Abstract
Recent work regarding chronic central neuropathic pain (CNP) following spinal cord injury (SCI) suggests that activation of key signaling molecules such as members of the mitogen activated protein kinase (MAPK) family play a role in the expression of at-level mechanical allodynia. Specifically, Crown and colleagues (2005, 2006) have shown that the development of at-level CNP following moderate spinal cord injury is correlated with increased expression of the activated (and thus phosphorylated) forms of the MAPKs extracellular signal related kinase and p38 MAPK. The current study extends this work by directly examining the role of p38 MAPK in the maintenance of at-level CNP following spinal cord injury. Using a combination of behavioral, immunocytochemical, and electrophysiological measures we demonstrate that increased activation of p38 MAPK occurs in the spinal cord just rostral to the site of injury in rats that develop at-level mechanical allodynia after moderate SCI. Immunocytochemical analyses indicate that the increases in p38 MAPK activation occurred in astrocytes, microglia, and dorsal horn neurons in the spinal cord rostral to the site of injury. Inhibiting the enzymatic activity of p38 MAPK dose dependently reverses the behavioral expression of at-level mechanical allodynia and also decreases the hyperexcitability seen in thoracic dorsal horn neurons after moderate SCI. Taken together, these novel data are the first to demonstrate causality that increased activation of p38 MAPK in multiple cell types play an important role in the maintenance of at-level CNP following spinal cord injury.
Keywords: p38 MAPK, electrophysiology, at-level mechanical allodynia, girdling, Western Immunoblotting, immunocytochemistry, central neuropathic pain
INTRODUCTION
Spinal cord injury (SCI) results in a graded disruption of both sensory and voluntary motor ability distal to the site of injury. Numerous recent reports indicate that one of the more prevalent negative outcomes following SCI is the development of chronic central neuropathic pain (CNP; Christensen and Hulsebosch 1997; Hulsebosch, 2005; Widerstrom-Noga et al. 2002). Central neuropathic pain manifests itself behaviorally as allodynia (a condition in which non-noxious stimuli become noxious) or hyperalgesia (a condition in which a noxious stimulus is perceived as more noxious) and can be classified in one of three categories based on the location of the pain relative to the site of injury: above-level, at-level, and below-level pain (Siddall et al 2001). These three types of regional pain are the result of different pathophysiological mechanisms and it is this complication, in part, that has led to the creation of numerous therapeutic regimens for the treatment of CNP following spinal cord injury that carry only limited efficacy (Attal et al. 2002; Capel et al. 2003; Cardenas et al. 2002; Dyson-Hudson, et al. 2001; Finnerup et al. 2002, 2005; Hicks et al. 2003; Vierck et al. 2000; Wade et al. 2003).
An emerging candidate mechanism for the generation of CNP following spinal cord injury involves the activation of members of the mitogen activated protein kinase (MAPK) family (Crown et al 2005, 2006; Yu and Yezierski 2005). In models of peripheral neuropathic pain and its presumed physiological correlate, central sensitization, MAPKs such as extracellular signal related kinase (ERK) and p38 MAPK contribute to dorsal horn hyperexcitability (Ji et al 2003; Ji 2004; Zhao et al 2007; Zhuang et al 2005). Recent findings from our laboratory suggest a similar phenomenon occurs during central neuropathic pain, as we previously found significant increases in spinal levels of the phosphorylated (and thus activated) forms of ERK 1/2 and p38 MAPK, but not JNK, in SCI rats experiencing at-level mechanical allodynia but not in SCI rats without mechanical allodynia (Crown et al. 2006). Supporting this finding, Hains and colleagues (2006) found that SCI rats treated with the second generation tetracycline derivative, minocycline, showed decreases in the expression of below-level pain that correlated with minocycline’s ability to decrease glial activation of p38 MAPK. The purpose of the current set of studies was to directly test the hypothesis that inhibiting the enzymatic activity of p38 MAPK would decrease the behavioral expression of at-level mechanical allodynia and decrease the dorsal horn hyperexcitability following spinal cord injury. The current studies provide the first converging evidence (combining behavioral, protein, cytochemical and electrophysiological analyses) that activation of p38 MAPK plays a critical role in “at level” mechanical allodynia, or chronic CNP following SCI.
MATERIAL AND METHODS
Subjects
Male Sprague Dawley rats weighing between 225-250 g (Harlan Inc, Houston TX) received a contusion injury at spinal level T10 using an Infinite Horizon impactor (150 kdynes, 1 s dwell), were given a laminectomy to serve as sham controls, or received no manipulation to serve as age-matched naïve rats. Data from naive, sham, and SCI rats that developed pain were examined (this level of injury routinely produces 90% of SCI rats that develop pain). SCI rats that did not develop pain were excluded from these analyses. For the study involving measurement of at-level mechanical allodynia, age matched rats were divided into 3 groups: 1) 10 naïve rats (naïve), 2) 10 rats given a laminectomy at T10 (sham) and 3) 10 injured rats (SCI). To test the effects of p38 MAPK inhibition on the expression of at-level allodynia, 24 injured rats and 12 age-matched sham rats were exposed to various doses of the inhibitor of enzymatic activity of p38 MAPK, SB203580. This compound has been shown to inhibit the enzymatic activity of p38MAPK on its downstream targets (Tong et al. 1997; also reviewed in Koistinaho & Koistinaho, 2002). For immunohistochemical and Western immunoblotting studies, 5 age-matched sham rats were compared to 5 SCI rats. For electrophysiological studies, 15 age matched sham rats were compared to 20 SCI rats. Following SCI surgery, rats were given supplemental injections of Baytril twice daily for 3 days to prevent infection, and bladders were also expressed twice daily until the rats began to void on their own. All procedures were reviewed by the UTMB Animal Care and Use Committee and are consistent with the guidelines of the International Association for the Study of Pain and the NIH Guide for the Care and Use of Laboratory Animals.
Behavioral Procedures
Recovery of locomotor function following 150 kdynes, 1 s dwell time impact with the Infinite Horizons device was monitored using the Basso, Beattie, Bresnahan scale of locomotor recovery (Basso et al. 1995). Briefly, this 21 point scale is used to quantify locomotor performance with a score of 0 meaning no observable hindlimb movement and a maximum score of 21 indicating normal locomotion. During the course of recovery, sham and injured rats were examined for the development of mechanical allodynia rostral to the injury and compared to responses from age matched naïve rats, using the procedure outlined in Crown and colleagues (2005, 2006) with a few modifications. Briefly, animals were restrained in Plexiglass tubes with slats cut in the tube to allow for the application of the tactile stimuli. The Plexiglass tubes were cleaned with Nolvasan between each animal. A von Frey hair with bending force of 204.14 mN (26 g-force) was applied to each point on the grid, and supraspinally mediated nociceptive responses (e.g., escape, biting, or vocalization) were recorded and mapped onto a grid map of that animal. The scoring of these complex behaviors excludes simple hyperreflexia, which is a segmental response (Vincler et al., 2001; Woolf, 1984). Since animals do not normally display supraspinal responses to this stimulus, a positive response was interpreted to demonstrate that a noxious stimulus was experienced. In mapping the area of response, the number of responses was recorded (Nr) and normalized by the following formula: (Nr × 100)/total number of applications, indicating the percent responding out of the total number of applications. For both behavioral studies, data were analyzed only for the dermatome corresponding to the site of injury. To test whether increases in activated p38 MAPK expression corresponded to the development of at-level mechanical allodynia, animals from each condition (naïve, sham, SCI) were baseline tested prior to injury and then were tested weekly until 35 days post injury. The percent of supraspinal responses to a 4 mm blunt probe, a more natural stimulus, were also recorded and analyzed using the same method. In all cases, test order (26 g force von Frey filament or probe) was counterbalanced across conditions, with half the subjects in each condition being exposed to the probe first and half the subjects being exposed to the probe last. Animals did not respond in the absence of mechanical stimulation. In addition, to test the effects of SB203580 (EMD Biosciences, which inhibits the enzymatic activity of p38 MAPK on its downstream targets) on the threshold to make a supraspinal response in SCI and sham rats, we have adapted a procedure for establishing the mechanical threshold on the animal’s back. In this procedure, a series of von Frey filaments (starting with 0.6 g) were applied to a spot on the rat’s back just rostral to the site of injury at T10. The force of the von Frey stimulus was increased until a vocalization response accompanied by stimulus avoidance was produced and the average response threshold was compared between sham and SCI rats prior to and for 2 hours after drug delivery. In all cases, behavior was assessed by the same experimenter who was blinded to the experimental condition.
For behavioral studies of the effects of p38 MAPK inhibition on at-level mechanical allodynia, SB203580 was dissolved in vehicle (1% DMSO in 0.9% saline). Four doses of SB203580 (0, 0.1, 1, or 10 uM in 50 uL) were administered to SCI rats. Sham rats were tested at the same time intervals but received either vehicle or the highest dose of SB203580 (10 uM in 50 uL). Each SCI and sham rat received one dose of the drug over the course of the experiment. All rats were tested for supraspinal nociceptive responses (escape behaviors, biting, or vocalization) to a 26 g force von Frey hair and a 4 mm blunt probe prior to drug administration (baseline). Following drug administration, rats were tested at 15, 30, 60, and 120 minutes post injection. SB203580 was given by intraspinal injection into the lumbar L4/L5 space as described in other studies (Zhuang et al. 2005). Briefly, rats were given inhalation anesthesia with isofluorane and the L4/L5 vertebral segment was identified by determining the location of the iliac crest. The injection needle was inserted into the L4/L5 space for drug delivery and a lateral tail flick response to needle insertion was taken as evidence for successful lumbar puncture. Prior work in our laboratory and others show that this protocol is effective for delivering drug via the intrathecal space as far as the upper thoracic region when 50uL of the drug is infused (Crown et al. submitted; Kim et al. 2006). To ensure that drug delivery into the lumbar space affected responses in the midthoracic region, a group of naïve rats were injected with 50 uL of a 2% lidocaine solution and tested for the loss of reflexive responses to a 100 gm-force von Frey stimulus applied to the rat’s dorsum. This volume of lidocaine was confirmed to produce a loss of reflexive and supraspinal responses throughout the region to be tested (data not shown). In addition, 50uL of methylene blue was injected into a group of naïve rats to monitor the spread of the drug. This volume of dye traveled to the upper thoracic region, indicating that the spread of the drug covers the spinal segments that innervate the dermatomes in which rats were tested for at-level mechanical allodynia.
Western Immunoblotting
Twenty four hours after the last behavioral testing time for at-level mechanical allodynia (35 days), rats were sacrificed for Western immunoblotting. To test for persistent changes in activated p38 MAPK expression following spinal cord injury by Western blot, all subjects were overdosed with pentobarbital (100 mg/kg) and perfused intracardially with 250 ml cold heparinized (1ml/1L) saline (0.9%) and 6 mm segments of spinal cord tissue from immediately rostral to the injury site were removed and dissected while on dry ice. The dorsal aspects of the spinal cord segments were microdissected and frozen on dry ice for subsequent analysis. The collected tissue was mechanically homogenized in ice-cold tris-buffered saline containing 40 mM Tris-HCl (pH 7.5), 2% SDS, 2 mg/ml aprotinin, 2 mg/ml antipain, 2 mg/ml chymostatin, 2 mg/ml bestatin, 2 mg/ml pepstatin-A, 2 mg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, and 1 mM EDTA. Homogenates were centrifuged at 10,000 × g for 10 min. The supernatant was collected and centrifuged again at 10,000 × g for 10 min and then stored at -80°C. Protein concentrations of the homogenate were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). We have shown previously that this extraction method is efficient at collecting both the cytoplasmic and nuclear protein fractions (Crown et al. 2005, 2006).
Once homogenized, the samples from 5 injured, 5 sham, and 5 naive rats were heated for 4 min at 95°C in an equal volume of sample buffer (100 mM Tris, pH 6.8, and 2% SDS, 2% 2-mercaptoethanol, 0.001% bromophenol blue, 20% glycerol) and then loaded onto a polyacrylamide gel in equal protein amounts (10 ug per lane). The stacking gel was 4% acrylamide, prepared in 0.13 M Tris, pH 6.8, and 0.1% SDS, and the separating gel was 10% acrylamide, prepared in 0.38 M Tris, pH 8.8, and 0.1% SDS. Samples were separated by electrophoresis in Tris-glycine buffer (25 mM Tris, 250 mM glycine, 0.1% SDS) at 300 V for approximately 30 min. Proteins were transferred overnight (12-14 hrs) to a PVDF membrane at 30 V in transfer buffer containing 20% MeOH, 20 mM Tris, 150 mM glycine, pH 8.0. Membranes were incubated for one hour at room temperature in blocking buffer containing 5% non-fat powdered milk in tris buffered saline (TBS)-Tween (20 mM Tris, 137 mM NaCl, 0.1% Tween-20), then washed for 10 min in TBS-Tween. Membranes were incubated overnight with primary antibodies to the activated (and thus phosphorylated) form of p38 MAPK (p-p38 MAPK; 1:1000). Serial dilutions of the primary antibody were first tested on the protein samples using dotblotting to ensure that the results were not influenced by a ceiling effect. To control for equal protein loading, both total p38 MAPK (1:2000) and GAPDH (1:5000) immunoreactivity were used to verify equal loading of proteins on the PVDF membrane and these methods found no significant differences between the naïve, sham, and SCI rats (all Fs < 1.0, p > 0.05). After washing off the primary antibody, membranes were incubated in horseradish peroxidase-conjugated anti-rabbit IgG diluted 1:20000 for one hour and washed 3 times in TBS for 5 min. Peroxidase activity was detected using an Amersham ECL Plus detection system, images were collected by exposing the membranes (exposure time varied from 30 s to 5 min) on chemiluminescence film (Hyperfilm ECL, Amersham Pharmacia Biotech, England), and integrated density values were calculated using LabWorks software (UVP, Upland, CA). Each membrane contained samples from sham, naïve, and SCI rats to allow for valid intergel comparisons. In addition, all membranes were exposed at the same time following p-p38 MAPK, p38 MAPK, and GAPDH immunoblotting to allow for valid intragel comparisons. Finally, for statistical purposes all subjects’ data were normalized to GAPDH expression prior to analysis.
Immunocytochemistry
Thirty-five days after injury, rats in the immunocytochemical study were overdosed with pentobarbital (75 mg/kg) and perfused intracardially first with 200 ml of heparinized warm 0.9% saline followed by 250 ml of cold 4% paraformaldehyde. The first spinal segment immediately rostral to the injury (T9) that showed no signs of direct damage from the injury was removed and post fixed for 4 hours in 4% paraformaldehyde prior to protection for 2 days in 30% sucrose at 4°C. The tissue was then embedded in OCT compound, frozen, mounted, and sectioned with a sliding microtome (model HM 400, Microm International, Waldorf Germany). Thirty micron sections from the T9 tissue were blocked in 5% normal goat serum for 30 minutes and incubated overnight in rabbit polyclonal p-p38 MAPK antibody (1:200, R&D Biosciences). The sections were then rinsed in phosphate buffered saline (PBS) and incubated in goat anti-rabbit antiserum conjugated to Alexa Fluor 568-Red (1:400, Molecular Probes). After rinsing, the floating sections were mounted on gelatin-coated slides and coverslipped with non-fade media. Expression of p-p38 MAPK was quantified in the spinal cord gray and white matter rostral to the site of injury (T9) and in the individual laminae of the dorsal horn in sham and injured rats by measuring the density of the reaction product for activated p38 MAPK and comparing between sham and SCI rats using MetaMorph Software coupled to a BIO-RAD Radiance 2100, K-2 system. To examine the cellular populations within the spinal cord that expresses p-p38 MAPK, double labeling with either the neuronal marker NeuN (Chemicon, MAB377, 1:500), the astrocytic marker GFAP (Chemicon, MAB360, 1:1000) or the microglial marker OX-42 (Serotec Ltd, MCA275G, 1:100) was performed. Omission of the primary antibodies or use of non-specific secondary IgGs in the immunostaining process served as a negative control for the tissue sections.
For double immunofluorescent staining, data from two or three channels (if DAPI image was included) were collected by sequential scan (BioRad Radiance 2100 Confocal Laser System coupled to a Nikon E800) to avoid bleeding through between channels. Localization of p-p38 MAPK and one of the cell marker images were collected with Krypton lasers of 568 nm excitation and 488 nm excitation. Red emission (a result of excitation of the conjugated AlexaFluor 568 to the p-p38 MAPK antibody) demonstrated localization of p-p38 MAPK; whereas, green emission (a result of excitation of the conjugated AlexaFluor 488 to the appropriate antibody) demonstrated localization of immunoreaction product in neurons, microglia or astrocytes. Blue emission (DAPI) demonstrated nuclear localization. The yellow structures in merged images indicated the co-localization of the two antigens (Red+Green). Digital images were collected, saved and labels added with Adobe Photoshop.
Electrophysiology
Extracellular single-unit recordings were made from sham and spinally contused rats 35 days after injury (n=20). Rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and supplemented with sodium pentobarbital (5 mg/kg/h) infused intravenously through a jugular vein catheter. Adequacy of anesthesia was monitored by the lack of withdrawal reflexes to noxious stimuli and the absence of corneal blink reflexes. Core temperature was maintained at 37°C by a thermostatically controlled heating blanket. Laminectomy was performed to expose the thoracic spinal segments T8-T10. Cells were isolated from thoracic segments (T8-T9), which lay immediately rostral to the site of contusion injury (T10). Extracellular single-unit recordings were made with a low impedance (0.4-0.8 MΩ at 1k Hz; Kation Scientific) glass carbon fiber microelectrode. Once a cell was identified, background activity was measured followed by cutaneous receptive field mapping with von Frey filaments and brief pinches. Wide dynamic range (WDR) neurons, responsive to both innocuous (brush) and noxious (press and pinch) stimuli were sought. Peripheral stimuli during electrophysiology included the following: brush (with a makeup brush; force range [±SEM] = 2-4 [±0.5] g), pressure (with a large arterial clip at 144 g/mm2), pinch (with a small arterial clip at 583 g/mm2), and von Frey filament application (with 5 filaments: 0.6g, 2g, 6g, 26g, and 60g). Each stimulus was applied over a 10 second period with a 20 second break between stimuli.
Electrical signals were amplified and input to a window discriminator, displayed on analog and digital storage oscilloscopes, processed by a data collection system (CED 1401+; Cambridge Instruments, Cambridge, UK; Pentium computer, Dell, Austin, TX, USA), and stored on a computer to construct peristimulus time histograms. The stored digital record of unit activity was analyzed offline with Spike 2 software (v4.07, Cambridge Electronic Design, Cambridge, UK). Responsiveness to peripheral stimulus was calculated by subtracting the baseline activity from evoked activity to calculate a net increase in discharge rate.
The effects of topical spinally applied SB203580 (0, 0.1, 1, or 10 uM in 50uL) were tested on cells immediately rostral to the site of contusion at 35 days post injury and in the same spinal segment in shams. Cells from this area have been described as having increased responsiveness to peripheral stimuli (Crown et al submitted; Tan et al. 2004). The 35 day time point was chosen for two reasons: 1) all SCI rats developed maximal at-level mechanical allodynia by this time and 2) we find activated p-p38 MAPK expression is significantly and maximally upregulated. Recordings in response to the set of peripheral stimuli were made and the same cells then received SB203580 application at one of the four doses and were recorded at 15, 30, 60, and 120 minutes after application.
Statistics
Supraspinal responses during the girdling test for at-level mechanical allodynia were analyzed using a one way analysis of variance (ANCOVA; with baseline reactivity as the covariate) using SPSS 14 for Windows (SPSS; Chicago, IL). To compare the group means for the effects of SB203580 administration on at level mechanical allodynia, laminar distributions of p-p38 MAPK positive cells in the dorsal horn, and the electrophysiological data for reactivity to brush, press, pinch, and von Frey stimuli, 1 between-1 within repeated measures ANOVAs were performed. To compare average intensity of p-p38 MAPK expression in both spinal cord and STT cells, Student’s t tests were performed using SigmaPlot 9 (Systat Software, Point Richmond CA). Western immunoblot analyses data were analyzed using a 1 between ANOVA. Post hoc tests were performed using Duncan’s New Multiple Range Test. In all cases, the alpha level for statistical significance was set at p < 0.05.
RESULTS
Recovery of Locomotor Function after SCI
Following a 150 kdynes, 1 s dwell time impact using the Infinite Horizons device, SCI rats slowly recovered locomotor function over the course of the 35 day recovery period. Consistent with our other work (e.g., Carter et al. 2004), rat given this severity of injury showed significant locomotor impairment as compared to naïve and sham rats (p < 0.0001; Figure 1A). SCI rats recovery of locomotor function reached asymptote at a BBB score of 11, indicating a recovery of hindlimb function and the ability to walk with weight support. Although it has been reported that chronic p38 MAPK inhibition using the SB compound can enhance locomotor recovery post SCI (Horiuchi et al. 2003), the acute nature of the treatment in the current study did not have any obvious effect on locomotor function when the drug was administered at 35 days post injury.
Figure 1.
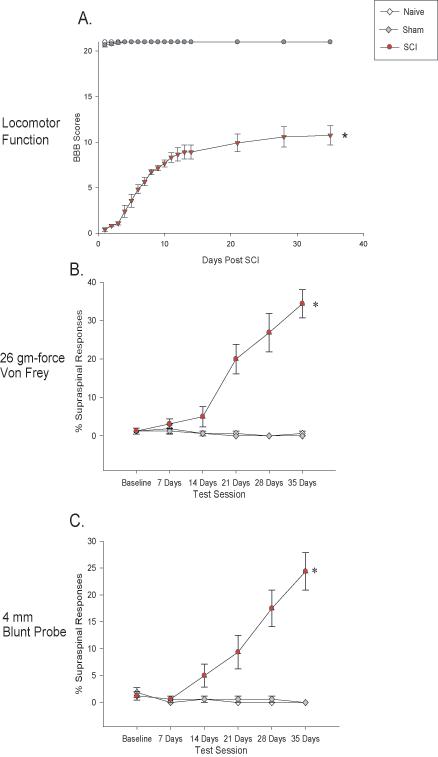
Recovery of locomotor function and development of at-level mechanical allodynia following contusive SCI. The recovery of locomotor function (as assessed using the BBB Score) following 150 kdyne, 1 s dwell time impact using the Infinite Horizons device. SCI rats displayed significant impairment in locomotor function relative to naïve and sham rats (panel A, p < 0.0001). The percentage of supraspinal nociceptive responses (y axis) made by naïve (Naïve), sham (Sham), and spinal injured rats (SCI) tested weekly for 35 days post injury is graphed. The SCI rats showed a significant increase in supraspinal responses to both von Frey stimulation (panel B, *p < 0.0001) and stimulation with a 4 mm blunt probe (panel C, *p < 0.0001).
Moderate SCI Produces At-Level Mechanical Allodynia by 35 Days after Injury
As in our prior studies (Crown et al., 2005, 2006, submitted) rats given moderate spinal cord injury (150 kdynes, 1 s dwell time) were interpreted to develop mechanical allodynia over the course of 35 days, as evidenced by significant increases in the percentage of supraspinal responses (e.g., escape, biting, vocalization) to both the 26 g force von Frey filament and the 4 mm blunt probe applied to the dermatomes of the back corresponding to the area rostral to the site of injury (Figures 1B and C). There were no significant differences in the baseline scores for supraspinal responses to the von Frey and blunt probe stimuli between the groups prior to injury (p > 0.05). SCI rats developed significant at-level mechanical allodynia after injury, as there were significant increases over time in supraspinal responses to the von Frey and blunt probe stimuli compared to presurgical and naïve and sham control responses (p < 0.0001, repeated measures ANCOVAs).
At-Level Mechanical Allodynia is Attenuated by the p38 MAPK Inhibitor, SB203580
To examine the effects of inhibiting the enzymatic activity of p38 MAPK on at-level mechanical allodynia, SCI and sham rats were tested prior to drug administration, exposed to one of four doses of the p38 MAPK inhibitor SB203580 ((0, 0.1, 1, or 10 uM in 50 uL) and then tested at 15 min, 30 min, 60 min, and 120 min after drug application. Sham rats were either given vehicle or the highest dose of SB230580, 10 uM in 50 uL. Prior to SB203580 administration, SCI rats displayed significant at-level mechanical allodynia relative to sham rats in response to both the 26 g von Frey stimulus and the blunt probe (p < 0.0001, ANOVA). Injections of SB203580 led to a significant dose-dependent attenuation of the at-level mechanical allodynia in SCI rats over the 120 minute testing interval (p < 0.0001 for all comparisons; repeated measures ANOVAs, Figures 2A&B). Post hoc analyses of the group means for reactivity to the von Frey and blunt probe stimuli determined that sham groups had significantly fewer responses compared to all of the SCI groups except for the SCI group given 10 uM of SB203580 (p < 0.05 for all comparisons). The highest dose of SB203580 delivered to SCI rats reversed responses to the level of the sham rats. In addition, administration of 1 and 10 uM of SB203580 led to a significant attenuation of at level mechanical allodynia, as these 2 groups had significantly fewer supraspinal responses to both the von Frey and blunt probe stimuli than either the SCI vehicle or SCI 0.1 uM SB203580 groups (ps < 0.05). The SCI rats given 1 uM had significantly greater responding that the sham or SCI rats given 10 uM SB203580 but had significantly fewer responses than the SCI vehicle and SCI 0.1 uM SB203580 groups (all ps < 0.05). The SCI vehicle and SCI 0.1 uM SB203580 groups had significantly greater response values compared to all other groups to both the von Frey and blunt probe stimuli, (ps < 0.05).
Figure 2.
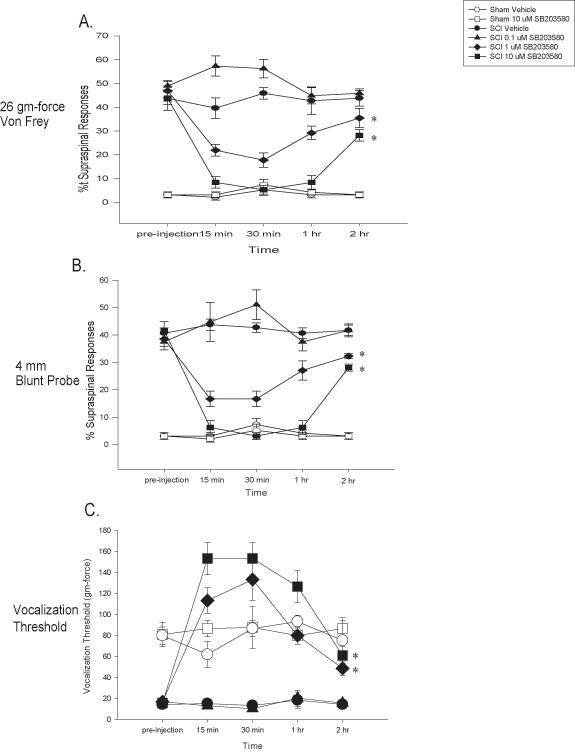
Inhibition of p38 MAPK activation reverses at-level mechanical allodynia after SCI. The inhibitor of p38 MAPK activation, SB203580, dose dependently reversed at-level mechanical allodynia when subjects are tested at 35 days post injury. The percentage of supraspinal nociceptive responses (y axis) made by sham (Sham), and spinal injured rats (SCI) tested is graphed. Prior to SB203580 injection or vehicle, SCI rats displayed at-level mechanical allodynia (as evidenced by an increased percentage of supraspinal nociceptive responses). SB203580 dose dependently reversed this at-level mechanical allodynia to both von Frey (panel A, * p < 0.0001) and blunt probe (panel B, * p < 0.0001) stimulation. Panel C demonstrates that SB203580 dose dependently reversed decreases in vocalization thresholds to mechanical stimuli at the level of the spinal cord injury (* p < 0.0001). In all cases, SB203580 had no effect on sham rats. All data are represented as mean ± SEM.
Another goal of the current study was to examine the effects of SB203580 administration on vocalization thresholds to mechanical stimulation after SCI. Prior to drug administration, SCI rats had significantly lower vocalization thresholds than sham rats (p < 0.001, ANOVA). SB203580 administration produced a significant dose dependent reversal of mechanical sensitivity as measured by the von Frey threshold needed to produce a vocalization response in sham and SCI rats (p < 0.0001, Figure 2C). Post hoc tests indicated that the 1 uM SB203580 dose reversed mechanical thresholds of SCI rats to control levels (all ps < 0.05). In addition 10 uM SB203580 significantly reversed mechanical thresholds to a level that was higher than the sham groups and the SCI rats given 1 uM SB203580 (all ps < 0.05). Finally, SCI rats given vehicle or 0.1 uM SB203580 had significantly lower mechanical thresholds than all the other groups (all ps < 0.05). Taken together, these data indicate that p38 MAPK inhibition significantly attenuates the expression of at-level mechanical allodynia following spinal cord injury.
Increases in Activated p38 MAPK Protein Levels in Dorsal Spinal Cord Correspond to At-Level Mechanical Allodynia Following SCI
By 35 days post SCI, the behavioral data indicated that at-level mechanical allodynia had reached a maximum in injured rats. Previously, our laboratory (e.g., Crown et al 2006) demonstrated that moderate SCI produced an upregulation in the expression of the activated (or phosphorylated) form of p38 MAPK in rats that developed at-level mechanical allodynia, but the study used spinal homogenates, so spinal laminar as well as cellular localization of p38 MAPK was not determined. The current immunocytochemical study confirmed our previous finding using Western immunoblot analyses and determined that SCI rats that developed at-level mechanical allodynia displayed significant upregulation in p-p38 MAPK expression following injury relative to either sham or naïve rats (Figure 3A, p < 0.05, ANOVA) and extends the findings by examining and identifying spinal and cellular localization. Figure 3B contains a representative Western blot comparing the differences between the groups.
Figure 3.
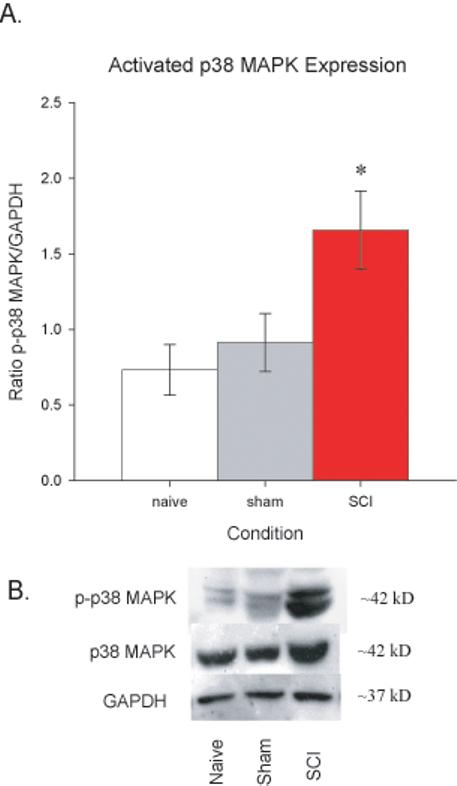
Activated p38 MAPK is upregulated in the spinal cord by 35 days post injury. A. Western blot data from naïve, sham, and SCI rats (normalized to GAPDH expression) indicated that SCI rats had significantly greater levels of activated p38 MAPK than the other groups (*p < 0.0001). B. Representative Western blots for p-p38 MAPK (top panel) and total p38 MAPK (middle panel), with GAPDH (bottom panel) included as the loading control. All data are represented as mean ± SEM.
Activated p38 MAPK Increases in the Dorsal Spinal Cord Gray Matter in Neurons, Astrocytes and Microglia Following SCI
To determine the laminar localization of p38 MAPK activation after SCI, the dorsal spinal cords of injured and sham rats were compared at 35 days post injury using immunocytochemistry. As is apparent in Figure 4A, SCI rats displayed significant increases in p38 MAPK expression in dorsal horn laminae I-V relative to sham rats (p < 0.0001, repeated measures ANOVAs, Figure 4B). Another important molecular event that has been tied to neuropathic pain following nervous system injury is the activation of astrocytes and microglia (Bigbee et al 2007; Hains et al. 2006; Raghavendra et al 2003; Zhuang et al 2005). Sections of T9 spinal cord were stained for increases in GFAP (Figure 5A&B) and OX-42 (Figure 5C&D) expression in SCI versus sham rats. The spinal cord injury produced a significant increase in GFAP and OX-42 expression in rostral spinal segments of SCI rats relative to sham rats in all five laminae of the spinal cord dorsal horn (all ps < 0.001, repeated measures ANOVAs).
Figure 4.
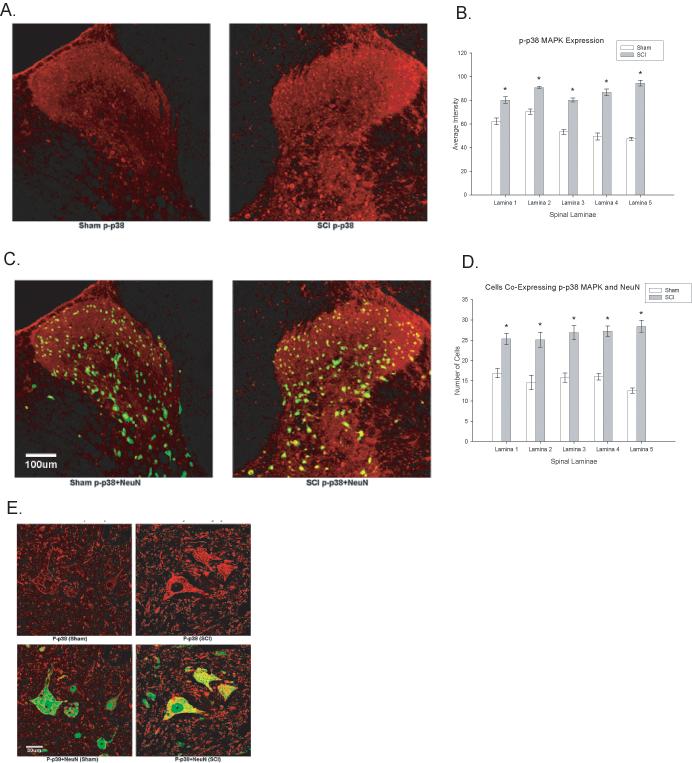
Spinal cord injury increases activated p38 MAPK expression within the spinal cord. A. Example of phosphorylated p38 MAPK (p-p38 MAPK) expression in sham (left panel) and SCI (right panel) rats. B. Quantification of the significant increase in p-p38 MAPK expression in laminae I-V following SCI (p < 0.05). C. Colocalization of NeuN with p-p38 MAPK in the spinal cord dorsal horn of sham (left panel) and SCI (right panel) rats. D. Quantification of the significant increase in p-p38 MAPK expression in the neurons following SCI (p < 0.05). E. High magnification images of NeuN positive cells in sham (left panels) and SCI (right panels) rats to illustrate differences in p-p38 MAPK expression. All data are represented as mean ± SEM.
Figure 5.
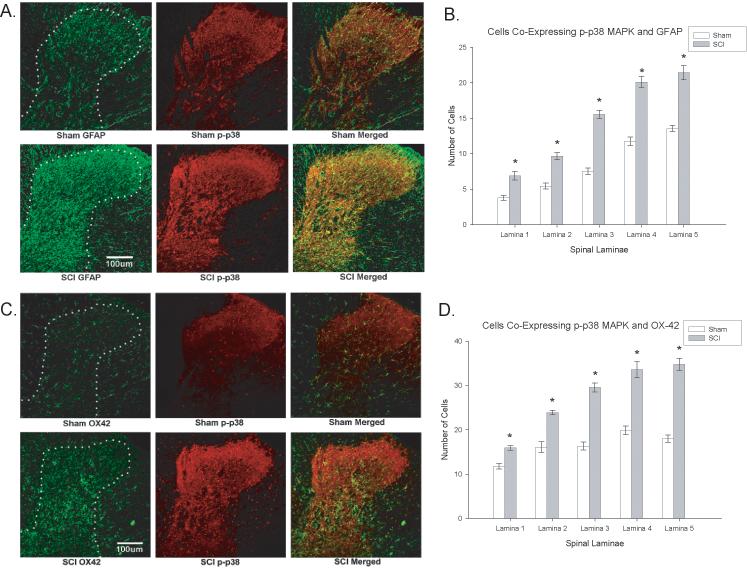
Activated p38 MAPK expression is seen in astrocytes and microglia of spinal cord injured rats that develop at-level neuropathic pain. Spinal cord injury increased expression of GFAP in astrocytes (Figure 5A, left panels) and significantly increased the expression of OX-42 in microglia (Figure 5C, left panels). Histogram density measures of immunofluorescent staining of GFAP (Figure 5B) and OX-42 Figure 5D) indicated significantly increased expression in laminae of the dorsal horn just rostral to the spinal injury. Colocalization with activated p38 MAPK was significantly upregulated and colocalized with astrocytes (Figure 5A, right panels) and microglia (Figure 5B, right panels) in the dorsal horn of injured (right panels) rats relative to sham (left panels) rats (*p < 0.0001). The colocalization is evident at the cellular level in Figure 6.
In order to determine the cellular populations that expressed activated p38 MAPK in the spinal cord after SCI, double immunofluorescent staining was performed using NeuN (to localize neurons), GFAP (to localize astrocytes) or OX-42 (to localize microglia) in laminae I-V of the dorsal horn. Figure 4C&D demonstrates the localization of p38 MAPK with NeuN, indicating that neurons in both the superficial and deeper laminae of the dorsal horn expressed activated p38 MAPK. Spinal cord injured rats that developed at-level neuropathic pain displayed significant increases in the number of NeuN positive cells in the dorsal horn that colocalized with activated p38 MAPK relative to sham controls (p < 0.0001, repeated measures ANOVA, Figure 4D). In addition to significant upregulation in neurons, we also observed a significant increase in p-p38 MAPK expression in astrocytes (GFAP labeled cells) and microglia (OX-42 labeled cells) within the dorsal horn (Figure 5A-D; Figure 6A-D; p < 0.0001, repeated measures ANOVA). These data indicate that multiple cell types contribute to the enhanced mechanical sensitivity seen at the level of the injury in SCI rats.
Figure 6.
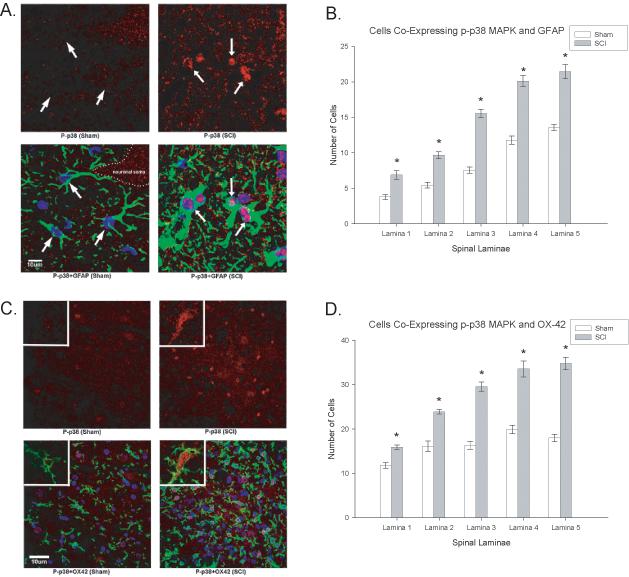
SCI demonstrates higher magnification of increased cellular localization of activated p38 (p-p38, red) in both astrocytes (Figure 6A, GFAP positive, green) and microglia (Figure 6B, OX-42 positive, green). Note that the p-p38 is increased in the nuclear compartment of both cell types. Quantification of the significant increases in p-p38 MAPK in astrocytes (GFAP, 6B) and microglia (OX-42, 6D) in the dorsal horn laminae of SCI rats relative to sham rats (*p<0.001). Quantification of the significant increases in p-p38 MAPK colocalization with GFAP (6B) and OX-42 (6D) positive cells (astrocytes and microglia, respectively) in the dorsal horn laminae of SCI rats relative to sham rats (*p < 0.001). All data are represented as mean ± SEM.
Dorsal Horn Neurons in the Thoracic Spinal Cord Rostral to the Site of Spinal Cord Injury Are Hyperexcitable Prior to p38 MAPK Inhibition
Before testing the role of inhibiting the activity of p38 MAPK on dorsal horn neuronal activity, we first examined whether dorsal horn neurons were hyperexcitable in the region rostral to the injury. At 35 days post injury, neuronal activity in response to brush, press, and pinch stimuli were compared between sham and SCI rats. As in our earlier studies (e.g., Crown et al. submitted) prior to drug administration SCI rats showed significantly greater neuronal activity to brush, press, and pinch stimuli than sham rats (all ps < 0.0001; ANOVA, Figure 7). In addition, similar analyses of neuronal responds to a graded series of von Frey stimuli found that SCI rats prior to drug application displayed significantly increased neuronal activity to the 0.6 g, 2 g, 6 g, 26 g, and 60 g force stimuli (p < 0.005, ANOVA, Figure 7).
Figure 7.
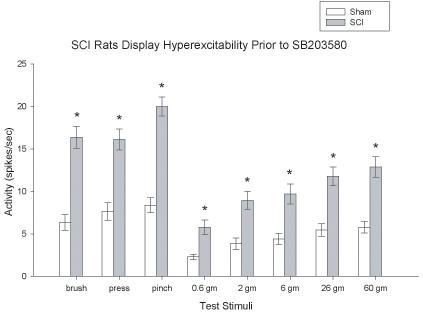
SCI produces dorsal horn neuronal hyperexcitability at the site of injury. SCI significantly increased evoked neuronal responses to brush, press, pinch and graded von Frey stimuli relative to sham rats (*p < 0.001) as demonstrated by the histograms of dorsal horn neuronal responses in spikes/sec. All data are represented as mean ± SEM.
Inhibition of p38 MAPK with SB203580 Attenuated Neuronal Hyperreactivity to Brush, Press, and Pinch
The next goal of the current set of experiments was to determine the effects of inhibiting the activity of p38 MAPK with SB203580 on neuronal hyperexcitability in SCI rats 35 days after injury. First, we tested the effects of three doses of SB203580 (0.1, 1, and 10 uM) on neuronal responses in sham rats. The p38 MAPK inhibitor, SB203580, produced no change in reactivity in sham rats, as none of the sham rats were significantly different in their neuronal responses to brush, press, and pinch stimuli (Figure 8E-H, all ps > 0.05.; repeated measures ANOVA). SB203580, on the other hand, produced a dose dependent attenuation of neuronal responses in SCI rats to the brush stimulus (p < 0.001; repeated measures ANOVA). Post hoc analysis indicated that SCI rats given the two highest doses of SB203580 (1 and 10 uM) were not significantly different than any of the sham groups (all ps < 0.05). SCI rats given the highest doses of SB203580 also displayed significantly fewer neuronal responses than vehicle treated SCI rats (both ps < 0.05). SB203580 also significantly decreased neuronal firing in SCI rats to the press and pinch stimuli in a dose dependent fashion (p < 0.0001, repeated measures ANOVA). For both measures, SCI rats given the highest doses of SB203580 also displayed significantly fewer neuronal responses than vehicle and 0.1 uM treated SCI rats (all ps < 0.05). Vehicle and 0.1 uM treated rats had significantly elevated neuronal responses that parallel responses seen in untreated SCI rats (all ps < 0.05). Taken together, these data indicate that inhibiting the activity of p38 MAPK with SB203580 dose-dependently attenuated neuronal hyperexcitability following SCI to both non-noxious (brush) and noxious (press, pinch) stimuli in dermatomes corresponding to the site immediately rostral to SCI. Figure 8A-D depicts neuronal responses in spikes per second over the 2 hour time course of testing to brush, press, and pinch stimuli from WDR neurons in rats with SCI.
Figure 8.
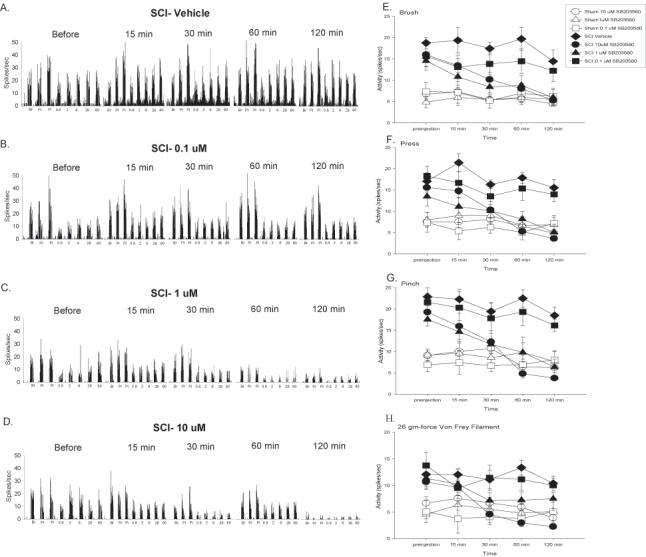
Inhibition of p38 MAPK activation significantly decreases background activity and evoked neuronal responses to brush, press, pinch, and graded von Frey stimulation at the level of SCI. A-D Spike frequency histograms of neuronal activity to brush, press, pinch and von Frey stimuli prior to SB203580 administration, and at 15, 30, 60, and 120 minutes after vehicle (A), 0.1 uM (B), 1 uM (C), or 10 uM (D) SB203580 administration. Mean activity (± SEM) for background and responses to brush (E), press (F), and pinch (G) for sham (open symbols) and SCI (filled symbols) rats given vehicle or SB203580 over the 120 minute recording period. SB203580 not only significantly depressed background activity but also dose dependently produced significant decreases in neuronal responding to brush, press, and pinch (*p < 0.001). H. Mean activity (± SEM) for responding to von Frey stimuli applied to the skin immediately rostral to the site of SCI for the 120 minutes following drug administration. SB203580 dose dependently depressed neuronal responding to the 2 gm, 6 gm, 26 gm, and 60 gm von Frey stimuli (*p < 0.001). For ease of presentation, only data for the 26 gm von Frey filament (the same filament used in the behavioral studies) is shown. All data are represented as mean ± SEM.
Inhibiting p38 MAPK Activation Also Diminishes Neuronal Responses to von Frey Stimulation
Next, we examined the effects of SB203580 on dorsal horn neuronal activity in response to graded von Frey stimuli applied to the dermatome immediately rostral to the site of SCI. As was the case with brush, press, and pinch stimuli, SB203580 had no impact on neuronal responses to the von Frey stimuli in sham rats at any of the three doses that were tested (all ps > 0.05; repeated measures ANOVA). In contrast, in the SCI rats, SB203580 produced a significant dose-dependent attenuation of neuronal responses to the 0.6 g, 6 g, 26 g, and 60 g force von Frey stimuli (p < 0.01; repeated measures ANOVA). Post hoc tests indicated that the 2 highest doses of SB203580 significantly reduced neuronal responses to the 0.6 g, 6 g, 26 g, and 60 g force von Frey stimuli, as these 2 doses were not significantly different over the two hour period that any of the sham groups (Figure 8H shows responding to the 26 gm-force von Frey stimulus, all ps < 0.05). In addition, the vehicle treated and 0.1 uM SB203580 SCI groups showed significantly greater responses to the von Frey stimuli than all the other groups (all ps < 0.05). These analyses also found that SB203580 dose dependently attenuated the responses to the 2 g force von Frey stimulus (p < 0.001; repeated measures ANOVA). Post hoc tests indicated that the vehicle-treated SCI group had significantly greater responses than all sham groups and the 1 uM and 10 uM SB203580 treated groups (all ps < 0.05). No other differences were significant (p > 0.05). Figure 8A-D shows neuronal responses in spikes per second over the 2 hour time course of testing to the von Frey stimuli.
DISCUSSION
Taken together, these data provide the first convergent evidence that persistent activation of the mitogen activated protein kinase, p38 MAPK, contributes to the maintenance of central neuropathic pain following spinal cord injury (SCI). As in our previous studies (Crown et al. 2005, 2006; Hulsebosch et al. 2000), we determined that rats given moderate SCI develop at-level mechanical allodynia. The current work extends these findings, demonstrating that persistent activation of p38 MAPK is causally related to the behavioral expression of at-level mechanical allodynia, a pathophysiological condition in which non-noxious stimuli become noxious. This was accomplished by demonstrating that an enzymatic inhibitor of p38 MAPK activity, SB203580, dose dependently attenuated at-level mechanical allodynia in SCI rats. SB203580 also was found to have no impact on nociceptive reactivity in sham rats, indicating that p38 MAPK signaling may only be important in producing changes in reactivity under pathological conditions. This observation is consistent with other work (Boyle et al 2006; Hains et al 2006; Svensson et al 2005, 2006). In addition, we report significant increases in p-p38 MAPK expression immediately rostral to the spinal lesion in dorsal horn laminae I-V, in microglia, astrocytes and neuronal populations. Lastly, multireceptive dorsal horn neurons rostral to the site of injury, in regions of the trunk where at-level mechanical allodynia developed in injured rats, display significant differences in evoked neuronal excitability to a broad range of stimuli (e.g., brush, press, pinch, and graded von Frey stimuli) following SCI relative to sham controls. SB203580 significantly reduced the aberrant hyperexcitability in multireceptive dorsal horn neurons, indicating a critical role for p38 MAPK phosphorylation in the altered membrane excitability. These results are the first to demonstrate causality in that persistent p38 MAPK activation plays a pivotal role in intracellular pathways that underlie the neuronal hyperexcitability that provides the substrate for maintained central neuropathic pain.
One interesting difference between the current study and prior work from other laboratories (e.g., Hains and Waxman, 2006; Yune et al. 2007) concerns the localization of activated p38 MAPK in the spinal cord after contusive SCI. Other authors (Hains and Waxman, 2006) detected activated p38 MAPK in microglia but not in neurons, whereas the current work clearly detected activated p38 MAPK in neurons, microglia, and astrocytes. There are a number of potential reasons for these discrepancies. First, the current work employed a different contusion procedure than has been used in other work. In this work, we used the Infinite Horizons impactor to apply 150 kdynes of force and hold that force onto the surface of the spinal cord for 1 s. Other work from our laboratory (e.g., Carter et al. 2004) has shown that holding the force against the surface of the cord increases the probability that rats will develop neuropathic pain after SCI. It could be the case that this methodological difference produces a different pathophysiology at the injury than other weight drop devices like the NYU impactor. For example, the sustained application of force to the spinal cord may add an ischemic component to the injury that alters the CNS response to the injury. It is known from other work (e.g., Bu et al 2007; Guo and Bhat 2007) that hypoxia can induce activation of p38 MAPK in both neurons and microglia. These findings suggest that differences in the injury paradigms used in the different studies may have lead to a different ischemic response in the tissue that could have lead to broader activation of p38 MAPK. This discrepancy is interesting and will require future studies to better understand the pathophysiological differences between the different injury paradigms. Secondly, the spinal regions examined are different in the current study compared to that of Hains and Waxman in which p38 expression was examined in lumbar regions 28 days after a low thoracic SCI (below level regional pain). Thirdly, the time after injury and methods vary in the current study compared to that of Yune et al., in which p38 expression was examined 5 days after SCI, at which time p38 MAPK is involved in intracellular signaling and cell death and cellular expression was tested in primary cultures. Thus, other studies did not rigorously tested a variety of cell types in the segment rostral to SCI (at level regional pain) in the chronically injured spinal cord, as was done in the current study, In summary, p38 MAPK is involved in cellular apoptotic pathways early after SCI injury, but appears to be a key factor in the maintenance of chronic pain weeks after SCI. Currently, we are working to examine the time course of activation of members of the MAP kinase family after SCI and better understand the cell types activated by this type of injury. Another area of interest involves the downstream targets of p38 MAPK activation in the context of SCI. Other work from our laboratory has focused on cyclic AMP responsive element binding protein (CREB; Crown et al. 2005), further work is needed to understand the role of downstream effectors in at-level neuropathic pain.
Recent work in our laboratory (e.g., Crown et al. 2005, 2006, submitted) has focused on the activation of key intracellular signaling cascades in the initiation and maintenance of central neuropathic pain following spinal cord injury. We show that a number of signaling cascades that have been implicated in long-term potentiation and central sensitization (e.g., calcium calmodulin dependent protein kinase II, other members of the MAPK family such as ERK1/2) are upregulated in dorsal horn neurons during the maintenance of at-level neuropathic pain following spinal cord injury. These findings are novel for spinal circuits just rostral to a chronic injury and are the first that implicate multiple cellular populations: microglia, astrocytes and neuronal populations in the p38 mediated responses. Previous work by others focused on below level signaling pathways and only the microglia involvement. For example, recent research suggested a role for p38 MAPK in the maintenance of below-level pain (Hains et al., 2006). A recent study by Peng and colleagues (2006) also demonstrated that decreases in p38 MAPK expression caused by soluble tumor necrosis factor alpha receptor administration also produced a decrease in below-level neuropathic pain after spinal hemisection. It is important to note, however, that the methods used to decrease p38 MAPK expression in both of these studies (minocycline administration or herpes simplex viral vector-mediated transfer of the cleaved soluble receptor for tumor necrosis factor-alpha) have a wide range of biological effects that could influence pain sensitivity. In addition to p38 MAPK, inhibiting the activation of ERK1/2 by PD98059 following excitotoxicity lesion of the spinal cord, was shown to diminish the development of excessive grooming behavior, which is one indication of central neuropathic pain (Yu and Yezierski, 2005).
The current data represent the first study to directly demonstrate that inhibition of the enzymatic activity of p38 MAPK leads to a decrease in at-level mechanical allodynia, a measure of chronic central neuropathic pain following SCI. This study combined behavioral, immunoblotting, immunocytochemical, and electrophysiological methods suggesting that increases in p38 MAPK expression in neuronal and glial populations play an important role in nervous system dysfunction following SCI. Recent research from other laboratories has determined other potential effects of p38 MAPK following SCI. Xu and colleagues (2006) report that p38 MAPK mediates the degeneration of spinal cord neurons produced by iNOS after SCI. In addition, Wang and colleagues (2005) have demonstrated that IL-1 beta induced apoptosis of spinal cord neurons following SCI is mediated by p38 MAPK signaling. Lastly, inhibiting the enzymatic activity of p38 MAPK has been shown to promote the recovery of locomotor function following spinal cord injury (Horiuchi et al. 2003). Taken together, these data suggest that p38 MAPK inhibition is beneficial in limiting the neuronal response to CNS trauma and improving locomotor outcomes following injury. In summary, our findings establish that p38 MAPK inhibition and multiple cell types play roles in the maintenance of central neuropathic pain following SCI.
Acknowledgements
This work was funded by CRPF grant CB1-0404-2 to EDC, Mission Connect of TIRR-Houston, the Dunn Foundation, the West Foundation, Mr. Frank Liddell and NIH grants NS11255 and NS39161 to CEH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Attal N, Guirimand F, Brasseur L, Gaude V, Chauvin M, Bouhassira D. Effects of IV morphine in central pain: a randomized placebo-controlled study. Neurol. 2002;58:554–563. doi: 10.1212/wnl.58.4.554. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bigbee AJ, Hoang TX, Havton LA. At-level neuropathic pain is induced by lumbosacral ventral root avulsion injury and ameliorated by root reimplantation into the spinal cord. Exp Neurol. 2007;204:273–282. doi: 10.1016/j.expneurol.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle DL, Jones TL, Hammaker D, Svensson CI, Rosengren S, Albani S, Sorkin L, Firestein GS. Regulation of peripheral inflammation by spinal p38 MAP kinase in rats. PLoS Med. 2006;3:e338. doi: 10.1371/journal.pmed.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Huang P, Qi Z, Zhang N, Han S, Fang L, Li J. Cell type-specific activation of p38 MAPK in the brain regions of hypoxic preconditioned mice. Neurochem Int. 2007;51:459–466. doi: 10.1016/j.neuint.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord. 2003;41:109–117. doi: 10.1038/sj.sc.3101401. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96:365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- Carter MW, Tan H, Johnson KM, Hulsebosch CE. Abstract Viewer/Itinerary Planner. Soc. Neurosci. Washington, DC: 2004. Effects of force and dwell-time in modeling chronic central neuropathic pain after contusive spinal cord injury (SCI) Program No. 458.7. 2004. [Google Scholar]
- Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Westlund KN, Hulsebosch CE. Upregulation of the phosphorylated form of CREB in spinothalamic tract cells following spinal cord injury: relation to central neuropathic pain. Neurosci Lett. 2005;384:139–144. doi: 10.1016/j.neulet.2005.04.066. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Westlund KN, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Tan H, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Calcium/calmodulin dependent kinase II plays a role in persistent central neuropathic pain following spinal cord injury. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson-Hudson TA, Shiflett SC, Kirshblum SC, Bowen JE, Druin EL. Acupuncture and Trager psychophysical integration in the treatment of wheelchair user’s shoulder pain in individuals with spinal cord injury. Arch Phys Med Rehabil. 2001;82:1038–1046. doi: 10.1053/apmr.2001.24888. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Biering-Sorensen F, Johannesen IL, Terkelsen AJ, Juhl GI, Kristensen AD, Sindrup SH, Bach FW, Jensen TS. Intravenous lidocaine relieves spinal cord injury pain: a randomized controlled trial. Anesthesiology. 2005;102:1023–1030. doi: 10.1097/00000542-200505000-00023. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS. Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain. 2002;96:375–383. doi: 10.1016/S0304-3959(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Guo G, Bhat NR. p38alpha MAP kinase mediates hypoxia-induced motor neuron cell death: a potential target of minocycline’s neuroprotective action. Neurochem Res. 2007;32:2160–2166. doi: 10.1007/s11064-007-9408-8. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, McCartney N. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord. 2003;41:34–43. doi: 10.1038/sj.sc.3101389. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Ogata T, Morino T, Chuai M, Yamamoto H. Continuous intrathecal infusion of SB203580, a selective inhibitor of p38 mitogen-activated protein kinase, reduces the damage of hind-limb function after thoracic spinal cord injury in rat. Neurosci Res. 2003;47:209–217. doi: 10.1016/s0168-0102(03)00216-5. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. From discovery to clinical trials: treatment strategies for central neuropathic pain after spinal cord injury. Curr Pharm Des. 2005;11:1411–1420. doi: 10.2174/1381612053507864. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. 2004;3:299–303. doi: 10.2174/1568010043343804. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou J-L, Lee I, Chung K, Chung JM. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–83. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- Peng X-M, Zhou Z-G, Glorioso JC, Fink DJ, Mara M. Tumor necrosis factor-α contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Siddall PJ, Loeser JD. Pain following spinal cord injury. Spinal Cord. 2001;39:63–73. doi: 10.1038/sj.sc.3101116. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Powell H, Sorkin LS. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci Lett. 2005;379:209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Schafers M, Jones TL, Yaksh TL, Sorkin LS. Covariance among age, spinal p38 MAP kinase activation and allodynia. J Pain. 2006;7:337–345. doi: 10.1016/j.jpain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Tan H, Johnson KM, Hulsebosch CE. Role of ionotropic glutamate receptors in a rodent model of central neuropathic pain. Neurosci Abstr. 2004:34. [Google Scholar]
- Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Biol. 1997;4:311–6. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Jr, Siddall P, Yezierski RP. Pain following spinal cord injury: animal models and mechanistic studies. Pain. 2000;89:1–5. doi: 10.1016/S0304-3959(00)00463-2. [DOI] [PubMed] [Google Scholar]
- Vincler M, Maixner W, Vierck CJ, Jr, Light AR. Effects of systemic morphine on escape latency and a hindlimb reflex response in the rat. J Pain. 2001;2:83–90. doi: 10.1054/jpai.2001.19560. [DOI] [PubMed] [Google Scholar]
- Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. doi: 10.1191/0269215503cr581oa. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Kong KM, Qi WL, Ye WL, Song PS. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol Sin. 2005;26:934–942. doi: 10.1111/j.1745-7254.2005.00152.x. [DOI] [PubMed] [Google Scholar]
- Widerstrom-Noga EG, Duncan R, Felipe-Cuervo E, Turk C. Assessment of the impact of pain and impairments associated with spinal cord injuries. Arch Phys Med Rehabil. 2002;83:395–404. doi: 10.1053/apmr.2002.28028. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang BR, Wang X, Kuang F, Duan XL, Jiao XY, Ju G. ERK1/2 and p38 mitogen-activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci. 2006;79:1895–1905. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Yu CG, Yezierski RP. Activation of the ERK1/2 signaling cascade by excitotoxic spinal cord injury. Brain Res Mol Brain Res. 2005;138:244–255. doi: 10.1016/j.molbrainres.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Yune TY, Lee JY, Jung GY, Kim SJ, Jiang MH, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–68. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]


