Abstract
The source of bloodmeals in 2,082 blood-fed mosquitoes collected from February 2002 through December 2003 in Memphis and surrounding areas of Shelby County, Tennessee were determined. Members of the genus Culex and Anopheles quadrimaculatus predominated in the collections. Members of the Cx. pipiens complex and Cx. restuans were found to feed predominately upon avian hosts, though mammalian hosts made up a substantial proportion of the bloodmeals in these species. No significant difference was seen in the host class of bloodmeals in mosquitoes identified as Cx. pipiens pipiens, Cx. p. quinquefasciatus, or hybrids between these two taxa. Anopheles quadrimaculatus and Cx. erraticus fed primarily upon mammalian hosts. Three avian species (the American Robin, the Common Grackle, and the Northern Cardinal) made up the majority of avian-derived bloodmeals, with the American Robin representing the most frequently fed upon avian host. An analysis of these host feeding data using a modification of a transmission model for Eastern Equine encephalitis virus suggested that the American Robin and Common Grackle represented the most important reservoir hosts for West Nile virus. A temporal analysis of the feeding patterns of the dominant Culex species did not support a shift in feeding behavior away from robins to mammals late in the summer. However, a significant degree of temporal variation was noted in the proportion of robin-derived bloodmeals when the data were analyzed by semi-monthly periods throughout the summers of 2002 and 2003. This pattern was consistent with the hypothesis that the mosquitoes were preferentially feeding upon nesting birds.
Keywords: Mosquito host preference, Mosquito bloodmeal identification, Culex pipiens pipiens, Culex quinquefasciatus, West Nile virus
INTRODUCTION
West Nile virus (WNV) has spread rapidly across the United States and southern Canada since its introduction into New York in 1999 (Hayes et al. 2005). WNV has become the most prevalent viral encephalitis in the United States, with 2,949 human cases reported in 2005 (CDC 2006b). WNV is a Flavivirus, and a member of a family of RNA positive stranded viruses that includes such human pathogens as Saint Louis Encephalitis virus (SLEV) and Yellow Fever virus. Like SLEV, WNV is transmitted in an enzootic cycle involving the endemic avifauna (Godsey et al. 2005).
Sixty species of mosquitoes have been shown to harbor WNV by various assays (CDC 2006a), of which roughly 20 species have been shown to be competent laboratory vectors (Turell et al. 2005). Species belonging to the genus Culex are thought to be key vectors for WNV in the United States, with members of the Cx. pipiens L. complex representing the major vectors throughout the Mississippi River basin and eastern portions of the United States (Andreadis et al. 2001, 2004, Godsey et al. 2005, Loftin et al. 2006). In North America, the Culex pipiens complex includes Cx. pipiens pipiens L., Cx. p. quinquefasciatus Say and their hybrids. Based on analysis of morphological features of the male genitalia, Barr (1957) presented a simple latitudinal model to explain the geographic distribution of these taxa in North America. Culex p. pipiens is assumed to occur in areas north of 39°N and Cx. p. quinquefasciatus is thought to be present in areas south of 36°N latitude. In the middle latitudes of the United States between 36°N and 39°N both nominal taxa and their hybrids (Barr 1957) and introgressed specimens (Tabachnick and Powell 1983, Miller et al. 1996, Cornel et al. 2003) may be present. In the Mississippi River basin, both nominal taxa and hybrids are known to occur in areas south of 36°N latitude in northern Alabama, Arkansas, and Memphis and other areas of southern Tennessee (Barr 1957, Jakob et al. 1979, Savage et al. 2006).
The transmission dynamics of WNV is often represented as consisting of two parallel cycles (Savage et al. 1999, Fyodorova et al. 2006). In the enzootic cycle, transmission occurs from bird-to-bird, mediated through a mosquito vector. This cycle is the primary way WNV is maintained during the transmission season, though vertical transmission to the egg stage may also play a role, both in over-wintering of the virus and maintaining infection in the vector population during the transmission season (Miller et al. 2000, Nasci et al. 2001, Goddard et al. 2003, Anderson and Main 2006). The enzootic cycle is maintained through mosquito species that feed primarily or exclusively upon birds. For example, in recent studies in Connecticut, it was reported that Culex restuans Theobald fed exclusively upon avian hosts, implicating it as an important enzootic vector in this area (Molaei et al. 2006). The virus, while being maintained in the bird population, occasionally escapes the bird-to-bird cycle to infect other vertebrates, including horses and humans. Virus escape from an enzootic cycle into an epidemic cycle is mediated through mosquito species with broad host utilization patterns, e.g. those that feed upon both birds and mammals. In some cases, a single mosquito species can function as both an enzootic and bridge vector. For example, Cx. p. quinquefasciatus and Cx. nigripalpus Theobald, function as both enzootic and epidemic vectors of SLEV and WNV in the southern United States (Mitchell et al. 1980, Savage et al. 1993b, Shaman et al. 2004). These species are responsible for virus amplification in the enzootic cycle and transmission to humans. Transmission to humans in urban areas by Cx. p. quinquefasciatus is thought to occur when mosquito population levels and infection rates are high allowing incidental feeding on humans to result in virus transmission (Mitchell et al. 1980, Savage et al. 1993b). Transmission of SLEV to humans in Florida is believed to result from meteorological events that result in increased Cx. nigripalpus–human contact following periods of enzootic virus amplification (Shaman et al. 2004). Similarly, Cx. p. pipiens has been hypothesized to act as both an enzootic and bridge vector in the mid-Atlantic states (Kilpatrick et al. 2005). Mammalian hosts of WNV, although they often suffer pathologies associated with infection, generally do not develop very high circulating titers of the virus, are therefore generally not efficient reservoir hosts for the virus and result in few infected mosquitoes.
For WNV to be maintained in the enzootic cycle, a mosquito vector must first be infected by feeding on an infectious bird. The mosquito must then survive long enough to develop a viral titer sufficient to infect a vertebrate host, and the mosquito must then find and feed upon a susceptible host in order to complete the transmission cycle. Conversely, the vertebrate host must develop a sufficient viral titer in its blood to be infectious, and must also survive long enough to be available to transmit the virus to the mosquito vector. Several variables determine if a particular avian species will be a significant vertebrate reservoir host for WNV. For example, laboratory infection studies have demonstrated that different bird species vary widely in their susceptibility to WNV, in the peak viral titers in sera, duration of infectious viremia, and in their survival after WNV infection (Komar et al. 2003). Similarly, the importance of a particular avian species as a reservoir will be particularly dependent upon the amount of contact between the bird species in question and the mosquito vector, since two feeding events are required to complete the cycle of transmission.
Previous research has suggested that mosquito vectors of the arboviral encephalitides feed predominately upon certain available bird species, and that some species are targeted to a greater extent than might be expected based upon their abundance alone (Hassan et al. 2003, Kilpatrick et al. 2006a). Similarly, recent studies have suggested that the American Robin is the most important avian host for Cx. p. pipiens and Cx. restuans in the Northeastern United States (Kilpatrick et al. 2006a, Molaei et al. 2006), and that a shift in feeding from robins to humans late in the summer in the Northeast may be an important factor in the escalation of human WNV infections during this period (Kilpatrick et al. 2006b). However, these conclusions were based upon an analysis of a relatively limited number of blood-fed mosquitoes. It is also not known if a similar pattern of mosquito feeding is typical of other areas in the United States or if this host-feeding pattern is specific to the Northeastern United States.
In this manuscript, we present an analysis of over 2,000 blood-fed mosquitoes collected in 2002 and 2003 at 70 sites located in Memphis and surrounding areas of Shelby County, Tennessee. The majority of mosquitoes analyzed were members of the Cx. pipiens complex and Cx. restuans; both taxa are significant vectors of WNV in the Eastern USA (Andreadis et al. 2001, 2004). Shelby County is located in the southern portion of the zone where Cx. p. pipiens and Cx. p. quinquefaciatus are sympatric and hybridize, allowing us to analyze the feeding patterns of both taxa and their hybrids (Barr 1957, Savage et al. 2006). Finally, the large number of individuals analyzed permitted us to conduct a detailed temporal and spatial analysis of the host-feeding choice of these mosquito species, and to model the reservoir capacity of avian hosts for WNV.
METHODS
Collection of mosquitoes
Mosquitoes were collected in Memphis and surrounding areas of Shelby Co., Tennessee, from 80 resting sites in concrete and galvanized metal storm water sewers. Collections were made with hand-held aspirators (Hausherr’s Machine Works, Toms River, NJ). Collections began on February 4, 2002 and continued at least once per month through December 12, 2003. Mosquitoes in sealed aspirator tubes were placed in small coolers and transported to the Memphis/Shelby County Vector Control (M/SCVC) laboratory where specimens were transferred to labeled cryotubes and held at −70°C until they were shipped on dry ice to the CDC, Ft. Collins, Colorado, for processing.
Classification of collection sites
At each site, the longitude and latitude were recorded with a hand-held Global Positioning System (GPS) unit (Garmin, Olathe, KS), and the type of construction (concrete or galvanized pipe) was recorded. The neighborhood surrounding each site was classified as rural, urban, or commercial and industrial. Rural and urban sites were also classified by general income class as either low income, middle income or upper income. Classification was done independently by two members of the M/SCVC program after inspecting the area within 200 m of the collection site.
Specimen processing, dissection, and morphological and molecular identification of mosquitoes
Mosquitoes were identified to species or lowest taxonomic unit using dissecting microscopes on refrigerated chill tables, and published taxonomic keys (Darsie and Ward 1981). For Culex L. mosquitoes additional morphological characters were used for species identification as previously described (Apperson et al. 2002). It was not always possible to reliably distinguish specimens of Cx. restuans from members of the Cx. pipiens complex; therefore, these specimens were morphologically identified as Cx. pipiens complex/restuans. Members of the Cx. pipiens complex can not be distinguished morphologically without examination of the male genitalia, and adults were identified morphologically as Cx. pipiens complex.
Specimens with a visible bloodmeal were assigned a unique number and processed individually. In a Biological Safety Level-2 cabinet, each specimen was placed on a clean microscope slide. An insect pin or forceps was used to hold the thorax and the abdomen was removed with a separate insect pin. Insect pins were used only for one specimen; forceps, when used, were used once, rinsed with ethanol, and washed with detergent before being reused. For each specimen, the head and thorax were placed in a tube, and the severed abdomen placed into a separate, identically labeled tube. Tubes were held on ice until being placed at −70°C for temporary storage. Abdomens were shipped on dry ice to North Carolina State University (NCSU), Raleigh, North Carolina, and then onto the University of Alabama at Birmingham (UAB), Birmingham, Alabama, and tested for bloodmeal host as described below. The head and thorax of each specimen was processed at CDC and tested for the presence of WNV, and specimens of Cx. (Culex) were processed for molecular species identification (Savage et al. 2006).
The head and thorax of each specimen were placed into 2.0-mL snap-cap tubes (Daigger and Co., Lincolnshire, IL) and a single copper-coated, steel bead (Copperhead, East Bloom-field, NY) and 0.5 mL of bovine albumin-1 solution (Savage et al. 2006) was added to the tube. Mosquitoes were homogenized using a Mixer Mill apparatus (QIAgen Inc., Valencia, CA) for 4 min at 20 cycles/sec. After centrifugation at 4,000 rpm for 3 min, 220 μL of supernatant was placed in an identically labeled 1.7-mL microfuge tube. Nucleic acids were extracted from this supernatant aliquot as indicated below and the remainder of the original mosquito suspension was held at −70°C for confirmatory tests. Nucleic acids, including mosquito DNA and viral RNA, were extracted from each supernatant aliquot using a QIAgen Biorobot 9604 and QIAgen’s Qiamp Viral RNA kit following the manufacturer’s protocols.
Specimens identified morphologically as members of the subgenus Cx. (Culex) were assayed by PCR using species-specific primers to refine, correct or verify morphological identifications. All Cx. (Culex) specimens were subject to a PCR assay using species-specific primers designed to identify Cx. restuans and members of the Cx. pipiens complex (Crabtree et al. 1995) based on differences in the internal transcribed spacers (ITS) of the ribosomal DNA gene array as previously described (Aspen et al. 2003). Specimens identified either morphologically or by the ITS primers as members of the Cx. pipiens complex were assayed with primer sets based on the ACE.2 gene to distinguish between the three members of the Cx. pipiens complex: Cx. p. pipiens, Cx. p. quinquefasciatus, and hybrids. For specimens collected in 2002, the ACE.2 assay followed the protocol of Aspen and Savage (Aspen and Savage 2003) except that approximately 300 ng of template DNA was used in each reaction mixture. For specimens collected in 2003, a revised hot start ACE.2 protocol was used that incorporated two significant changes and improved sensitivity and specificity (Gordon and Savage, unpublished data): use of a hot start Taq polymerase and the required activation step, and an approximate four-fold increase in primer concentrations. Each 25-μL reaction contained 1× of GeneAmp PCR Buffer I (10 mM Tris-HCl pH 8.3, 50mM KCl, 1.5mM MgCl2, 0.01% w/v gelatin [Applied Biosystems, Foster City CA]), 0.2 mM of each deoxyribonucleic triphosphate (Roche Diagnostics Corp., Indianapolis IN), 330 nM of the reverse-sense primer B1246, 2 μL of template DNA, and 0.025 U of Qiagen Hot-StarTaq (QIAgen, Valencia, CA). Each reaction also contained either 330 nM of PACEF290 or 1315 nM of QACEF290 forward primers. The reaction mixtures were placed in either a PTC-100 or PTC-200 thermal cycler (MJ Research, Inc., Incline Village, NV) programmed for 1 cycle at 95°C for 15 min, 94°C for 1 min, 52°C for 1 min, 72°C for 1 min, followed by 30 cycles of 94°C for 1 min, 52°C for 1 min, 72°C for 1 min, and completed by 1 cycle at 72°C for 10 min. PCR products were visualized and taxonomic status of specimens assessed as described in Aspen and Savage (2003).
Detection of WNV in mosquitoes
The head and thorax of each specimen was tested for the presence of WNV-RNA using a TaqMan RT-PCR assay performed as described by Lanciotti et al. (2000). Five microliters of template RNA from each sample were added to primers and probes, specific to the 3′ non-coding region of the WNV genome (WN3′ NC [Lanciotti et al. 2000]), and reagents in QIA-gen’s Quantitect Probe RT-PCR kit. Samples were subjected to 45 amplification cycles in the iCycler iQ™ Real-time PCR Detection System (Bio-Rad, Hercules, CA) according to cycling conditions described previously (Lanciotti et al. 2000). Presumptive positives were confirmed by hand-extraction of RNA from 100 μL of the original mosquito suspension (QIAgen Viral RNA Minikit) followed by WNV detection in a TaqMan RT-PCR assay using a second primer set based on the envelope gene, WNENV (Lanciotti et al. 2000). Mosquitoes positive in both TaqMan assays were reported as WNV positive.
Bloodmeal identification
Abdomens of blood-fed mosquitoes were initially processed for bloodmeal identification by indirect ELISA as previously described (Irby and Apperson 1988). Abdomens of blood-fed mosquitoes were homogenized individually in 500 μL of phosphate buffered saline (pH 7.4) and the homogenate subjected to centrifugation at 6,225 × g for 5 min. A total of 400 μL of the supernatant was removed for use in the ELISA. The remaining solution was brought to 10 mM EDTA, the pelleted material resuspended and the samples stored at −80°C. The supernatants were initially classified using a panel of broadly reactive antisera (anti-mammal, reptile, avian, and amphibian) as previously described (Irby and Apperson 1988). Extracts positive for mammalian blood were further characterized using a panel of species-specific antisera.
Samples testing positive for avian blood were further classified by PCR, employing a previously described PCR-based assay for the specific amplification of a portion of the cytochrome B gene encoded in the DNA present in avian derived bloodmeals (Hassan et al. 2003). Amplicons were classified by heteroduplex analysis (HDA), as previously described (Lee et al. 2002), using two drivers derived from Northern Cardinal or Carolina Chickadee. Samples were grouped on the basis of a comparison of the relative mobility of the HDA bands in both assays carried out with the two different drivers. Amplicons from representative samples from each HDA group were purified using the Qiaquick PCR purification kit (Qiagen) and their DNA sequence directly determined. The origin of the bloodmeal was then determined by comparison of the DNA sequence to the sequences present in the Gen-bank DNA sequence bank, as previously described (Apperson et al. 2004).
Statistical analysis
The significance of observed differences in the number of bloodmeals derived from different species or classes was assessed using a χ2 test. The 95% confidence intervals surrounding the estimated proportion of blood-meals taken from a given class or species were calculated as described previously (Apperson et al. 2004).
Non-parametric models were used to assess the significance of observed temporal shifts in host feeding. This analysis was restricted to the 2002 data, as semi-monthly collections were not carried out in 2003. The analysis concentrated on shifts in feeding on robins compared to all other hosts, as previous studies have suggested that such a shift from robins to other hosts may be an important factor in driving infection of humans with WNV (Kilpatrick et al. 2006b). Non-parametric models were used to develop “predicted” numbers of robin and non-robin derived bloodmeals for each semi-monthly period. Concordance between the modeled curves for the robin and non-robin derived meals was assessed using Kendall’s Tau test. The significance of the differences between the predicted and observed values for each semi-monthly period were assessed using a χ2 test.
In analyzing this sort of seasonal data, one would expect to see two types of variation. The first of these would result from the normal seasonal variation in the total number of blood-fed mosquitoes present, simply reflecting those months when the mosquitoes are most active. The second potential source of variation would result from actual temporal shifts in host choice. To separate these two sources of variation, the curves derived from the non-parametric regression models were subtracted from the observed data, producing residual curves that highlighted trends associated with shifts in host choice. The concordance of these residual curves was assessed using a Kendall’s Tau test.
Modeling analysis
The model used to assess the relative reservoir competence of various avian species was modified from a recently published step-flow model (Unnasch et al. 2006), whose purpose was to test the hypothesis that young of the year birds played a significant role in the transmission dynamics of Eastern Equine Encephalitis virus. This model was created with the Stella® Software package (Wallis et al. 2002). The model was designed to run in 200 daily steps, simulating a 200-day transmission season (March 15 to October 1). To adapt this model to assess the importance of various avian species as reservoirs for WNV, the young of the year module present in the original model was replaced by a second adult bird module. Feeding success on individual bird species were estimated by normalizing the number of bloodmeals taken from a given bird species with a global estimate of the overall abundance of that species in the Memphis/Shelby County area, as estimated from data collected by the U.S. Geological Survey (USGS) breeding bird survey (BBS) (Sauer et al. 2005). To obtain as global an estimate as possible, data from the five BBS routes located within a 40-kM radius of the center of the city of Memphis were combined to estimate bird abundance in the area. Feeding success values were further normalized to the species that exhibited the greatest value (the Grey Catbird). The feeding success on the Grey Catbird was set as 1.0 (i.e., a mosquito attempting to feed upon this species succeeded 100% of the time). The feeding success estimates for all other species were thus estimated as less than 1.0, based upon their feeding index relative to that of the Grey Catbird. Relative encounter rates were set at 0.5, meaning that if two birds of different species were encountered by a questing mosquito, there was an equal probability that the mosquito would attempt to feed upon either. Data on mortality rates, peak serum viremia levels and duration of viremia for each bird species were obtained from published laboratory studies (Komar et al. 2003). All of the remaining parameters in the model relating to infection in the mosquito vector (e.g., vector competence, extrinsic incubation period and daily mosquito mortality rates) were derived from published sources (Nasci and Edman 1984, Mokgweetsinyana 1987, Johnson 1998, Turell et al. 2001, Dohm et al. 2002).
Two approaches were taken to evaluate the reservoir capacity of each species. In the first analyses, birds that were frequently targeted by mosquitoes were analyzed individually, to estimate the reservoir capacity of each species. In conducting this analysis, the model was first seeded with 500 identically parameterized adult birds, which were divided equally between the two modules. The modules were parameterized identically, using values for the avian parameters (e.g., duration of infectious viremia, mortality rate in infected birds and mosquito feeding success) derived from published laboratory studies (Komar et al. 2003). Several output values from the model indicative of the predicted intensity of transmission were then examined to estimate the reservoir capacity of each species.
The studies involving the individual bird species provided an indication of the predicted reservoir capacity of each species. However, these studies did not indicate the relative role that each species might play in the transmission of WNV in the context of the avifauna as a whole. To address this question, the model was run with the two modules parameterized differently. In these analyses, one module was parameterized with the values derived from the individual species to be studied, while the second module was parameterized with weighted averages for all parameters derived from the entire observed bird population, minus the species under consideration. The number of adult birds seeded into the individual module was based upon the relative abundance of the species under study relative to all other birds observed, so that the number of birds in both modules totaled 500.
A free-standing version of the model, a text file describing how to navigate the model’s interface, an Excel spreadsheet detailing the parameters used in the analysis and a link to a free engine that may be used to run the model can be obtained from Dr. Thomas R. Unnasch at tunnasch@uab.edu.
RESULTS
In total, 8,440 mosquitoes were collected from 80 resting sites. Among these 8,440 specimens, 2,346 (28%) had visible bloodmeals under the dissecting microscope and were processed for bloodmeal identification. The bloodmeals of 2,082 (89%) of 2,346 processed mosquitoes were identifiable to host class. These 2,082 specimens were collected at 70 of the 80 sampled sites. The locations of these 70 sites are depicted in Figure 1.
FIG. 1.
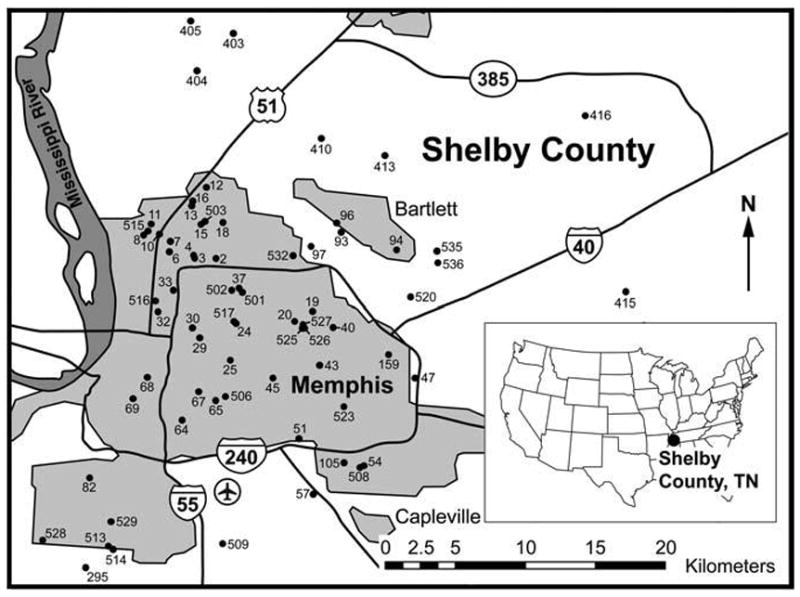
Location of the 70 mosquito resting sites in Shelby County, Tennessee, that yielded mosquito specimens with blood-meal hosts identifiable to host class.
Members of the genus Culex made up 79% of the collection, with members of the Cx. pipiens complex (29%), Cx. restuans (21%), and Cx. erraticus (22%) predominating among the Culex mosquitoes. The only species other than Culex mosquitoes that was collected in significant numbers was Anopheles quadrimaculatus, which made up 19% of the total collection.
Mosquitoes with identifiable bloodmeals were collected on at least one day per month from February 4 through December 31, 2002, and from March 24 through November 13, 2003. No blood fed specimens were detected during 12 site visits conducted between January 8, 2003 and February 18, 2003, and on seven site visits conducted between November 20 and December 12, 2003. The seasonal abundance of blood-fed specimens for the five most common mosquito taxa are summarized in Figure 2. The numbers of blood-fed Cx. pipiens complex mosquitoes peaked in July in both 2002 and 2003, as did the number of blood-fed An. quadrimaculatus collected (Fig. 2). In contrast, the numbers of blood-fed Cx. restuans collected peaked earlier in both years, in April and May. The numbers of blood engorged Cx. erraticus collected peaked in late summer (August–October) in 2002 and in July 2003 (Fig. 2). In 2003, the majority (72%) of the Cx. erraticus collected were obtained from a single collection taken from a single site.
FIG. 2.
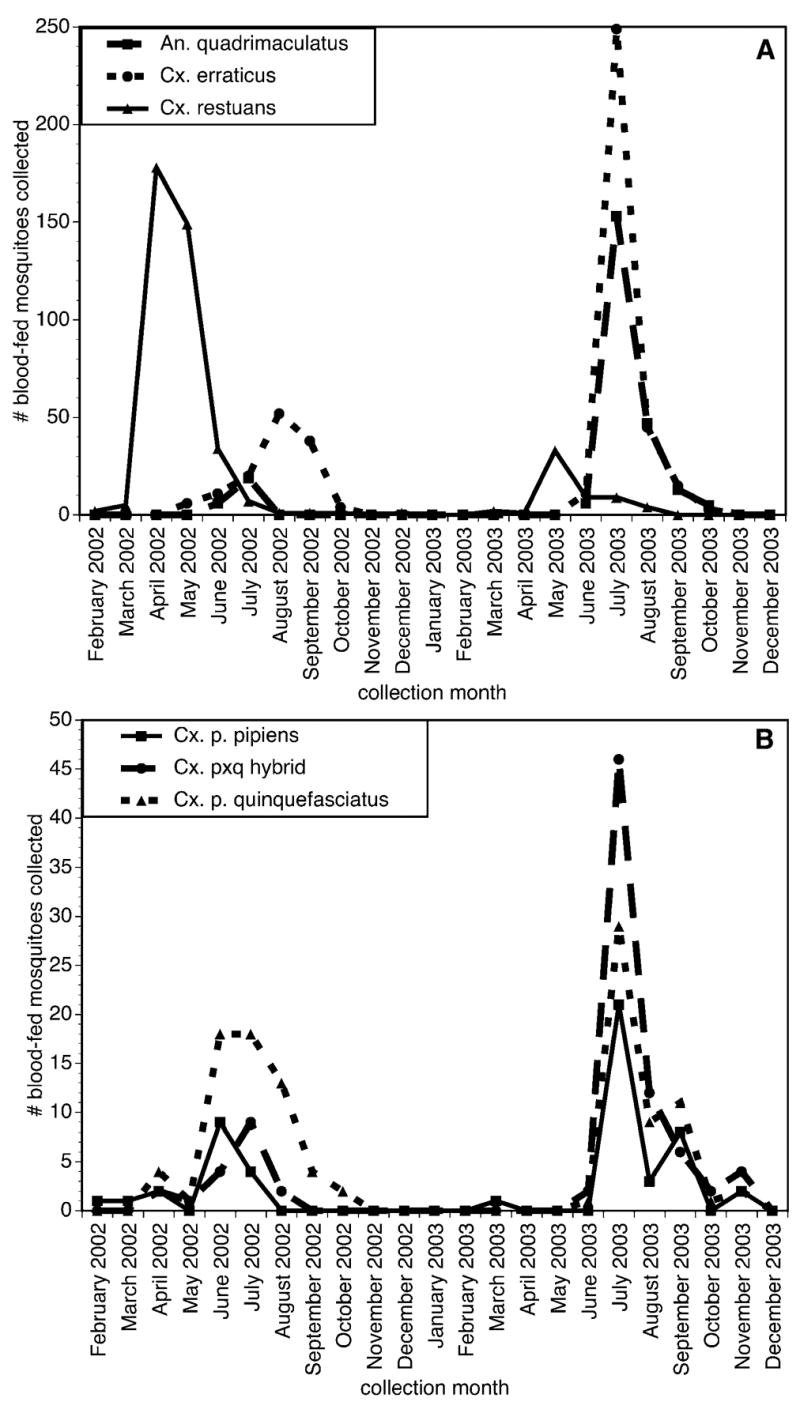
Number of blood-fed mosquitoes collected, for species and members of the Culex pipiens complex represented by more than 50 specimens, by monthly intervals from Shelby County, Tennessee, 2002–2003. (A) Species other than members of the Cx. pipiens complex. (B) Taxa in the Cx. pipiens complex.
Forty-eight of the mosquitoes with blood-meals identifiable to host class were not identified below the generic level, for example, Culex spp., and were excluded from further analysis. Host class utilization data on the remaining 2,034 specimens, representing 13 mosquito taxa, are summarized in Table 1. Of the 2,034 mosquitoes with bloodmeals identified to host class, 1,956 (96%) were identified as having come from a single class of host (Table 1). The remaining 4% of the meals were found to have been derived from more than one host class, with mixed avian-mammalian meals accounting for 3.5% of the meals. Members of the Cx. pipiens complex were ornithophilic, with 73% (95% CI, 70–76%) of the individuals from these taxa taking meals from avian hosts. The majority (14%) of non-avian meals taken by members of the Cx. pipiens complex were mammalian in origin, and 24 (3.6%) bloodmeals were of mixed avian-mammalian origin (Table 1). No statistically significant (p >0.05; χ2 test) differences in the class of hosts fed upon were noted among members of the Cx. pipiens complex that were classified as Cx. p. pipiens, Cx. p. quinquefaciatus or hybrids of the two taxa, based upon polymorphisms in the ACE.2 gene (Table 1). Culex restuans also fed primarily upon birds, taking 62% (95% CI, 58–67%) of its meals from avian hosts (Table 1). In contrast, both Cx. erraticus and An. quadrimaculatus took the majority of their meals from mammals. However, both species were found to occasionally feed upon avian hosts (Table 1). Culex territans was the only species that fed predominantly on cold-blooded hosts with 63% of its meals taken from amphibians (95% CI, 43–81%).
Table 1.
Number and Percent of Bloodmeals by Host Class for 2,034 Mosquitoes from Shelby County, Tennessee, 2002–2003
| Taxon | Amphibian | Avian | Mammal | Reptile | Mixed avian-mammal | Other mixed feeds | Total |
|---|---|---|---|---|---|---|---|
| An. punctipennis | 1 (10) | 8 (80) | 1 (10) | 10 | |||
| An. quadrimaculatus | 1 (<1) | 15 (6) | 220 (88) | 3 (1) | 10 (4) | 249 | |
| Cx. (Cux.) pipiens complex/restuans | 15 (8) | 134 (73) | 25 (14) | 5 (3) | 5 (3) | 184 | |
| Cx. (Cux.) pipiens complex | 38 (9) | 284 (69) | 64 (15) | 10 (2) | 15 (4) | 2 (<1)a | 413 |
| Cx. (Cux.) p. pipiens | 42 (81) | 7 (13) | 3 (6) | 52 | |||
| Cx. (Cux.) p. quinquefasciatus | 5 (4) | 89 (79) | 15 (13) | 2 (2) | 2 (2)b | 113 | |
| Cx. p.p.–p.q. hybrids | 74 (82) | 10 (11) | 2 (2) | 4 (4) | 90 | ||
| Cx. (Cux.) restuans | 53 (12) | 273 (62) | 101 (23) | 9 (2) | 1 (<1) | 437 | |
| Cx. (Mel.) erraticus | 5 (1) | 70 (15) | 341 (75) | 6 (1) | 31 (7) | 1 (<1)c | 454 |
| Cx. (Neo.) territans | 15 (63) | 3 (13) | 5 (21) | 1 (4)d | 24 | ||
| Other taxae | 2 (25) | 6 (75) | 8 |
One mixed amphibian-mammal and one mixed avian-reptile.
Two mixed amphibian-avian.
One mixed mammal-reptile.
One mixed amphibian-mammal.
Total for taxa in which less than 10 individual bloodmeals were identified. These included Aedes sticticus (n = 3), Ae. triseriatus (n = 1), Ae. vexans (n = 1), Ps. ferox (n = 1), and Ps. horrida (n = 2)
Of the 802 meals taken from mammals, 786 (98%) were identified to a single species or were of mixed mammalian origin (Table 2). Nine different mammalian species served as hosts for the various mosquito species. However, three to four mammalian hosts made up the vast majority of the mammalian blood-meals identified (Table 2). The domestic dog (Canis familarius) was the most frequently utilized host for An. quadrimaculatus and Cx. erraticus, making up 72% of the mammalian-derived bloodmeals identified in these two species. In contrast, the raccoon (Procyon lotor) was the preferred host for members of the Cx. pipiens complex and Cx. restuans, with 56% of the mammalian-derived meals having been taken from this host in these two taxa. Humans were rarely fed upon by any of the species collected, and only 2.5% (20/786) of all of the mammalian-derived meals were from humans. Mosquitoes identified as either Cx. restuans, or a member of the Cx. pipiens complex yielded 45% (9/20) of the human feeds; thus, approximately one out of a hundred (9/852) of these mosquitoes fed on humans. Additionally, one mixed cat-human feed was observed for Cx. restuans. The mammalophilic species, An. quadrimaculatus, yielded the largest proportion of human feeds (8/20) of any nominal species.
Table 2.
Number and Percent of Bloodmeals Identified to One Mammalian or Mixed Mammalian Hosts for 786 Mosquitoes from Shelby County, 2002–2003
| Taxon | Cat | Deer | Dog | Horse | Human | Opossum | Rabbit | Raccoon | Squirrel | Mixed | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| An. punctipennis | 7 (88) | 1 (13) | 8 | ||||||||
| An. quadrimaculatus | 29 (14) | 128 (60) | 23 (11) | 8 (4) | 2 (1) | 11 (5) | 3 (1) | 1 (<1) | 7 (3)a | 212 | |
| Cx. (Cux.) pipiens complex/restuans | 2 (9) | 5 (23) | 1 (5) | 14 (64) | 22 | ||||||
| Cx. (Cux.) pipiens complex | 9 (15) | 11 (18) | 4 (6) | 1 (2) | 4 (6) | 30 (48) | 2 (3) | 1 (2)b | 62 | ||
| Cx. (Cux.) p. pipiens | 2 (29) | 2 (29) | 2 (29) | 1 (14) | 7 | ||||||
| Cx. (Cux.) p. quinquefasciatus | 3 (20) | 5 (33) | 1 (7) | 6 (40) | 15 | ||||||
| Cx. p. pipiens-quinquefasciatus hybrids | 1 (10) | 1 (10) | 1 (10) | 3 (30) | 4 (40) | 10 | |||||
| Cx. (Cux.) restuans | 1 (1) | 1 (1) | 32 (33) | 3 (3) | 60 (61) | 1 (1)c | 98 | ||||
| Cx. (Mel.) erraticus | 8 (2) | 8 (2) | 275 (81) | 5 (1) | 3 (1) | 2 (1) | 10 (3) | 20 (6) | 1 (<1) | 9 (3)d | 341 |
| Cx. (Neo.) territans | 2 (40) | 2 (40) | 1 (20)e | 5 | |||||||
| Other taxae | 1 (17) | 2 (33) | 1 (17) | 1 (17) | 1 (17)f | 6 |
Seven mixed meals: one cat-deer, one deer-dog, one dog-horse, two dog-rabbit, two dog-raccoon.
One mixed dog-raccoon meal.
One mixed cat-human meal.
Nine mixed meals: one cat-dog, one deer-human, five dog-rabbit, one dog-raccoon, one rabbit-squirrel.
One mixed dog-raccoon meal.
One mixed dog-raccoon meal.
Taxa in which fewer than five mammalian bloodmeals were identified. These included Aedes sticticus (n = 2), Ae. vexans (n = 1), Ps. ferox (n = 1), and Ps. horrida (n = 2).
Of the 987 bloodmeals identified as from avian sources by the ELISA-based assay, 709 (72%) were identified to a single avian species using a PCR assay (Table 3). An additional 46 mosquitoes (5%) were found to have taken meals from multiple avian species and were not characterized further, due to technical limitations. Although 23 different avian species were identified in the bloodmeals, three species (the American Robin, the Common Grackle and the Northern Cardinal) made up over 81% of the avian-derived meals (Table 3). The American Robin was the most commonly fed upon avian host, making up 54% of the identified avian-derived meals.
Table 3.
Number and Percent of Bloodmeals Identified to One Avian or Mixed Avian Hosts for 755 Mosquitoes from Shelby County, 2002–2003
| Avian species |
An. quadrimaculatus | Cx. pipiens complex/ restuans |
Cx. pipiens complex |
Cx. p. pipiens |
Cx. p. quinquefasciatus |
Cx. p. pipiens-quinquefasciatus hybrids |
Cx. restuans |
Cx. erraticus |
Cx. territans |
Total |
|---|---|---|---|---|---|---|---|---|---|---|
| American Robin | 2 (17) | 42 (52) | 108 (49) | 24 (71) | 49 (62) | 31 (46) | 112 (53) | 11 (22) | 1 (50) | 380 |
| Common Grackle | 3 (25) | 15 (19) | 31 (14) | 4 (12) | 10 (13) | 11 (16) | 37 (17) | 18 (36) | 1 (50) | 130 |
| Northern Cardinal | 2 (17) | 7 (9) | 22 (10) | 1 (3) | 4 (5) | 7 (10) | 18 (8) | 4 (8) | 65 | |
| Blue Jay | 10 (4) | 4 (5) | 2 (3) | 1 (2) | 17 | |||||
| European Starling | 2 (2) | 2 (1) | 1 (3) | 1 (1) | 11 (5) | 17 | ||||
| House sparrow | 1 (8) | 2 (2) | 4 (2) | 1 (3) | 2 (3) | 2 (3) | 3 (1) | 15 | ||
| Grey Catbird | 3 (1) | 2 (3) | 4 (6) | 4 (2) | 13 | |||||
| Northern Mockingbird | 1 (1) | 5 (2) | 1 (1) | 5 (2) | 12 | |||||
| Green Heron | 1 (3) | 2 (3) | 1 (1) | 2 (1) | 2 (4) | 8 | ||||
| Brown-Headed Cow Bird | 1 (1) | 2 (1) | 2 (3) | 1 (<1) | 1 (2) | 7 | ||||
| Brown Thrasher | 1 (1) | 4 (2) | 1 (<1) | 1 (2) | 7 | |||||
| Mourning Dove | 1 (1) | 2 (1) | 1 (1) | 3 (1) | 7 | |||||
| Tufted Titmouse | 2 (2) | 2 (1) | 1 (1) | 1 (<1) | 6 | |||||
| Cedar Waxwing | 1 (1) | 4 (2) | 5 | |||||||
| House Finch | 1 (1) | 3 (1) | 1 (<1) | 5 | ||||||
| Chicken | 3 (1) | 3 | ||||||||
| Field Sparrow | 1 (1) | 2 (1) | 3 | |||||||
| American Kestrel | 1 (1) | 1 (1) | 2 | |||||||
| Red Winged Blackbird | 1 (<1) | 1 (2) | 2 | |||||||
| Yellow-billed Cuckoo | 2 (1) | 2 | ||||||||
| American Crow | 1 (<1) | 1 | ||||||||
| Barn Swallow | 1 (2) | 1 | ||||||||
| Common Yellowthroat | 1 (<1) | 1 | ||||||||
| Mixed aviana | 4 (33) | 2 (2) | 13 (6) | 2 (6) | 3 (4) | 5 (7) | 7 (3) | 10 (20) | 46 | |
| Total | 12 | 80 | 219 | 34 | 79 | 67 | 212 | 50 | 2 | 755 |
Mixed blood meals of two or more avian species that were not identified to individual species.
Avian host preference did not differ significantly among members of the Cx. pipiens complex or between members of the Cx. pipiens complex and Cx. restuans (Table 3; p > 0.05 χ2 test). In all of these taxa, American Robins were the most frequently fed upon hosts. In contrast, the proportion of avian hosts in Cx. erraticus differed significantly from the remaining Culex species (p < 0.01; χ2 test). In Cx. erraticus, the positions of the Common Grackle and Robin were reversed, with the grackle representing the most commonly fed upon avian host (Table 3).
There was no significant difference in the feeding pattern of Cx. pipiens complex and Cx. restuans mosquitoes collected at sites in urban residential neighborhoods with different income levels (p > 0.05; χ2 test). At urban sites of all income levels, the American Robin, Common Grackle and Northern Cardinal were the three most commonly utilized avian hosts. All human-derived bloodmeals were collected in sites classified as urban residential middle-income.
West Nile virus was detected in the head and thorax, indicating a disseminated infection, in 13 mosquitoes, representing six taxa (Table 4). Nine (69.2%) of the WNV-positive mosquitoes were members of the Cx. pipiens complex, and two (15.4%) were Cx. restuans. The only non-Culex (Culex) mosquitoes that were WNV-positive were two (15.4%) specimens of An. quadrimaculatus. Virus was detected at 10 different sites; eight sites yielded a single positive mosquito, while site 67 yielded two positive mosquitoes and site 8 yielded three positive mosquitoes. Twelve (92.3%) of WNV-positive mosquitoes were detected from sites located in urban residential neighborhoods with nine (69.2%) positive mosquitoes being collected from urban residential middle income sites (URMI). Virus-positive mosquitoes of the Cx. pipiens complex were collected from May 6 through September 12, while both WNV-positive Cx. restuans specimens were collected in April, supporting a role for this species in early spring amplification.
Table 4.
Mosquitoes with Head and Thorax Positive for West Nile Virus from Shelby County, Tennessee, 2002–2003
| Positive mosquito | Date collected | Number collecteda | Site number | Site classification | Bloodmeal host |
|---|---|---|---|---|---|
| Anopheles quadrimaculatus | 8/5/2002 | 8 | 404 | RMI | Not determined |
| An. quadrimaculatus | 7/8/2003 | 10 | 13 | URMI | Dog |
| Culex pipiens complex/restuans | 5/6/2002 | 38 | 513 | URMI | Frog |
| Cx. pipiens complex/restuans | 5/29/2002 | 5 | 517 | URUI | Raccoon |
| Cx. pipiens complex/restuans | 8/6/2002 | 6 | 67 | URMI | Raccoon |
| Cx. pipiens complex | 6/18/2002 | 51 | 8 | URMI | Robin |
| Cx. pipiens complex | 8/6/2002 | 5 | 8 | URMI | Mockingbird |
| Cx. pipiens complex | 8/6/2002 | 1 | 20 | URMI | Robin |
| Cx. pipiens complex | 8/6/2002 | 5 | 295 | URMI | Frog |
| Cx. p. quinquefasciatus | 9/12/2003 | 3 | 68 | URLI | Robin |
| Cx. p. pipiens-quinquefasciatus hybrid | 8/14/2003 | 2 | 67 | URMI | Cardinal |
| Cx. restuans | 4/22/2002 | 8 | 24 | URUI | Raccoon |
| Cx. restuans | 4/29/2002 | 29 | 8 | URMI | Avian |
Number of blood-fed mosquitoes collected of the positive species at the site and on the date when the positive mosquito was collected.
RMI, Rural Middle Income; URLI, Urban Residential Low Income; URMI, Urban Residential Middle Income; URUI, Urban Residential Upper Income.
To address the question of relative reservoir capacity of the different avian species fed upon in Shelby County, a step-flow model was first used to evaluate the reservoir potential of the most commonly fed upon birds. We also used the model to estimate the reservoir capacity of the American Crow, a species that has experienced widespread mortality from WNV (Komar et al. 2003, Caffrey et al. 2005). A schematic of this model is shown in Figure 3. Results of these studies are summarized in Table 5. Several avian species exhibited a low predicted reservoir capacity, including the American Crow, the House Sparrow, the Northern Mockingbird and the European Starling. In all of these species, the infection rate in the mosquito population declined asymptocally from its initial setpoint of 0.33 infected mosquitoes per 1,000 mosquitoes, indicating little or no WNV transmission was occurring (Table 5). In keeping with this finding, little evidence for infection in the avian population was noted in these four species, with final infection rates of 0.08–0.9% (Table 5). In contrast, three species (the American Robin, the Blue Jay, and the Common Grackle), exhibited relatively high predicted reservoir capacities. In these species, the mosquito infection rate increased from its initial value of 0.33 per 1,000 to between 9.26 per 1,000 and 70.9 per 1,000, indicating that these species were capable of supporting transmission of WNV. In keeping with this finding, these three species exhibited high predicted avian infection rates, ranging from 72.3% to 100% of the susceptible population (Table 5).
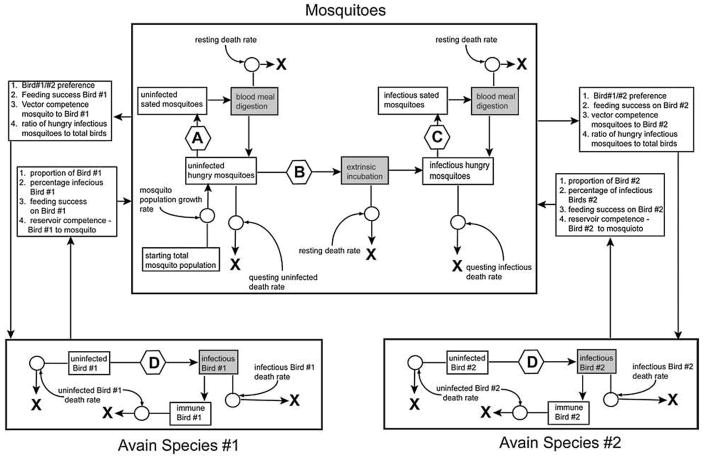
FIG. 3.
Schematic of model used to compare reservoir capacities of different avian species. Large boxes highlight the avian and mosquito modules that make up the model. Variables that affect the interaction of the three modules are listed in small boxes interrupting the arrows that connect the modules. The arrows connecting the modules indicate the direction of the interactions among the modules. Within each module, the white boxes indicate stocks of the avian reservoir and vector mosquito species in different states (e.g., uninfected or infectious). Gray boxes indicate conveyors, in which animals moving from one stock to another must rest for a defined period of time before moving to the next stock. The bold Xs indicate death of animals in a given stock, resulting in removal from subsequent iterations of the model. Arrows indicate the direction that animals may move from stock to stock. Arrows interrupted by a circle indicate that movement from one stock to another stock is governed by a single variable. This variable is indicated by the curved arrow pointed to the circle in question. Arrows interrupted by hexagons symbolize that movement between the stocks indicated is controlled by multiple variables, most of which affect the interactions between the modules. Variables affecting the transitions in each hexagon are as follows. Hexagon A: Number of uninfected birds of species 1 (susceptible and immune), number of uninfected birds of species 2 (susceptible and immune), and relative feeding upon species 1 and species 2, calculated from the relative feeding indices of the two species. Hexagon B: Number of infectious birds of species 1, number of infectious birds of species 2 and relative feeding upon species 1 and species 2. Hexagon C: Total number of birds of species 1, total number of birds of species 2, and relative feeding upon species 1 and species 2. Hexagon D: Number of infectious mosquitoes, number of susceptible birds, and relative feeding preference on species 1 and species 2.
Table 5.
Output Values Indicative of Transmission Intensity Derived from Iterations of the Step-flow Model Parameterized with Values Derived from Individual Avian Species
| Avian species | Peak IR in vector | Day of peak IR in vector | Final cumulative %of infected + immune in avian population | Day cumulative maximum % of infected + immune reached in avian population | No. birds surviving | No. infected birds succumbing |
|---|---|---|---|---|---|---|
| American Crow | 0.33 | 0 | 0.08 | 27 | 409 | 1 |
| Blue Jay | 11.77 | 183 | 72.26 | 200 | 329 | 85 |
| House Sparrow | 0.33 | 0 | 0.77 | 68 | 409 | 1 |
| Common Grackle | 9.26 | 54 | 100 | 77 | 380 | 37 |
| Northern Cardinal | 1.04 | 186 | 26.38 | 198 | 408 | 1 |
| Northern Mockingbird | 0.33 | 0 | 0.90 | 54 | 409 | 0 |
| American Robin | 70.90 | 23 | 100 | 17 | 409 | 2 |
| European Starling | 0.33 | 0 | 0.81 | 54 | 409 | 0 |
IR, infection rate per 1000 mosquitoes.
The studies involving the individual bird species summarized in Table 5 provided an indication of the reservoir capacity of each species. However, these studies did not indicate the relative role that each species might play in the transmission of WNV in the context of the avifauna as a whole. To address this question, the model was run with the two modules parameterized differently, with one module parameterized to represent the individual species in question and the second module parameterized with weighted averages for all other species. The results from these studies are summarized in Table 6. Here, the analysis suggested that just two species (the American Robin and the Common Grackle), when considered in the context of the avifauna as a whole, were capable of supporting WNV transmission. Interestingly, these species behaved somewhat differently (Table 6). The robin population induced a rapid enzootic of virus transmission, with a peak infection rate of 1.52 per 1,000 mosquitoes, occurring on day 35. The model predicted a rapid spread of virus infection that appeared to be confined primarily to the robin population, as 100% of the robins became infected by day 73, but just 18% of the avifauna overall were infected. In contrast, the Common Grackle population induced a more widespread, late season pattern of viral activity. Here, the maximal level of infection in the vector population reached 3.22 per 1000 mosquitoes on day 182. A more widespread pattern of infection was also noted, as the model predicted that 71% of the Common Grackles and 42% of all birds became infected with the virus.
Table 6.
Output Values Indicative of Transmission Intensity Derived from Iterations of the Step-flow Model Parameterized with Values Derived from Placing Individual Avian Species in One Module and a Weighted Average for all Other Birds in the Second Module
| Avian species | Percentage of all birds | Peak IR in vector | Day of peak IR in vector | Cumulative % of infected + immune in the avian population | cumulative % of Day maximum infected + immune reached in avian population | Cumulative %of infected + immune birds in study species | Day cumulative maximum % of infected + immune birds in study species reached |
|---|---|---|---|---|---|---|---|
| American Crow | 1.5% | 0.33 | 0 | 2.83 | 200 | 0 | na |
| Blue Jay | 3.4% | 0.33 | 0 | 2.89 | 198 | 0 | na |
| House Sparrow | 5.0% | 0.33 | 0 | 2.68 | 192 | 0 | na |
| Common Grackle | 9.0% | 3.22 | 182 | 42.38 | 200 | 68.6 | 200 |
| Northern Cardinal | 5.5% | 0.33 | 0 | 2.67 | 183 | 4.5 | 58 |
| Northern Mockingbird | 3.7% | 0.33 | 0 | 2.77 | 191 | 0 | na |
| American Robin | 4.4% | 1.52 | 35 | 18.12 | 192 | 100 | 73 |
| European Starling | 5.9% | 0.33 | 0 | 2.71 | 199 | 0 | na |
IR, infection rate per 1000 mosquitoes; na, not applicable.
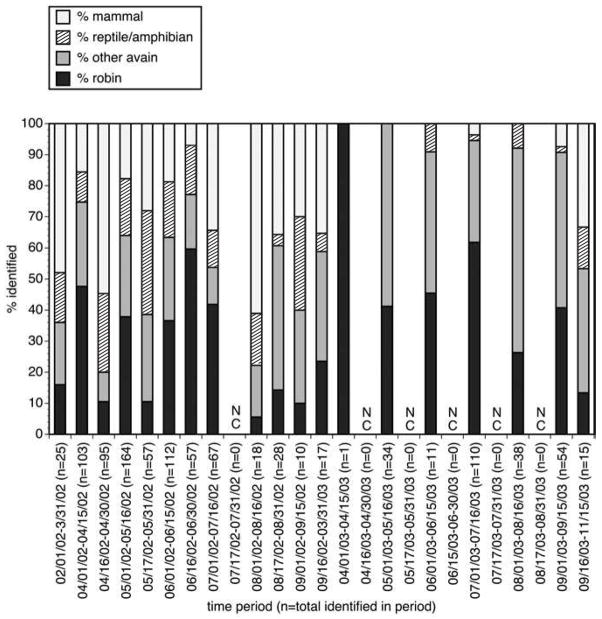
The semi-monthly distribution of blood-meals by host groups for Cx. pipiens complex and Cx. restuans mosquitoes are summarized in Figure 4. These data suggested that a large level of variation occurred in the proportion of robin-derived bloodmeals from semi-monthly period to semi-monthly period. The data also indicated a slight trend away from feeding on robins to other hosts, a pattern that has been noted in previous studies (Kilpatrick et al. 2006b). To assess the significance of these patterns, non-parametric models were used to prepare “smoothed” curves for the number of robin and non-robin-derived blood meals. For both robins and all other hosts, the observed data exhibited some variation about the trend line predicted by the models (Fig. 5A,B). However, variation was much greater for the robin-derived blood meals than it was for the non-robin-derived blood meals, with significant deviations from the smoothed prediction of the number of robin-derived meals seen during each semi-monthly period from March 1, 2002 through June 15, 2002 (Fig. 5A).
FIG. 4.
Temporal distribution of bloodmeals taken by Cx. pipiens complex and Cx. restuans mosquitoes for host groups (robin, other avian, mammal, and amphibian/reptilian combined) at semi-monthly intervals, Shelby County, Tennessee, 2002–2003. Time intervals during which no collections were made are indicated by “NC.”
FIG. 5.
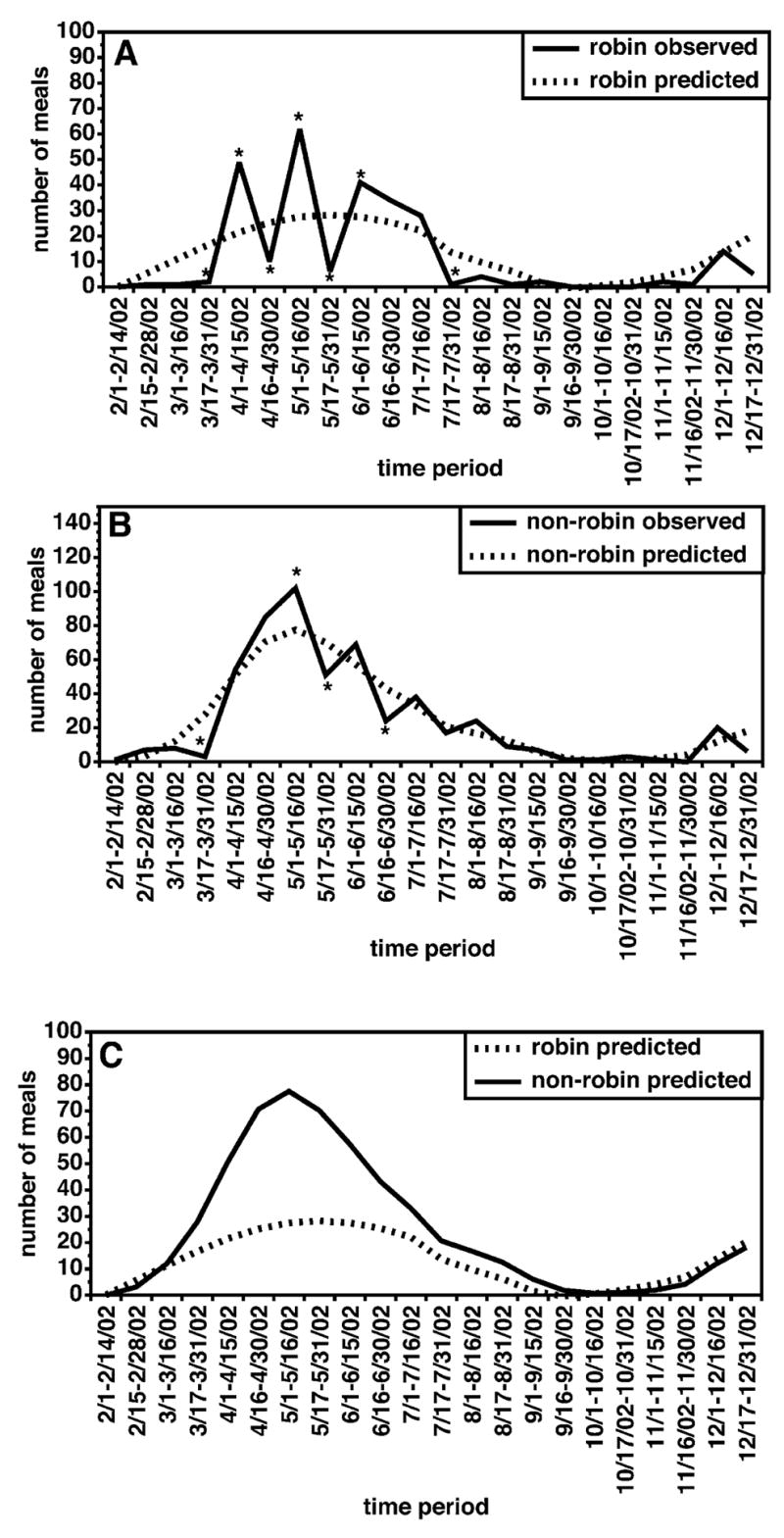
Non-parametric analysis of the numbers of robin and non-robin avian bloodmeals by semi-monthly period. (A) Predicted and observed numbers of robin blood meals. (B) Predicted and observed numbers of avian blood-meals derived from species other than robin. (C) Predicted numbers of blood-meals from robins and other avian species. In A and B, asterisks (*) indicate time periods where the observed numbers of meals differed significantly from those predicted by the models (p < 0.05; χ2 test).
When the modeled curves for the number of robin-derived and non-robin-derived curves were compared, there was some suggestion that feeding on robins peaked slightly earlier than did feeding upon other hosts (Fig. 5C). However, when the robin and non-robin derived curves were compared for concordance using Kendall’s Tau, the two curves were found to be highly concordant (τ = 0.81, p < 0.0001). Furthermore, when the effect of the normal seasonal variation in the number of blood-fed mosquitoes was removed as described in the Methods and the resulting residual time histories were again compared by Kendall’s Tau, the value for concordance remained very high (τ = 0.49, p < 0.0003). Taken together, these analyses suggested that perceived shift from feeding on robins early in the season to other hosts later in the season was not statistically significant. However, as discussed above, significant differences in the number of robin-derived meals were noted in contiguous semi-monthly periods. This pattern was inconsistent with the hypothesis that the mosquitoes were feeding upon free-ranging adult robins, because if this were the case, one would predict that the frequency of feeding upon robins would be stable throughout the spring and early summer months. However, a variable pattern of feeding with peaks lasting one to two semi-monthly periods might be expected if the mosquitoes were targeting nesting birds. Robins nest throughout the spring and summer in the Shelby County area, and tend to raise two to three broods of nestlings per season (Gough et al. 1998). Nestlings fledge in 14–16 days (Gough et al. 1998). Thus, if mosquitoes were targeting nesting birds, one might predict that the frequency of feeding upon robins would increase during the period when nests were active.
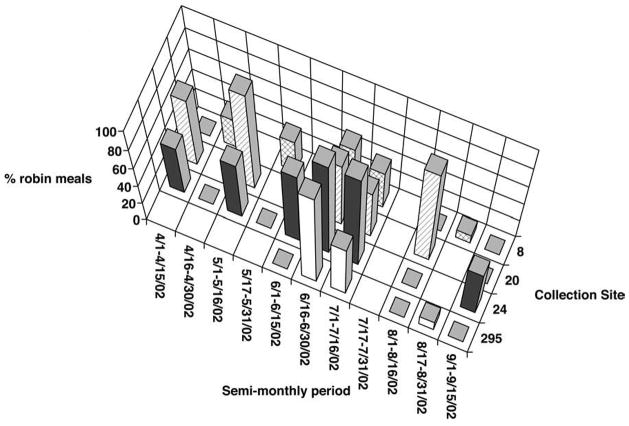
Because breeding in robins is not synchronized and the birds are capable of raising more than one brood per year, a pattern of preferential feeding upon nesting birds would be difficult to discern when mosquitoes were collected from multiple sites. However, such a pattern might become more evident if the data were analyzed by site, as this would presumably limit the number of active nests capable of being sampled by the resident mosquitoes. The data were therefore re-analyzed for collection sites where more than 10 robin-derived blood-meals were detected, where more than 25 blood-fed mosquitoes were identified overall, and where blood-fed mosquitoes were collected in at least six of the 11 semi-monthly periods during the 2002 transmission season. When broken down by site and time, the proportion of robin-derived bloodmeals was seen to vary widely over time at most sites, with peaks in the proportion of robin derived blood-meals lasting one to two semi-monthly periods (Fig. 6).
FIG. 6.
Percent of American Robin derived bloodmeals taken during semi-monthly periods during the 2002 transmission season, May 1, 2002 to September 15, 2002, at four collection sites. Only sites where greater than 10 robin-derived bloodmeals and at least 25 total bloodmeals were identified, and where blood-fed mosquitoes were found during at least six of the 11 possible semi-monthly periods are included.
DISCUSSION
The host-feeding data presented above support previous studies (Apperson et al. 2004, Molaei et al. 2006) that show that members of the Cx. pipiens complex and Cx. restuans are primarily ornithophilic. However, it is clear from the data presented here that neither species is exclusively ornithophilic. Furthermore, it appears that the degree of preference for avian hosts varies from location to location. For example, the percentage of avian derived meals in Cx. p. pipiens in New Jersey was reported to be just 35%, while in New York it was found to be 85% (Apperson et al. 2004). Similarly, Cx. restuans fed upon avian hosts 62% of the time in this study, while it was reported to be exclusively ornithophilic in Connecticut (Molaei et al. 2006) and highly mammalophilic in Delaware (Gingrich and Williams 2005). This level of variation may in part reflect the limited number of specimens analyzed in some of the previous studies (Apperson et al. 2004, Gingrich and Williams 2005). However it is also possible that these data reflect the fact that host feeding choice may vary widely within a single species from location to location, which may in turn affect the role that each species plays both as an enzootic and as a bridge vector in different regions. The reason for such a difference in feeding behavior remains to be resolved. However, it appears that the innate host preference of many mosquito species is modulated by the spatial and temporal abundances of potential hosts (Savage et al. 1993a, Niebylski et al. 1994, Richards et al. 2006), and by regional differences in host reproductive cycles and other behaviors.
Shelby County is located in the southernmost portion of the hybrid zone for members of the Cx. pipiens complex (Barr 1957, Savage et al. 2006). The results of our study suggest that there are no significant differences in host utilization among members of the Cx. pipiens complex within Shelby County. One possible explanation for this finding rests upon the fact that the mosquitoes involved in this study were classified using a single molecular marker, which would not have detected evidence of hybridization at other unlinked loci (Aspen and Savage 2003). More detailed multi-locus classification methods (Fonseca et al. 2004, Smith and Fonseca 2004) may provide an answer to this question. Alternatively, studies of the host-feeding preferences of genetically pure Cx. p. pipiens and Cx. p. quniquefaciatus from areas outside the hybrid zone might be able to address this issue. However, it is likely that such studies would be complicated by regional differences in host feeding such as those described above.
Similar to the results from previous studies (Apperson et al. 2002, 2004, Hassan et al. 2003, Ngo and Kramer 2003, Kilpatrick et al. 2006a, Molaei et al. 2006), mosquitoes that fed upon avian hosts targeted a limited number of species most of the time. In keeping with other recently published studies from the Northeastern and mid-Atlantic states, (Kilpatrick et al. 2006a, Molaei et al. 2006) the American Robin was by far the most preferred avian host. The only exception to this finding was the case of Cx. erraticus, which fed upon the Common Grackle more frequently than the American Robin. However, the distribution of Common Grackle meals in Cx. erraticus was highly focal, as the majority of grackle-fed Cx. erraticus were collected from a single site on a single date. This focality may have resulted in a feeding pattern that was not reflective of the overall host preference in this species.
Several laboratory studies have been conducted that have estimated many of the parameters involved with reservoir competency in different bird species, while the host-feeding data presented above permitted us to estimate the level of host-vector contact. All of these parameters were employed in a step-flow model to gain insight into the relative reservoir capacity of the avian species that were important bloodmeal sources of the Culex mosquitoes in our study. The results suggested that two species (the Common Grackle and the American Robin) may play major roles as reservoir hosts for WNV in Shelby County. However, these species differed somewhat in the pattern of viral transmission that they supported. The robins were predicted to be very susceptible to WNV infection, with the entire robin population becoming infected less than half way through the season. In contrast, the grackles produced a late-season pattern of transmission, which also seemed to involve a wider variety of other avian species. This difference is likely to be related to the relative intensity of vector-host contact in these two species. Robins are a highly preferred host for the Culex species, and as such would be expected to become rapidly exposed to the virus. Furthermore, the high level of feeding upon robins would tend to exclude other species from being fed upon, thereby tending to confine the infection to the robin population, resulting in an intensive, but limited enzootic of infection within the robin population. In contrast, grackles, although exhibiting a relatively high feeding index, were less likely to be chosen as hosts when compared to robins. This may have resulted in a pattern in which the level of infection built more slowly and became more widespread among the avian population as a whole, when compared to the intensive enzootic seen in the robin simulations.
A recent study has suggested that a temporal shift in feeding by Cx. pipiens complex mosquitoes from robins to humans occurs late in the summer (Kilpatrick et al. 2006b). The authors of that study suggested that this shift might play an important role in permitting WNV to move from an avian cycle of transmission to infect humans. However, the data presented above suggest that such a shift to feeding upon humans may not be occurring in Shelby County, as the percentage of robin-derived meals in Cx. pipiens complex and Cx. restuans mosquitoes did not decline significantly in the late summer period. Furthermore, human-derived bloodmeals in Culex pipiens complex mosquitoes made up just over 1% of the total meals identified in these taxa, and the human-derived meals were spread throughout the semi-monthly collection periods (data not shown). There are several possible explanations for the difference in the temporal pattern of robin feeding seen here and the host shift reported to occur in the northeastern United States. First, robins are year-round residents in Shelby County (National Audubon Society 2002) and therefore may differ in behavior from the robin populations in the Northeast. It is also possible that the level of human contact with the Cx. pipiens complex is lower in Shelby County than in the Northeastern and mid-Atlantic states. Shelby County is a southern urban area with very warm and humid summers, and many residents stay indoors in air-conditioned buildings during the months of July through early September. This behavior would limit the exposure of humans to the mosquito population, and focus Culex mosquito feeding on other more available mammals, such as dogs, horses and raccoons.
Despite the relative lack of targeting of humans, there were 40 human WNV cases reported in 2002 (USGS 2003) and 10 human WNV cases reported in 2003 (USGS 2004) in Shelby County. These cases occurred during a period marked by extensive public health messages and an intense mosquito control program targeting Culex mosquitoes. These data indicate that seasonal or targeted feeding by Cx. pipiens complex and Cx. restuans mosquitoes on humans is not necessary to support significant WNV transmission to humans in this area. The very high rates of WNV infection in Cx. pipiens complex and Cx. restuans mosquitoes in Memphis observed in this study and elsewhere (Savage et al. 2006), combined with extremely high mosquito population levels and an observed human feeding rate of roughly 1% suggest that these species account at least in part for the observed WNV transmission to humans. However, other mosquito species may also play an important role in acting as bridge vectors of WNV from birds to humans. Our WNV infection and abundance data suggest a possible bridge vector role for An. quadrimaculatus in Memphis, while Cx. salinarius has been implicated as a potential bridge vector in Connecticut (Andreadis et al. 2004) and has been found to be frequently infected with WNV in studies conducted in the Southeastern United States (Godsey et al. 2001, 2005, Cupp et al. 2007).
It has been hypothesized that the reason for the shift in feeding from robins to other hosts is related to the dispersal of adult robins late in the summer (Kilpatrick et al. 2006b). As mentioned above, we could detect no such late-summer shift away from robins in Shelby County. However, we were able to demonstrate a significant difference in the proportion of robin-derived bloodmeals in contiguous semi-monthly periods in the spring and early summer periods. Such shifts in feeding would not be predicted to occur if the mosquitoes were targeting free-ranging adult birds, which are present throughout the spring and early summer. However, this pattern is consistent with the hypothesis that the mosquitoes were targeting nesting birds. As mentioned above, robins in Tennessee generally produce more than one brood of young per year, and the young fledge in 14–16 days (Gough et al. 1998). If unfledged birds were particularly targeted by mosquitoes, it would be predicted that one would see a pulse of feeding upon robins after hatching and before the young fledged. It is also possible that mosquitoes are targeting brooding adults, which while on the nest may be unable to exhibit the full range of defensive and avoidance behaviors available to them while they are free-ranging. In either case, the prediction that nesting birds are preferentially targeted is completely consistent with the feeding pattern described above. Furthermore, a similar pattern of tightly temporally regulated feeding upon a preferred avian host has been noted in studies of host choice in Cx. erraticus at a site endemic for Eastern Equine Encephalitis virus in Alabama (Unnasch et al. 2005). Several previous studies have suggested that nestlings may play an important role in the amplification of Saint Louis Encephalitis (Scott et al. 1988, Mahmood et al. 2004), and recent modeling studies have suggested that nestlings may be instrumental in the amplification of Eastern Equine Encephalitis virus as well (Unnasch et al. 2006). Taken together, these studies suggest that preferential feeding upon and infection of young of the year birds may be an important amplifier in the transmission of WNV and other arboviruses.
Acknowledgments
We thank the entire Centers for Disease Control laboratory team, which included Stephen Aspen, K. Burkhalter, D. Charnetzky, L. Colton, M. Godsey, J. Gold, E. Gordon, H.M. Savage, and G. Sutherland. We thank the entire Memphis-Shelby County Vector Control Field Team, which included M. Anderson, W. Arnold, P. Birkholz, W. Crutcher, J. Clifford, Y. Gatewood, E. Hessin, M. Jones, L. McMillen, J.K. Medford, D.W. Mitchell, and K. Thornton. We thank L. Lacey, Director, Memphis-Shelby County Vector Control Program, and N. LaChapelle, Memphis-Shelby County Health Department, for their support and cooperation in conducting this study. We would like to acknowledge the excellent technical assistance of Shawn Kennedy, of North Carolina State University. We also thank Ana Oliviera and Amy Schuh of the University of Alabama at Birmingham for help with the statistical analysis, and Naomi Lang-Unnasch for critically reading the manuscript. The work was supported by funding from the Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention to C.S.A. and T.R.U., and by a grant from the Centers for Disease Control and Prevention to T.R.U. (project R01 CI000226).
References
- Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the northeastern United States. J Infect Dis. 2006;194:1577–1579. doi: 10.1086/508754. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR. Mosquito surveillance for West Nile virus in Connecticut, 2000: isolation from Culex pipiens, Cx. restuans, Cx. salinarius, and Culiseta melanura. Emerg Infect Dis. 2001;7:670–674. doi: 10.3201/eid0704.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Vossbrinck CR, Main AJ. Epidemiology of West Nile virus in Connecticut: a five-year analysis of mosquito data 1999–2003. Vector-Borne Zoonotic Dis. 2004;4:360–378. doi: 10.1089/vbz.2004.4.360. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, et al. Host feeding patterns of probable vector mosquitoes of West Nile virus in the eastern United States. Vector-Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspen S, Crabtree M, Savage H. Polymerase chain reaction assay identifies Culex nigripalpus: part of an assay for molecular identification of the common Culex (Culex) mosquitoes of the eastern United States. J Am Mosq Cont Assoc. 2003;19:115–120. [PubMed] [Google Scholar]
- Aspen S, Savage H. Polymerase chain reaction assay identifies North American members of the Culex pipiens complex based on nucleotide sequence differences in the acetylcholinesterase gene Ace.2. J Am Mosq Cont Assoc. 2003;19:323–328. [PubMed] [Google Scholar]
- Barr AR. The distribution of Culex p. pipiens and C. p. quinquefasciatus in North America. Am J Trop Med Hyg. 1957;6:153–165. doi: 10.4269/ajtmh.1957.6.153. [DOI] [PubMed] [Google Scholar]
- Caffrey CS, Smith CR, Weston TJ. West Nile virus devastates the American Crow population. Condor. 2005;107:128–132. [Google Scholar]
- CDC (Centers for Disease Control and Prevention) West Nile virus: entomology. [Accessed July 15, 2007];2006a Available at: www.cdc.gov/ncidod/dvbid/westnile/mosquitoSpecies.htm.
- CDC (Centers for Disease Control and Prevention) West Nile virus: statistics, surveillence and control—2005 case count. [Accessed July 15, 2007];2006b Available at: www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount05_detailed.htm.
- Cornel AJ, McAbee RD, Rasgon J, Stanich MA, et al. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- Crabtree MB, Savage HM, Miller BR. Development of a species-diagnostic polymerase chain reaction assay for the identification of Culex vectors of St. Louis encephalitis virus based on interspecies sequence variation in ribosomal DNA spacers. Am J Trop Med Hyg. 1995;53:105–109. [PubMed] [Google Scholar]
- Cupp EW, Hassan HK, Yue X, Oldland WK, et al. West Nile virus infection in mosquitoes in the mid-south USA, 2002–2005. J Med Entomol. 2007;44:117–125. doi: 10.1603/0022-2585(2007)44[117:wnviim]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitoes of North America, north of Mexico. Mosq Syst. 1981;1S:1–313. [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, et al. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Fyodorova MV, Savage HM, Lopatina JV, Bulgakova TA, et al. Evaluation of potential West Nile virus vectors in Volgograd region, Russia, 2003 (Diptera: Culicidae): Species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. J Med Entomol. 2006;43:552–563. doi: 10.1603/0022-2585(2006)43[552:eopwnv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Gingrich JB, Williams GM. Host-feeding patterns of suspected West Nile virus mosquito vectors in Delaware, 2001–2002. J Am Mosq Cont Assoc. 2005;21:194–200. doi: 10.2987/8756-971X(2005)21[194:HPOSWN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol. 2003;40:743–746. doi: 10.1603/0022-2585-40.6.743. [DOI] [PubMed] [Google Scholar]
- Godsey MS, Blackmore MS, Panella NA, Burkhalter K, et al. West Nile virus epizootiology in the southeastern United States, 2001. Vector-Borne Zoonotic Dis. 2005;5:82–89. doi: 10.1089/vbz.2005.5.82. [DOI] [PubMed] [Google Scholar]
- Godsey MS, Nasci R, Savage HM, Aspen S, et al. West Nile virus-infected mosquitoes, Louisiana, 2002. Emerg Infect Dis. 2005;11:1399–1404. doi: 10.3201/eid1109.040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough GA, Sauer JR, Iliff M. Patuxent Bird Identification Infocenter. [Accessed July 15, 2007]; Available at: www.mbr-pwrc.usgs.gov/id/framlst/infocenter.html.
- Hassan HK, Cupp EW, Hill GE, Katholi CR, et al. Avian host preference by vectors of Eastern Equine Encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- Jakob WL, Daggers SA, Francy DB, Mullenix J, et al. The Culex pipiens complex in Memphis, Tennessee. Mosq Syst. 1979;11:179–186. [Google Scholar]
- Johnson RT. Viral Infections of the Nervous System. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, et al. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, et al. Host heterogeneity dominates West Nile virus transmission. Proc R Soc Lond B Biol Sci. 2006a;273:2327–2333. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, et al. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006b;4:e82. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a Taqman reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hassan H, Hill G, Cupp EW, et al. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599–604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin KC, Diallo AA, Herbert MW, Phaltankar PG, et al. Five-year surveillance of West Nile and Eastern Equine Encephalitis viruses in southeastern Virginia. J Environ Health. 2006;68:33–40. [PubMed] [Google Scholar]
- Mahmood F, Chiles RE, Fang Y, Barker CM, et al. Role of nestling mourning doves and house finches as amplifying hosts of St. Louis Encephalitis virus. J Med Entomol. 2004;41:965–972. doi: 10.1603/0022-2585-41.5.965. [DOI] [PubMed] [Google Scholar]
- Miller BR, Crabtree MB, Savage HM. Phylogeny of fourteen Culex mosquito species, including the Culex pipiens complex, inferred from the internal transcribed spacers of ribosomal DNA. Ins Mol Biol. 1996;5:93–107. doi: 10.1111/j.1365-2583.1996.tb00044.x. [DOI] [PubMed] [Google Scholar]
- Miller BR, Nasci RS, Godsey MS, Savage HM, et al. First field evidence for natural vertical transmission of West Nile virus in Culex univittatus complex mosquitoes from Rift Valley Province, Kenya. Am J Trop Med Hyg. 2000;62:240–246. doi: 10.4269/ajtmh.2000.62.240. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Francy DB, Monath TP. Arthropod vectors. In: Monath TP, Reeves WC, editors. St Louis Encephalitis. Washington, DC: American Public Health Association; 1980. pp. 313–379. [Google Scholar]
- Mokgweetsinyana SS. MS thesis. Colorado State University; 1987. Survival rates of the inland flood-water mosquito Aedes vexans (Meigen) in Northern Colorado. [Google Scholar]
- Molaei G, Andreadis TA, Armstrong PM, Anderson JF, et al. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg Infect Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasci RS, Edman JD. Culiseta melanura (Diptera: Culicidae): Population structure and nectar feeding in a freshwater swamp and surrounding areas in southeastern Massachusetts, USA. J Med Entomol. 1984;21:567–572. doi: 10.1093/jmedent/21.5.567. [DOI] [PubMed] [Google Scholar]
- Nasci RS, Savage HM, White DJ, Miller JR, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Audubon Society. The Christmas bird count: historical results. [Accessed July 15, 2007];2002 Available at: www.audubon.org/bird/cbc.
- Ngo KA, Kramer LD. Identification of mosquito blood-meals using polymerase chain reaction (PCR) with order-specific primers. J Med Entomol. 2003;40:215–222. doi: 10.1603/0022-2585-40.2.215. [DOI] [PubMed] [Google Scholar]
- Niebylski ML, Savage HM, Nasci RS, Craig GB., Jr Blood hosts of Aedes albopictus in the United States. J Am Mosq Cont Assoc. 1994;10:447–450. [PubMed] [Google Scholar]
- Richards SL, Ponnusammy L, Unnasch TR, Hassan HK, et al. Host-feeding patterns of Aedes albopictus (Skuse) (Diptera: Culicidae) in relation to the availability of human and domestic animals in suburban landscapes of central North Carolina with notes on blood meal hosts of sympatric mosquito species. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JR, Hines JE, Fallon J. The North American breeding bird survey, results and analysis 1966–2004. Laurel, MD: USGS Patuxent Wildlife Research Center; 2005. [Google Scholar]
- Savage HM, Niebylski ML, Smith GC, Mitchell CJ, et al. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J Med Entomol. 1993a;30:27–34. doi: 10.1093/jmedent/30.1.27. [DOI] [PubMed] [Google Scholar]
- Savage HM, Smith GC, Moore CG, Mitchell CJ, et al. Entomologic investigations of an epidemic of St. Louis Encephalitis in Pine Bluff, Arkansas, 1991. Am J Trop Med Hyg. 1993b;49:38–45. doi: 10.4269/ajtmh.1993.49.38. [DOI] [PubMed] [Google Scholar]
- Savage HM, Ceianu C, Nicolescu G, Karabatsos N, et al. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Am J Trop Med Hyg. 1999;61:600–611. doi: 10.4269/ajtmh.1999.61.600. [DOI] [PubMed] [Google Scholar]
- Savage HM, Anderson M, Gordon E, McMillen L, et al. Oviposition activity patterns and West Nile virus infection rates for members of the Culex pipiens complex at different habitat-types within the hybrid zone, Shelby County, TN, 2002 (Diptera: Culicidae) J Med Entomol. 2006;43:1227–1238. doi: 10.1603/0022-2585(2006)43[1227:oapawn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Scott TW, Edman JD, Lorenz LH. Effects of disease on vertebrates’ ability behaviorally to repel host-seeking mosquitoes. In: Scott TW, Grumstrup-Scott J, editors. Proceedings of a Symposium: The Role of Vector-Host Interactions in Disease Transmission: Misc Pub. Vol. 68. Entomology Society of America; Lanham, MD: 1988. pp. 9–17. [Google Scholar]
- Shaman J, Day JF, Stieglitz M. The spatial-temporal distribution of drought, wetting, and human cases of St. Louis Encephalitis in south central Florida. Am J Trop Med Hyg. 2004;71:251–261. [PubMed] [Google Scholar]
- Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- Tabachnick WJ, Powell JR. Genetic analysis of Culex pipiens populations in the central valley of California. Ann Entomol Soc Am. 1983;76:715–720. [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, Oguinn ML, et al. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- USGS (U.S. Geological Survey) West Nile virus—Tennessee cumulative human map. [Accessed July 15, 2007];2003 Available at: http://westnilemaps.usgs.gov/2002/tennessee/tn_human_apr_22.html.
- USGS (U.S. Geological Survey) West Nile virus—Tennessee cumulative human map. [Accessed July 15, 2007];2004 Available at: http://westnilemaps.usgs.gov/2003/tn_human.html.
- Unnasch RS, Cupp EW, Unnasch TR. Host selection and its role in transmission of arboviral encephalitides. In: Collinge SK, Ray C, editors. Disease Ecology: Community Structure and Pathogen Dynamics. Oxford, UK: Oxford University Press; 2005. pp. 73–89. [Google Scholar]
- Unnasch RS, Sprenger T, Katholi CR, Cupp EW, et al. A dynamic transmission model of Eastern Equine Encephalomyelitis virus. Ecol Model. 2006;192:425–440. doi: 10.1016/j.ecolmodel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J, Chichakly K, Peterson S, Richmond B. Stella 7.03. Hanover, NH: High Performance Systems, Inc; 2002. [Google Scholar]