuff and an 18 gauge catheter was inserted into a subcutaneous vein (for repeated blood draws, kept patent by infusion of a dextrose (5%) solution at 100 cc/h) on the participant's writing arm, ECG and heart rate monitor leads were applied to the chest, and EEG leads were placed on the scalp, and skin conductance and temperature leads were placed on the participant's fingertips on the non-writing arm. Initially, heart rate and blood pressure was measured, blood samples were taken, and the participant was given the Cocaine Session Rating Scale and the Mood Analog Scale. Then 10 minutes (minute 0-5, eyes closed; minute 5-10, eyes open) of baseline resting EEG, skin conductance, and skin temperature were collected. Thereafter, each participant underwent a 15 minute cocaine cue exposure procedure that involved viewing and handling crack cocaine related items (previously used glass stems, $20 bill, lighter, small plastic “dime bags” containing simulated crack) placed in front of him/her on a table top (minute 0-5, eyes open), viewing a video tape of scenes depicting the preparation, sales and smoking of crack cocaine (minute 5-10, eyes open), and undergoing a guided imagery session during which participants were instructed to think of the scenario they most commonly associate with the purchase and subsequent use of crack cocaine (time, place, and person) (minute 10-15, eyes closed). This procedure was designed to replicate the conditioned stimuli (CS+) that participants associate with crack cocaine. Immediately after cue presentation (within 1 minute), participants completed the Cocaine Session Rating Scale and the Mood Analog Scale and heart rate and blood pressure was measured. Twenty minutes later another blood sample was obtained.
2.2.2. Cocaine Dose Testing
Participants smoked from a standardized glass stem, a commonly used form of crack cocaine smoking paraphernalia in the New York City metropolitan area. The stems were made from glass Pyrex tubes (8 mm diameter) (Fischer Scientific) cut into 10 cm lengths with a metal screen placed approximately 1 cm from one end. In order to obtain oral stimulation (smell and taste) similar to that of a used stem, three 10 mg cocaine base pellets had been burned through the tubes under a ventilated laboratory hood prior to use. Stems were replaced after every four participants.
Dose testing began 20 minutes after collection of the post-cue blood sample (40 minutes after cue exposure was completed). All settings and leads (blood pressure cuff, EEG, ECG, skin conductance and temperature) remained on as positioned during the cocaine cue testing and were checked for recording validity before commencing. Initially, blood samples were taken, the participant was given the Cocaine Session Rating Scale and the Mood Analog Scale, and then 10 minutes (minute 0-5, eyes closed; minute 5-10, eyes open) of baseline resting EEG, and skin conductance and skin temperature were collected. Thereafter, each participant underwent a 1 minute CS+ exposure that involved briefly viewing crack cocaine related items placed in front on a table top, being instructed that they “are now going to smoke a dose of crack cocaine”, and selecting a glass stem from which to smoke crack cocaine (this procedure was included for placebo/CS+ response testing – see Reid et al., 2006).
Following the brief CS+ procedure, heart rate and blood pressure were assessed to assure that cardiovascular activity was not above criteria for safe drug administration (HR<130, DP<100, SP<165) and then the cocaine smoking session began. The research staff member placed a blindfold over the participant's eyes, handed a prepared glass stem with a cocaine dose (50 mg, cocaine base) or an empty stem (placebo) to the participant, and then, holding a flame over the stem, instructed the participant to take one large inhalation and hold it as long as they would under routine smoking practices. Once the participant had inhaled the dose and then exhaled, the staff member took the stem from the participant and removed the blindfold. ECG was monitored continuously, and heart rate and blood pressure were assessed monitored every 5 min, beginning 30 min prior to cocaine dosing and continuing for 1 hr following dosing (every 2.5 min for 20 min following dosing). The Cocaine Session Rating Scale and the Mood Analog Scale were completed, and blood pressure measures, at 5, 10, 20, 40 and 60 minutes post-dose administration, followed by blood samples collected at the same time points (except for the 5 minute time point). The heart rate and EEG recordings were recorded continuously throughout the session. Eyes open, resting EEG data were obtained during minute 0-5, 15-20, 35-40, and 55-60 post-dose administration, and eyes closed, resting EEG were obtained during minute 5-10, 20-25, 40-45, and 60-65 post-dose administration.
2.3. Assessments
Cocaine craving, high, and related subjective effects were assessed using the Cocaine Session Rating Scale, based on the work of Foltin and colleagues (Foltin and Fischman, 1991; Foltin and Haney, 2000) and our previous cocaine craving and dosing studies (Reid et al, 2003, 2006), while mood variables were assessed using the Mood Analog Scale (Berger et al., 1996; Reid et al, 1998).
2.3.1.Cocaine Session Rating Scale (CSRS)
Participants rated the intensity of cocaine-like high (Cocaine Reward), their desire to use cocaine and likelihood to use cocaine if it were available (desire and likelihood to use were averaged to obtain an index score for Cocaine Craving), and cocaine-like withdrawal on a 1 to 100 visual analog scale.
2.3.2. Mood Analog Scale
Participants rated 16 adjectives describing their current feelings on a 1 to 100 visual analog scale where above the appropriate numbers were the adjectives, not at all, mildly, moderately, and extremely.
2.3.3. Plasma Sampling
Blood samples were spun for plasma extraction at 4 degrees Celsius and immediately frozen on dry ice before transferring to storage at -70° C. Samples were analyzed for cocaine by mass spectrometer and for cortisol by radioimmunoassy at the Analytical Psychopharmacology Laboratory of Mr. Thomas Cooper (Nathan Kline Institute, Orangeburg, NY), and for ACTH by radioimmunoasssay in the laboratory of Dr. Mary Jeanne Kreek (Laboratory of the Biology of Addictive Disorders, The Rockefeller University, New York, NY).
2.3.4. Psychophysiological Measures
Blood pressure and pulse were recorded using an automated vital signs monitoring unit (ProPaq Emcore, Protocol System Inc., Beaverton, OR).
2.3.5. EEG Acquisition
EEG data were collected from the 19 monopolar electrode (NaCl disc electrodes) sites of the International 10/20 System referenced to linked earlobes. In addition, transorbital electrodes were used to detect eye movement (EOG) and a neck electrode was used to monitor electrocardiogram activity (ECG). The electrophysiological data acquisition system used was a CadwellEasy EEG System 1.7 (Kennewick, WA) which has a bandpass of 0.016 to 70 Hz, a 60 Hz notch filter, and digitizes at 2400 Hz with 16 bit resolution.
2.3.6. EEG Quantification
Using neurometric analysis, the quantitative features of the EEG were extracted, log transformed to obtain normal (Gaussian) distributions, age-regressed and Z transformed relative to age appropriate population norms. Neurometric qEEG norms with high test-retest reliability have been derived and published (John et al., 1988; Ahn et al., 1980) from 550 normally functioning subjects aged 6-90 at the co-investigator's laboratory. During recordings raw data were continuously monitored for movement artifact, EOG activity, evidence of drowsiness, and other sources of artifact for later deletion. Captured EEG data was then recorded onto an optical disk for subsequent visual review and the selection of artifact-free epochs by a blinded rater. Artifact removal was performed by visual analysis of the raw EEG, EOG and ECG data to determine movement artifact from muscle tension and eye movement. Any epoch in which any region exceeded the artifact levels was excluded from analyses. Between one and two minutes of baseline, cue exposure, and post dose artifact free EEG data, from pre-defined intervals, were obtained and subjected to quantitative analyses using Fast Fourier Transform (FFT). The cue exposure recording periods were: minute 0-5 (paraphernalia handling, eyes open), minute 5-10 (video viewing, eyes open), minute 10-15 (guided imagery, eyes closed). The post-dosing recording periods were: minute 0-5 (eyes open), minute 5-10 (eyes closed), minute 15-20 (eyes open), minute 20-25 (eyes closed), minute 35-40 eyes open), and minute 40-45 (eyes closed). For each of the 19 monopolar derivations absolute power were computed for the delta (1.5-3.5 Hz), theta (3.5-7.5), alpha (7.5-12.5 Hz) and beta (12.5-25 Hz) frequency bands. Inter-hemispheric coherence and symmetry between homologous leads in the four frequency bands were also computed, taking into account the linked ear reference electrodes. The qEEG data were scaled in the metric of probability as standard deviation units (Z-score) relative to the age appropriate normal population, and displayed in color coded Z-score images for each condition. In estimating the confidence level of Z scale values for group data, the square root of sample size and the standard deviation of each group must be taken into account. For the current study (n=13) the range of Z-scores presented is ± 1.25, where p<0.05 is equivalent to a Z-score difference of 0.5.
2.4. Statistical Analyses
The effects of cocaine cue vs. cocaine dose were analyzed for this manuscript (the effects of placebo dose are presented elsewhere (Reid et al., 2006). Primary outcome measures of cue and dose responding were the CSRS scores for cocaine high, and cocaine craving and the neurometric qEEG activity profiles from leads over prefrontal cortex (FP1, FP2). The brain region selected for qEEG analysis (FP1 and FP2) was chosen a priori based on earlier studies demonstrating activation of prefrontal cortex during cocaine craving and cocaine high (Reid et al., 2003, 2006). Secondary outcome measures included the Mood Analog Scale items “anxious”, “nervous”, and “irritable”, plasma cortisol and ACTH levels, heart rate, blood pressure, skin conductance and skin temperature. Tests for order effects were performed comparing cue and dose responding between the first and second sessions, and none were found.
2.4.1. Subjective and Physiological Measures
Initially, tests comparing measures pre- to post-cue and pre- to post-cocaine dose were performed to determine the stimulation effects obtained in each condition. The subjective, cardiovascular, and neuroendocrine effects were analyzed by ANOVA comparing pre- and post-stimulation values. Skin conductance and skin temperature analyzed by repeated measures ANOVA comparing the first 15 minutes of stimulation (cue presentation or post-dose) analyzed with 1 minute averages. For the assessment of dose effects on subjective, cardiovascular, and neuroendocrine measures the maximal change was employed (this occurred within the first 10 minutes in all participants).
For the comparison of cue versus cocaine responding, conditions were analyzed in a 2 (cue condition, cocaine condition) by 2 (pre-stimulation, post-stimulation) within subjects ANOVA design. In each case, the cue session immediately preceding the dose session was included in the statistical model. Skin conductance and skin temperature were compared using a 2 (cue condition, cocaine condition) by 15 (one minute measurement intervals) within subjects repeated measures ANOVA. Bonferroni corrections for multiple comparisons of cue and cocaine data were applied to these analyses.
2.4.2. Neurophysiological Measures
For the qEEG data, the Z-scores for each recording condition (baseline, cocaine cue, cocaine dose) relative to the age matched normal population were used for statistical analyses. Z-scores for absolute power and coherence at each bandwidth (delta, theta, alpha, and beta) obtained from the leads over the prefrontal cortex (FP1 and FP2) were analyzed using Hotellings T2 statistics to compare the multivariate differences. Cue and dose intervals analyzed included the pre-cue baseline, 5-10 and 10-15 minute cue exposure intervals (0-5 minute paraphernalia handling qEEG was not analyzed due to movement artifact), and the pre-dose baseline, 0-5 and 5-10 minute post-dose intervals (period when maximum cocaine craving and high were reported). Comparisons between different recording sessions were always made in the same condition, eg. eyes closed pre-cue baseline (minute 5-10) versus eyes closed guided imagery cocaine craving (min 10-15). Initially, cocaine cue versus baseline and cocaine dose versus baseline analyses were performed to determine the stimulation effects obtained in each test condition. Thereafter, cocaine cue versus cocaine dose comparisons were performed. In all comparisons of cue versus cocaine effects the corresponding cue session immediately prior to dosing was employed for the analysis.
2.4.3. Correlation Analyses
The increase (relative to baseline) in the subjective and physiological measures that were significantly different between pre- and post-cocaine cue, or between pre- and post-cocaine dose, were calculated for correlation analyses. For the analysis of cocaine cue responding, the pre- to post-cue difference value of each measure was used. For the analysis of cocaine dosing effects, the peak increase of each measure (relative to pre-dose baseline) during the first 10 minutes after dosing was used. Pearson's correlations compared these subjective and physiological measures within each testing condition. In addition, these measures were also compared with qEEG data obtained within the same testing condition. For the analysis of cue responding, Z-transformed qEEG data obtained from the FP1 and FP2 leads during the 5-10 minute video viewing and the 10-15 minute guided imagery session were used for correlation analyses. For the analysis of cocaine dose responding, Z-transformed qEEG data obtained from the FP1 and FP2 leads during the 0-5 and 5-10 minute post dosing interval was used for correlation analyses. Canonical (absolute power) and Pearson's (coherence) correlations compared the subjective and physiological measures with qEEG in each bandwidth. To limit the number of comparisons, the subjective and physiological response measures employed were limited to those significantly affected by cue exposure or cocaine dose.
3. Results
3.1. Individual Stimulus Conditions
3.1.1. Cocaine Cue Reactivity
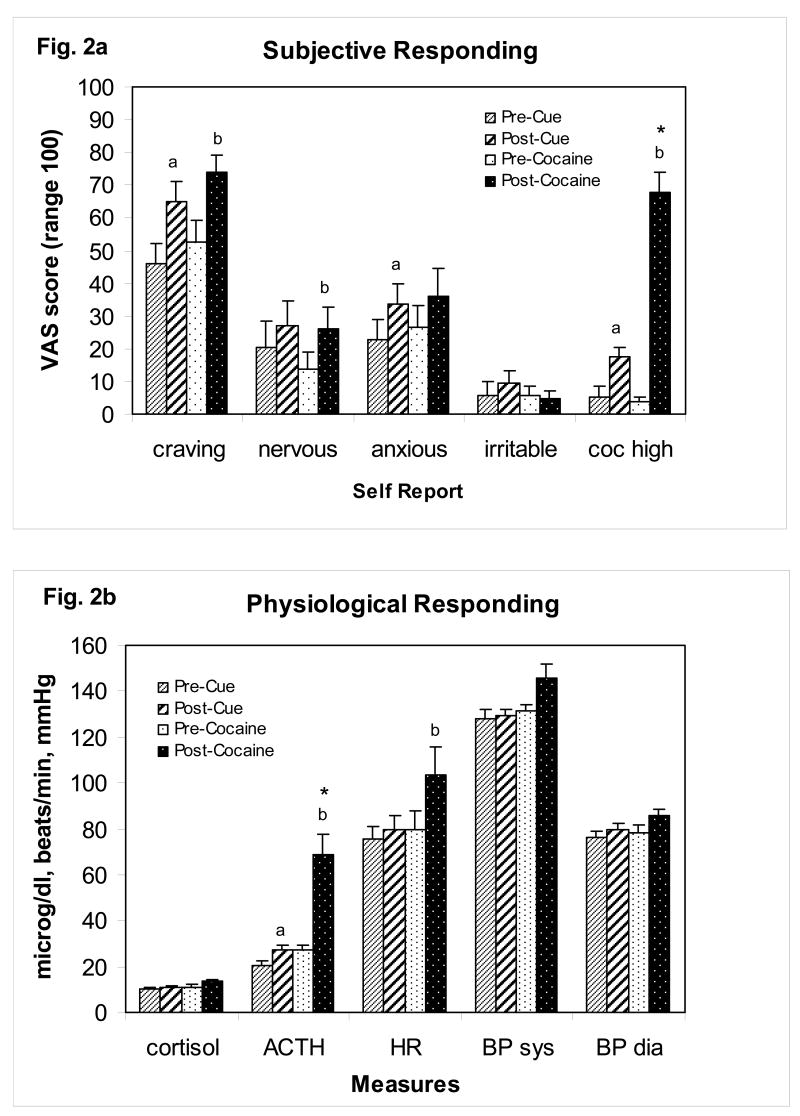
The effects of cocaine cue exposure on subjective and physiological measures relative are displayed in Figure. 2a,b. Relative to pre-cue baseline levels, cocaine cue exposure elicited an increase in subjective ratings for cocaine craving (F(1,24)=5.68, p<0.05), cocaine-like high (F(1,24)=8.27, p<0.01) and “anxious” (F(1,24)=4.98, p<0.05), an increase in skin conductance (F(1,230)=3.72, p<0.001) (data not shown) and plasma ACTH levels (F(1,20)=4.12, p=0.05), and a decrease in skin temperature (F(1,199)=9.99, p<0.001) (data not shown). Correlation analyses of subjective and physiological responding revealed a positive association of cue-induced cocaine craving with ratings of “anxious” (r=0.56, p<0.05) and a positive association between cue-induced ACTH and cortisol levels (r=0.60, p<0.05). Cue-induced cocaine-like high was not associated with any other cue responses.
Figure 2a,b.
The subjective and physiological effects of cocaine cue exposure or smoked cocaine base (50 mg) dose in cocaine dependent patients (n=13). For cocaine cue testing, measures were collected 10 minutes prior to cue exposure and within 1 minute after completion of cue exposure. For cocaine dose testing, measures were collected 10 minutes prior to dosing (baseline) and again at 5 and 10 minutes following dosing, and the peak increase in each measure following dosing is presented. Cue testing preceded dose testing, separated by a 40 minute rest period, for all patients. In a) the subjective ratings are presented. In b) the physiological measures are presented. The full time course for the cardiovascular and subjective effects of cocaine were previously reported (Reid et al., 2006). Abbreviations and symbols - coc high: cocaine high, ACTH: adrenocorticotropin hormone, HR: heart rate, BP: blood pressure, sys: systolic, dia: diastolic, a indicates p<0.05 for comparison with pre-cue value, b indicates p<0.05 for comparison with pre-cocaine value, and * indicates p<0.05 for ANOVA comparison between cocaine and cue responding.
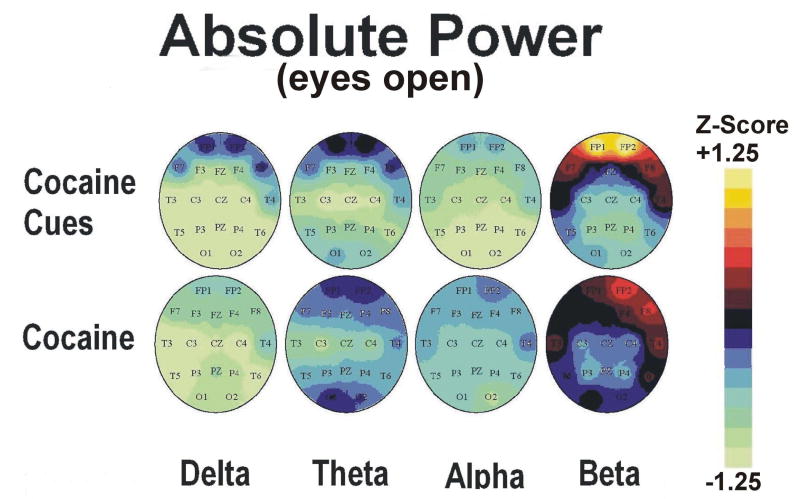
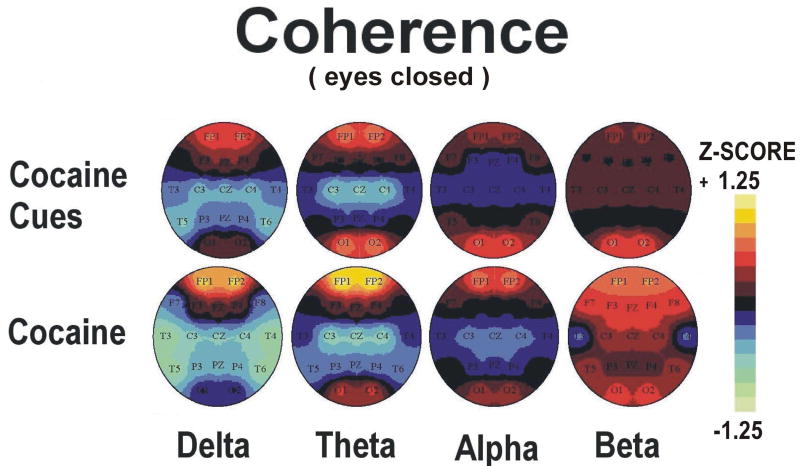
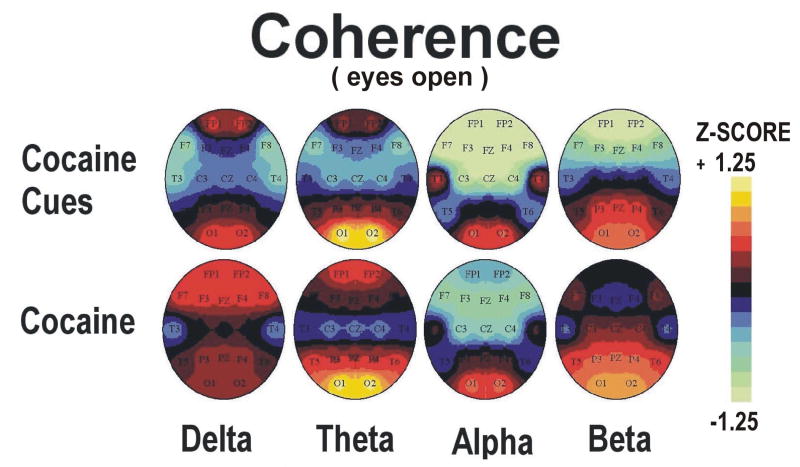
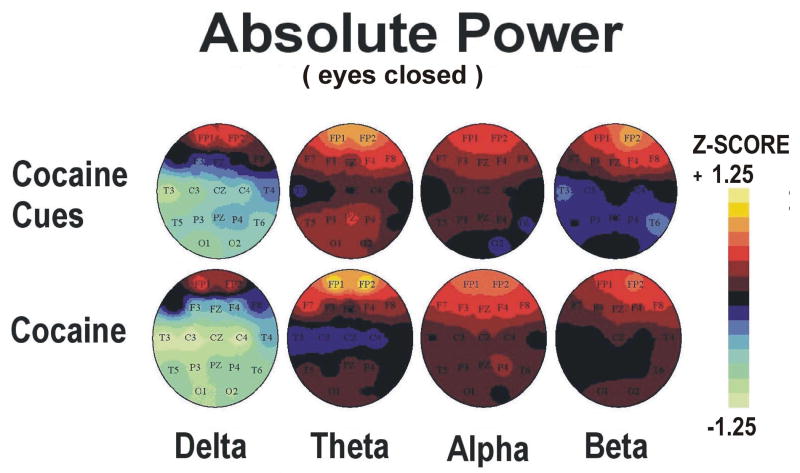
Topographic maps of qEEG absolute power and coherence Z scores during minutes 5-10 (video viewing) and 10-15 (guided imagery) phases of cocaine cue exposure are displayed in Figures 3-6 (upper panels). Statistical analyses of the effects of cocaine cue exposure relative to pre-cue baseline are displayed in Table 2a,b. Briefly, cocaine cue, eyes open (video viewing) produced an increase in delta, theta and beta power, but a decrease in delta, theta, and beta coherence, over the prefrontal cortex (Figures 3 and 4). Cocaine cue, eyes closed (guided imagery) sessions produced an increase in delta, theta, alpha and beta power, and a decrease in alpha coherence over the prefrontal cortex (Figures 5 and 6). Correlation analyses of qEEG over the prefrontal cortex during the video viewing sessions revealed a negative association of theta (r=-0.66, p<0.05) and alpha (r=-0.77, p<0.01) coherence with ratings of cocaine-like high, and a positive association of delta (r=0.58, p<0.05) and theta (0.67, p<0.05) coherence with ratings of “anxious”. Similar correlation analyses during the guided imagery sessions revealed a negative association of alpha coherence with ratings of cocaine-like high (r=-0.63, p<0.05).
Figure 3.
Group average topographic head maps of Z-scores for absolute power in delta (1.5-3.5 Hz), theta (3.5-7.5), alpha (7.5-12.5 Hz) and beta (12.5-25 Hz) frequency bands averaged across five min recording periods in an eyes open recording condition: Upper panel: Cocaine Cue during video viewing, Lower panel: Cocaine Dose during minutes 0-5 following dosing (50 mg cocaine base). Color coding, in steps corresponding to those shown in the Z-scale (range ± 1.25), is proportional to the difference in Z-scores from a healthy normative population. In estimating the confidence level of Z scale values for this group dataset (n=13), the significance level p<0.05 applies to a Z-score difference between conditions of 0.5 or greater.
Figure 6.
Group average topographic head maps of Z-scores for coherence in delta (1.5-3.5 Hz), theta (3.5-7.5), alpha (7.5-12.5 Hz) and beta (12.5-25 Hz) frequency bands averaged across five min recording periods in an eyes closed recording condition: Upper panel: Cocaine Cue during guided imagery, Lower panel: Cocaine Dose during minutes 5-10 following dosing (50 mg cocaine base). Color coding, in steps corresponding to those shown in the Z-scale (range ± 1.25), is proportional to the difference in Z-scores from a healthy normative population. In estimating the confidence level of Z scale values for this group dataset (n=13), the significance level p<0.05 applies to a Z-score difference between conditions of 0.5 or greater. Head maps in the lower panel are reprinted with permission from the American College of Neuropsychopharmacology from the publication; Neuropsychopharmacology 2006 31, 872-884.
Table 2. Cocaine Cue and Dose Effects: Hotellings T2 Tests.
| Cocaine Cue Exposure | |||||
|---|---|---|---|---|---|
| A |
Comparison of qEEG Measures: FP1 & FP2
Eyes Open – Baseline vs. Video Viewing |
t | df | P value | change |
| Delta Power | 5.34 | 2,11 | <0.05 | increase | |
| Delta Coherence | 6.06 | 1,12 | <0.05 | decrease | |
| Theta Power | 7.11 | 2,11 | <0.05 | increase | |
| Theta Coherence | 7.20 | 1,12 | <0.05 | decrease | |
| Alpha Power | 1.51 | 2,11 | 0.262 | n.s. | |
| Alpha Coherence | 0.68 | 1,12 | 0.427 | n.s. | |
| Beta Power | 5.38 | 2,11 | <0.05 | increase | |
| Beta Coherence | 12.86 | 1,12 | <0.05 | decrease | |
| B |
Comparison of qEEG Measures: FP1 & FP2
Eyes Closed - Baseline vs. Imagery |
t | df | P value | change |
| Delta Power | 9.02 | 2,10 | <0.01 | increase | |
| Delta Coherence | 2.05 | 1,11 | 0.180 | n.s | |
| Theta Power | 18.38 | 2,10 | <0.01 | increase | |
| Theta Coherence | 1.38 | 1,11 | 0.265 | n.s | |
| Alpha Power | 9.14 | 2,10 | <0.01 | increase | |
| Alpha Coherence | 7.14 | 1,11 | <0.01 | decrease | |
| Beta Power | 7.53 | 2,10 | <0.05 | increase | |
| Beta Coherence | 2.07 | 1,11 | 0.178 | n.s. | |
| Cocaine Dose | |||||
| C |
Comparison of qEEG Measures: FP1 & FP2
Eyes Open, Baseline vs. Post-Dose (min 0-5) |
t | df | P value | change |
| Delta Power | 0.41 | 2,9 | 0.675 | n.s. | |
| Delta Coherence | 2.17 | 1,10 | 0.227 | n.s. | |
| Theta Power | 2.80 | 2,9 | 0.113 | n.s. | |
| Theta Coherence | 1.27 | 1,10 | 0.711 | n.s. | |
| Alpha Power | 3.84 | 2,9 | 0.062 | n.s.* (up) | |
| Alpha Coherence | 1.24 | 1,10 | 0.746 | n.s. | |
| Beta Power | 0.69 | 2,9 | 0.527 | n.s. | |
| Beta Coherence | 1.02 | 1,10 | 0.959 | n.s. | |
| D |
Comparison of qEEG Measures: FP1 & FP2
Eyes Closed, Baseline vs. Post-Dose (min 5-10) |
t | df | P value | change |
| Delta Power | 1.97 | 2,11 | 0.186 | n.s. | |
| Delta Coherence | 1.35 | 1,12 | 0.613 | n.s | |
| Theta Power | 4.37 | 2,11 | <0.05 | increase | |
| Theta Coherence | 1.59 | 1,12 | 0.433 | n.s | |
| Alpha Power | 2.37 | 2,11 | 0.139 | n.s. | |
| Alpha Coherence | 1.70 | 1,12 | 0.372 | n.s. | |
| Beta Power | 6.38 | 2,11 | <0.05 | increase | |
| Beta Coherence | 2.92 | 1,12 | 0.076 | n.s.* (up) |
Figure 4.
Group average topographic head maps of Z-scores for coherence in delta (1.5-3.5 Hz), theta (3.5-7.5), alpha (7.5-12.5 Hz) and beta (12.5-25 Hz) frequency bands averaged across five min recording periods in an eyes open recording condition: Upper panel: Cocaine Cue during video viewing, Lower panel: Cocaine Dose during minutes 0-5 following dosing (50 mg cocaine base). Color coding, in steps corresponding to those shown in the Z-scale (range ± 1.25), is proportional to the difference in Z-scores from a healthy normative population. In estimating the confidence level of Z scale values for this group dataset (n=13), the significance level p<0.05 applies to a Z-score difference between conditions of 0.5 or greater.
Figure 5.
Group average topographic head maps of Z-scores for absolute power in delta (1.5-3.5 Hz), theta (3.5-7.5), alpha (7.5-12.5 Hz) and beta (12.5-25 Hz) frequency bands averaged across five min recording periods in an eyes closed recording condition: Upper panel: Cocaine Cue during guided imagery, Lower panel: Cocaine Dose during minutes 5-10 following dosing (50 mg cocaine base). Color coding, in steps corresponding to those shown in the Z-scale (range ± 1.25), is proportional to the difference in Z-scores from a healthy normative population. In estimating the confidence level of Z scale values for this group dataset (n=13), the significance level p<0.05 applies to a Z-score difference between conditions of 0.5 or greater. Head maps in the lower panel are reprinted with permission from the American College of Neuropsychopharmacology from the publication; Neuropsychopharmacology 2006 31, 872-884.
3.1.2. Cocaine Dose Effects
The effects of cocaine dosing on subjective and physiological measures are displayed in Figure. 2a,b. Relative to pre-dose baseline levels, cocaine produced a significant increase in subjective ratings for cocaine craving (F(1,24)=4.14, p<0.05), cocaine high (F(1,24)=46.10, p<0.01) and “nervous” (F(1,24)=4.00, p<0.05). In addition, there were significant increases in heart rate (F(1,16)=4.95, p<0.05), plasma ACTH levels (F(1,16)=8.98, p<0.01), skin conductance (F(1,230)=6.63, p<0.001) (data not shown) and skin temperature (F(1,199)=22.03, p<0.001) (data not shown). These effects peaked within the first 10 minutes after dosing. The subjective and cardiovascular effects generally returned to baseline levels within 40 minutes while the changes in autonomic arousal (skin conductance and temperature, ACTH) decreased gradually across the 60 minute recording period. The full time course for the cardiovascular and subjective effects of cocaine were previously reported (Reid et al., 2006). Correlation analyses of subjective and physiological responding following cocaine revealed a positive association between cocaine-induced ratings of high and “anxious” (r=0.57, p<0.05), a positive association between cocaine-induced ACTH and cortisol levels (r=0.67, p<0.05), and a positive association between cocaine-induced ratings of craving and both “anxious” (r=0.71, p<0.01) and skin temperature (r=0.71, p<0.05).
Topographic maps of qEEG absolute power and coherence Z scores during minutes 0-5 (eyes open) and 5-10 (eyes closed) following cocaine are displayed in Figures 3-6 (lower panels). The min 5-10 (eyes closed) qEEG data were previously reported (Reid et al., 2006). Statistical analyses of the effects of cocaine relative to pre-dose baseline levels are displayed in Table 2c,d. Cocaine, min 0-5 post dosing, produced a trend level increase in alpha power over the prefrontal cortex(Figures 3 and 4). Cocaine, min 5-10 post dosing, produced an increase in theta and beta power over the prefrontal cortex and a trend level increase in beta coherence (Figures 5 and 6). Correlation analyses of qEEG over the prefrontal cortex during minutes 0-5 post cocaine revealed a positive association of delta power with ratings of cocaine craving (r=0.76, p<0.05), a positive association of alpha power with ratings of “nervous”(r=0.85, p<0.05), and a negative association of beta coherence with the decrease in skin temperature (r=-0.71, p<0.05). Similar correlation analyses during minutes 5-10 post cocaine revealed a positive association of theta power (r=0.72, p<0.05) with ratings of “good drug effect”, a negative association of delta (r=-0.78, p<0.05) and theta (r=-0.77, r<0.05) coherence with ratings of “nervous”, and a positive association of alpha power with ratings of “nervous” (r=0.71, r<0.05). The r values for these correlations were previously reported (Reid et al., 2006).
3.2. Comparison of Stimulus Conditions
3.2.1. Cocaine Cue vs. Cocaine Dose: Subjective and Physiological Responding
Peak subjective and physiological responses produced by cocaine cue exposure and cocaine dosing are represented in Figure 2a,b Both stimuli produced statistically similar increases in subjective ratings for cocaine craving, “nervous”, “anxious” and “irritable”. In addition, heart rate, systolic blood pressure, diastolic blood pressure, and plasma cortisol level responding were similar. However, cocaine dosing produced a greater increase in subjective ratings for cocaine high (F(1,12) 42.280, p<0.001) and in plasma ACTH levels (F(1,11)=9.111, p<0.01). Skin conductance, compared across the first 15 minutes after the onset of stimulation, was statistically similar across both conditions. Skin temperature, however, showed an opposite reduction during cocaine cue exposure (F(14, 140)=3.650, p<0.001).
3.2.2. Cocaine Cue vs Dosing qEEG: Eyes Open Recording Condition
Topographic maps of qEEG absolute power and coherence during cocaine cue video viewing and during minutes 0-5 following cocaine (eyes open) are displayed in Figures 3-4. Hotellings T2 test comparisons of absolute power over the prefrontal cortex (FP1 and FP2) revealed no significant differences in EEG power during cocaine cue video viewing relative to the cocaine dose condition. Hotellings T2 test comparisons of coherence over the prefrontal cortex (FP1 and FP2) revealed significantly lower levels of alpha (F=10.32, p<0.01) and beta (F=41.29, p=0.001) coherence during cocaine cue video viewing relative to the cocaine dose condition.
3.2.3. Cocaine Cue vs Dosing qEEG: Eyes Closed Recording Condition
Topographic maps of qEEG absolute power and coherence during cocaine cue guided imagery and during minutes 5-10 following cocaine dose (eyes closed) are displayed in Figures 5-6. Hotellings T2 test comparisons of absolute power over the prefrontal cortex (FP1 and FP2) revealed lower (at a trend levels) alpha power (F=3.83, p=0.058) during cocaine cue guided imagery relative to the cocaine dose condition. Hotellings T2 test comparisons of coherence over the prefrontal cortex (FP1 and FP2) revealed significantly lower levels of theta (F=5.50, p<0.01) coherence during cocaine cue guided imagery relative to cocaine dose.
4. Discussion
This study sought to compare and contrast the physiological and neurobiological substrates of cocaine craving and reward. The experimental model included a sequence of cocaine cues, dosing self-expectancy, and then cocaine smoking resulting in a naturalistic model of human cocaine abuse. Conditions were designed to elicit a clear increase in craving during cocaine cue exposure, and a clear increase in cocaine reward following an acute dose of cocaine, and in each case the intended response was reliably induced. The response patterns elicited in each condition were consistent with previous reports on the subjective, physiological and regional neuronal activation effects of cocaine cue exposure (Ehrman et al., 1992; O'Brien et al., 1990; Berger et al., 1996; Reid et al., 1998, Robbins et al., 1999; Maas et al., 1998; Garavan et al., 2000; Grant et al., 1996; Childress et al., 1999; Kilts et al., 2001; Bonson et al., 2002) and acute cocaine (Herning et al., 1985, 1994; Jaffe et al., 1989; Ward et al., 1997; Foltin and Fischman, 1991, 1997; Evans et al., 1999; Breiter et al., 1997; Donny et al., 2004, Risinger et al., 2005; Kufahl et al., 2005) in cocaine dependent individuals, in which direct evidence for cocaine craving and high, autonomic arousal, and enhanced neurophysiological activity in the limbic cortex was obtained in both conditions.
The subjective response to cocaine cues was similar to that of cocaine dose on numerous measures. In both cocaine cue and dose conditions subjective measures of craving, “nervous” and “anxious” were reported at similar levels, and the increase in cocaine craving was positively associated with ratings of anxiousness. However, while cocaine cues produced a mild increase in self-reported “cocaine-like high” cocaine dosing produced a significantly greater increase in cocaine high, and only in this condition was cocaine high positively associated with other subjective measures. It must be recognized, however, that the subjective response to cocaine dose lasted for up to 40 minutes (see also Reid et al., 2006) while responding to cocaine cue exposure, though not assessed beyond the first minute following cue exposure in the present study, does not generally last more than 10-15 minutes (Ehrman et al., 1992; O'Brien et al., 1990; Berger et al., 1996; Reid et al., 1998, 1999, 2003; Robbins et al., 1999).
Cocaine dosing produced strong increases in cardiovascular output and autonomic arousal, as reflected by increases in heart rate (see also Reid et al., 2006), skin conductance, skin temperature, and plasma ACTH levels. Some of these autonomic arousal effects were also produced by cocaine cue exposure, though cardiovascular activity remained unaffected and skin temperature showed an opposing downward response. The only statistically significant differences in physiological responding between cue and cocaine conditions were seen with plasma ACTH levels and skin temperature. The stronger ACTH response to cocaine dose indicates a potent pharmacological effect on the HPA system, and is consistent with previous studies on acute cocaine in humans (Ward et al., 1999) and primates (Broadbear et al., 2004). The decrease in skin temperature during cue exposure indicates activation of the sympathetic nervous system. Moreover, cue-induced craving was positively associated with this decline in skin temperature. Studies comparing cue- and stress-induced craving have reported a robust, positive association of subjective anxiety and cocaine craving (Sinha et al., 2003). On the other hand, others have reported that the rewarding and cocaine craving effects of acute cocaine are not modulated by cortisol blockade (Ward et al., 1997). These reports, and the current findings, indicate that autonomic responding, while elicited in both stimulus conditions, is more closely associated with cue-induced craving than with cocaine-induced reward and craving.
Cocaine cue exposure and cocaine dosing produced similar increases in theta and beta EEG power over the prefrontal cortex (see also Reid et al., 2006). These findings are consistent with prior neuroimaging studies which have reported increased EEG power, metabolism and BOLD signals in the medial cingulate and prefrontal cortex in response to cue exposure (Maas et al., 1998; Garavan et al., 2000; Grant et al., 1996; Childress et al., 1999; Kilts et al., 2001; Bonson et al., 2002) and cocaine dosing (Herning et al., 1985, 1994; Breiter et al., 1997; Risinger et al., 2005; Kufahl et al., 2005) in cocaine dependent study participants. The current findings expand upon these prior studies by demonstrating that the amplitude of neurophysiological activation in this brain region is similar in both conditions.
There was, however, a difference in alpha EEG power over the prefrontal cortex which was higher following cocaine relative to cue exposure. Increases in alpha EEG following cocaine dosing have been shown in previous studies (Herning et al., 1994; Lukas et al.,1990, 1991). In contrast, our group (Reid et al., 2003) and others (Liu et al., 1998) have reported decreases in alpha EEG during cocaine cue exposure. These findings indicate that alpha power may be used to distinguish between cocaine cue and dose conditions. Moreover, alpha power was positively associated with cocaine-induced nervousness while alpha coherence was negatively associated with cue-induced cocaine-like high. Alpha power was also increased following placebo dosing, a condition in which drug reward was not observed, and alpha coherence was positively associated with plasma cortisol levels following cocaine dosing (see Reid et al, 2006). Elevated alpha activity has also been demonstrated in obsessive compulsive disorder (John and Prichep, 2006). These findings indicate that alpha EEG is a marker for cocaine induced arousal and anxiety, but not for the rewarding effects of cocaine. Consistent with this, others have reported a negative correlation between alpha power and ratings of “good drug” or “use again” following acute cocaine (Herning et al., 1994). While some have suggested that alpha power is associated with drug reward (Lukas et al., 1990, 1991) these findings were related to changes in EEG localized over parietal and occipital cortex.
In contrast to the findings on EEG power, EEG coherence over the prefrontal cortex decreased during cocaine cue exposure but increased following cocaine dosing. EEG coherence during wakefulness is suggested to represent functional connectivity of widespread brain regions (John and Prichep, 2006). Statistical comparison of the two conditions revealed a significant difference in the theta and alpha bandwidths. The higher levels of theta coherence, and the finding that theta power was positively associated with “good drug effect”, indicate a potential neurophysiological substrate for cocaine reward; rhythmic, slow wave EEG over the prefrontal cortex. This ability of cocaine to induce slow wave, oscillatory, neural activation is consistent with prior animal studies reporting cocaine induced synchronous oscillation of central noradrenergic neurons (Harris et al., 1992) and amphetamine induced delta rhythm in nucleus accumbens neurons (Leung and Yim, 1992). Hippocampal theta, a primary source of cortical theta activity (Erdi et al., 2005), is associated with cocaine seeking behavior in rats (Vorel et al., 2001). In functional MRI studies with cocaine dependent participants, BOLD activation positively associated with cocaine high was observed in the orbitofrontal cortex, plus subcortical structures including the insula, basal forebrain/ventral pallidum, thalamus, ventral tegmental area and substantia nigra, as well as the temporal gyrus (Breiter et al., 1997; Kufahl et al., 2005; Risinger et al., 2005), demonstrating that cocaine activates of multiple brain regions simultaneously. Based on this model, the increase in EEG coherence in prefrontal cortex following cocaine in the present study could represent afferent inputs from subcortical structures such as the thalamus, basal forebrain, and the mesencephalic dopamine nuclei acting in a synchronous manner. This is consistent with animal studies on EEG rhythmicity, which have demonstrated that EEG synchronization is orchestrated by oscillating pacemaker neurons in the thalamus and mesolimbic system (Steriade et al., 1990, 1993; Buzsake, 2002). In this model, theta rhythm is mediated via input from the medial dorsal thalamic nucleus, septal nuclei, and anterior cingulate, while delta rhythm is primarily mediated via thalamic nuclei, reflecting a condition where cortical neurons are disengaged from information processing (John et al., 2005).
The functional correlates of theta and delta EEG activity were distinct based on the condition in which they were recorded. Theta and delta coherence were positively associated with subjective ratings of “anxious” during cocaine cue exposure, but negatively associated with ratings of “nervous” following cocaine dosing. In addition, theta coherence was negatively associated with ratings of cocaine-like high during cue exposure, while theta power was positively associated with ratings of “good drug effect” following cocaine dosing. Previous fMRI studies on the acute effects of cocaine have reported a positive association of cocaine craving, and a negative association of cocaine high, with BOLD signal in the prefrontal cortex, anterior cingulate, putamen, nucleus accumbens and inferior frontal/orbitofrontal gyrus (Kufahl et al., 2005; Risinger et al., 2005). This functional polarity in BOLD signal is similar to the functional polarity of slow wave EEG activity, in conditions designed to elicit cocaine craving versus cocaine high, seen in the present study.
There are limitations to the present study that must be acknowledged. Multiple comparisons of subjective, physiological, and EEG measures were required by the need to investigate distinct response profiles in each condition, and then between conditions. This was addressed by employing Bonferroni corrections for the comparisons of cue- and dose-induced subjective and physiological measures. The lack of difference in cardiovascular output following cues versus cocaine is likely due to the variability of this measure in our small sample of participants, and indicates the potential for Type II error in this study's dataset in general. The effects of cocaine were tested at only one dose, and would have been better evaluated with multiple doses. The assessment schedule included only one post-cue time point, which limited our ability to compare cue and dose response duration, and the cocaine cues always preceded the cocaine dose to enhance CS+ reactivity (see Reid et al., 2006). As a result, comparisons between conditions are limited to the peak effects on each response measure, which were collected within the first 10-15 minutes after stimulation onset. Finally, there were no measures of reinforcement, such as a choice procedure following each stimulus condition, which limits the functional relevance of the data in terms of this critically important measure of drug abuse behavior.
In summary, the present study confirmed previous neuroimaging and psychopharmacological studies on the effects of cocaine cue exposure and cocaine dosing. Based on independent observations, these distinct behavioral and pharmacological stimuli resulted in similar response patterns. However, closer examination of the respective response profiles (eg, EEG amplitude vs. coherence) and their relationship to function revealed numerous distinctions, as was originally hypothesized. Cocaine cue responding was associated with subjective anxiety and autonomic arousal and, due to the reduction in coherence, possibly reflects local cortico-cortical EEG activation in the mesolimbic cortex. Cocaine dose responding was associated with subjective reward and, due to the increase in coherence, possibly reflects broader activation throughout the cortex and subcortex, including non-mesolimbic structures such as the thalamus. In both conditions, self-reported cocaine craving was associated with autonomic responding and subjective measures of anxiety. Further studies on subcortical contributors to the cortical EEG measures are needed to further investigate these differences in cocaine craving and reward.
Acknowledgments
Study supported by NIDA R21's DA 12277 and DA017556 to Malcolm S. Reid, NIDA RO1 DA07707 to Leslie Prichep, and by grant NIH/NCRR MO1RR00096 to New York University School of Medicine, General Clinical Research Center. We wish to acknowledge the support of Dr. Mary Jeanne Kreek and the Laboratory of the Biology of Addictive Disorders at The Rockefeller University for assistance in the plasma ACTH assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest: The authors declare that, except for salary/income from their primary employers, no financial support or compensation has been received from any corporate entity, professional service, or private individual associated with this study and that there are no personal financial holdings that may be perceived as a potential conflict of interest.
References
- Ahn H, Prichep L, John ER, Baird H, Treptin M, Kaye H. Developmental equations reflect brain dysfunctions. Science. 1980;210:1259–1262. doi: 10.1126/science.7434027. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall SM, Mickalian JD, Reid MS, Crawford CA, Delucchi KL, Carr K, Hall S. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman M, Kantor HL, Gastfried DR, Riorden JP, Mathew RT, Rosen BR, Hyman S. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;9:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of fentanyl, cocaine, and ketamine: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2004;176:398–406. doi: 10.1007/s00213-004-1891-x. [DOI] [PubMed] [Google Scholar]
- Buzsake G. Theta oscillations in the hippoampaus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley D, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Comparing the physiological and subjective effects of self-administered vs yoked cocaine in humans. Psychopharmacology. 2006;186:544–552. doi: 10.1007/s00213-006-0312-8. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacol. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Erdi P, Huhn Z, Kiss T. Hippocampal theta rhythms from a computational perspective: code generation, mood regulation and navigation. Neural Networks. 2005;18:1202–1211. doi: 10.1016/j.neunet.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Fischman MW, Foltin RW. Limited sex differences in response to “binge” smoked cocaine use in humans. Neuropsychopharmacology. 1999;21:445–454. doi: 10.1016/S0893-133X(98)00120-1. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Smoked and Intravenous Cocaine in Humans: Acute Tolerance, Cardiovascular and Subjective Effects. Journal of Pharmacology and Experimental Therapeutics. 1991;257:247–261. [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Residual effects of repeated cocaine smoking in humans. Drug and Alcohol Dependence. 1997;47:117–124. doi: 10.1016/s0376-8716(97)00093-8. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Effects of “binge” use of intravenous cocaine in methadone-maintained individuals. Addiction. 1998;93:825–36. doi: 10.1046/j.1360-0443.1998.9368254.x. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology. 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Ward AS, Collins ED, Haney M, Hart CL, Fischman MW. The effects of venlafaxine on the subjective, reinforcing, and cardiovascular effects of cocaine in opioid-dependent and non-opioid-dependent humans. Exp Clin Psychopharmacology. 2003;11:123–130. doi: 10.1037/1064-1297.11.2.123. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: Neuroanatomical specificty for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration. Psychopharmacology. 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- Harris GC, Hausken ZE. Cocaine induced synchronous oscillations in central noradrenergic neurons in vitro. Lett Neurosci. 1992;50:253–257. doi: 10.1016/0306-4522(92)90420-7. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug Alcohol Dependence. 2004;73:279–287. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Herning RI, Jones RT, Hooker WD, Mendelson J, Blackwell L. Cocaine increases EEG beta: A replication and extension of Hans Berger's historic experiments. Electroencephal Clin Neurophysiol. 1985;60:47–477. doi: 10.1016/0013-4694(85)91106-x. [DOI] [PubMed] [Google Scholar]
- Herning RI, Glover BJ, Koeppl B, Phillips RL, London ED. Cocaine-induced increases in EEG alpha and beta activity: evidence for reduced cortical processing. Neuropsychopharmacology. 1994;11:1–9. doi: 10.1038/npp.1994.30. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Cascell NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology. 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Freidman J, Easton P. Neurometrics: computer assisted differential diagnosis of brain dysfunction. Science. 1988;293:162–169. doi: 10.1126/science.3336779. [DOI] [PubMed] [Google Scholar]
- John ER. From synchronous neuronal discharges to subjective awareness? Prog In Brain Res. 2005;150:143–171. doi: 10.1016/S0079-6123(05)50011-6. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS. The relevance of QEEG to the evaluation of behavioral disorders and pharmacological interventions. Clinical EEG and Neurocience. 2006;37:135–143. doi: 10.1177/155005940603700210. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely T, Hoffman JM, Drexler PG. Neural activity related to drug craving in cocaine addiction. Archives Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kouri EM, Stull M, Lukas SE. Nicotine alters some of cocaine's subjective effects in the absence of physiological or pharmacokintic changes. Pharmacology Biochemistry Behavior. 2001;69:209–217. doi: 10.1016/s0091-3057(01)00529-9. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ. Neural responses to acute cocaine administration in the human brain detected by fMRI. NeuroImage. 2005;28:904–914. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Leung LS, Yim CYC. Rhythmic delta-frequency activities in the nucleus accumbens of anesthetized and freely moving rats. Can J Physiol Pharmacol. 1992;71:311–320. doi: 10.1139/y93-049. [DOI] [PubMed] [Google Scholar]
- Liu X, Vaupel BD, Grant S, London E. D Effect of cocaine-related environmental stimuli on the spontaneous electroencephalogram in polydrug abusers. Neuropsychopharmacology. 1998;19:10–17. doi: 10.1016/S0893-133X(97)00192-9. [DOI] [PubMed] [Google Scholar]
- Lukas SE. Topographic mapping during cocaine-induced intoxication and self-administration. In: Racagni G, Brunello N, Fukuda T, editors. Biological Psychiatry. Vol. 2. Elsevier Science Publishers; New York: 1991. pp. 25–29. [Google Scholar]
- Lukas SE, Mendelson JH, Amass L, Benedikt R. Behavioral and EEG studies of acute cocaine administration: comparisons with morphine, amphetamine, pentobarbital, nicotine, ethanol and marijuana. NIDA Research Monograph. 1990;95:146–151. [PubMed] [Google Scholar]
- Maas LC, Scott EL, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addictive Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KR, Kowalik SC, John ER, Merkin HA, Tom M, Rosenthal MS. Quantitative electroencephalographic characteristics of crack cocaine dependence. Biol Psychiary. 1996a;40:986–93. doi: 10.1016/0006-3223(95)00575-7. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KR, Kowalik SC, Rosenthal MS. Neurometric qEEG studies of crack cocaine dependence and treatment outcome. J Addictive Diseases. 1996b;15:39–53. doi: 10.1300/J069v15n04_03. [DOI] [PubMed] [Google Scholar]
- Prichep LS, Alper KR, Kowalik SC. Prediction of treatment outcome in cocaine dependent males using quantitative EEG. Drug and Alcohol Dependence. 1999;54:35–43. doi: 10.1016/s0376-8716(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug and Alcohol Dependence. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Prichep L, O'Leary S, Ciplet D, Tom ML, Howard B, Rotrosen J, John R. Quantitative electroencephalographic studies of cue-induced cocaine craving. Clinical EEG. 2003;34:110–123. doi: 10.1177/155005940303400305. [DOI] [PubMed] [Google Scholar]
- Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology. 2006;31:872–884. doi: 10.1038/sj.npp.1300888. [DOI] [PubMed] [Google Scholar]
- Risinger RC, Dalmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffman RG, Bloom AS, Garavan H, Stein EA. Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. NeuroImage. 2005;26:1097–1108. doi: 10.1016/j.neuroimage.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug and Alcohol Dependence. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Steriade M, Gloor P, Llina RR, Lopes Da Silva F, Mesulam MM. Basic mechanisms of cerebral rhythmic activities. Electroencephal Clin Neurophysiol. 1990;76:481–508. doi: 10.1016/0013-4694(90)90001-z. [DOI] [PubMed] [Google Scholar]
- Steriade M, Curro Dossi R, Pare D. Electrophysiological properties of intralaminar thalamocortical cells discharging rhythmic (40 Hz) spike bursts at 1000Hz during waking and rapid-eye-movement sleep. Neuroscience. 1993;56:1–19. doi: 10.1016/0306-4522(93)90556-u. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Ward AS, Haney M, Fischman MW, Foltin RW. Binge cocaine self-administration by humans: smoked cocaine. Behavioural Pharmacology. 1997;8:736–744. doi: 10.1097/00008877-199712000-00009. [DOI] [PubMed] [Google Scholar]
- Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. Blockade of cocaine-induced increases in adrenocorticotrophic hormone and cortisol does not attenuate the subjective effects of smoked cocaine in humans. Behav Pharmacol. 1999;5:523–529. doi: 10.1097/00008877-199909000-00010. [DOI] [PubMed] [Google Scholar]