Abstract
The CD8 co-receptor can modulate CD8+ T cell function through its contributions to T cell receptor (TCR) binding and signaling. Here we show that IFN-γ and IL-4 exert opposing effects on the expression of CD8α mRNA and surface CD8 protein during CD8+ T cell activation. IL-4 caused down-regulation of surface CD8 on ovalbumin (OVA)257–264-specific TCR-transgenic OT-I CD8+ T cells activated with OVA257–264-coated antigen presenting cells or polyclonal stimuli, and on wild type CD8+ T cells activated with polyclonal stimuli. This effect was enhanced in each case when the cells lacked a functional IFN-γ or IFN-γR gene. When WT or IFN-γ-deficient OT-I CD8+ T cells were analyzed 9 days after co-injection with control or IL-4-expressing OVA+ tumor cells into RAG-2−/−γc−/− mice, CD8 levels were highest on WT donor cells from mice that received the control tumor and lowest on IFN-γ-deficient donor cells from mice that received the IL-4-expressing tumor. The latter CD8low cells displayed markedly impaired binding of OVA257–264/MHC tetramers and peptide/MHC-dependent degranulation. The data reveal an unexpected role for IFN-γ in tuning the CD8 co-receptor during primary CD8+ T cell activation both in vitro and in vivo.
Keywords: CD8low, co-receptor tuning, T cell activation, cytotoxic T lymphocytes, cytokines
The CD8αβ co-receptor amplifies the CD8+ T cell response to peptide/MHC Class I complexes on antigen-presenting cells (APC) by at least four mechanisms. First, CD8 binding to nonpolymorphic regions of the MHC molecule and β2-microglobulin is thought to stabilize the interaction between the TCR and peptide/MHC complexes (1); second, CD8 binding to the MHC augments TCR signaling via activation of p56lck and LAT (2, 3); third, CD8 is thought to induce the co-localization of receptor complex molecules onto lipid rafts (4); and fourth, CD8 induces a conformational change in CD3 that is necessary for signal transduction (5). The effect can be dramatic: for example, transfection of CD8-negative T cells bearing low-affinity TCR with CD8α or CD8α/CD8β has been reported to amplify peptide sensitivity by factors of 107 to 109 (6). Modulation of surface CD8 expression on naive and effector cells can therefore alter the threshold peptide/MHC levels required to trigger proliferation, cytokine production and target cell lysis (7, 8), effectively “tuning” the T cell response.
Surface CD8 levels can be controlled by distinct antigen- and cytokine-dependent mechanisms. The former has been observed as a transient response to TCR activation (9) and in peripheral tolerance where chronic antigen exposure can be associated with stable CD8 down-regulation at the T cell surface (7, 10, 11). Cytokine-dependent CD8 down-regulation was first reported by Erard et al. (12) who demonstrated that exposure of naïve CD8+ T cells to IL-4 during activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin in vitro led to loss of surface CD8 and CD8α RNA, induction of a type 2 cytokine profile and the absence of cytolytic activity. We extended these studies to show that the presence of IL-4 in the first few days of primary activation with antibodies to the TCR, CD8 and CD11a (anti-receptor Ab) or alloantigens promoted the development of poorly cytolytic, type 2 effector cells that retained their TCR but displayed reduced or undetectable surface CD8 expression; essentially all conventional CD8+ T cells could eventually give rise to committed CD8low cells that maintained this phenotype in the absence of continued IL-4 exposure (13, 14). By contrast, Park et al. have recently reported that IL-4 and other γc cytokines up-regulated surface CD8 levels when CD8+ T cells were cultured in vitro without a TCR stimulus (7).
IL-4 and IFN-γ reciprocally regulate a number of lymphocyte functions, including the expression of type 1 and type 2 cytokines by CD4+ and CD8+ effector cells (15, 16). The present study was therefore undertaken to determine whether this was also true for CD8 expression in newly activated CD8+ T cells. We show here that IFN-γ not only counteracts the down-regulatory effect of IL-4 on CD8 mRNA and protein levels but also contributes to the maintenance of CD8 levels in the absence of exogenous IL-4. The reciprocal effects of IL-4 and IFN-γ were observed both in vitro and in the response to an IL-4-expressing tumor in vivo and point to an unexpected role for these key immunoregulatory cytokines in tuning TCR signaling thresholds during the primary CD8+ T cell response.
Results
CD8 Expression Is Reduced in CD8+ T cells Activated in the Absence of IFN-γ and the Presence of IL-4.
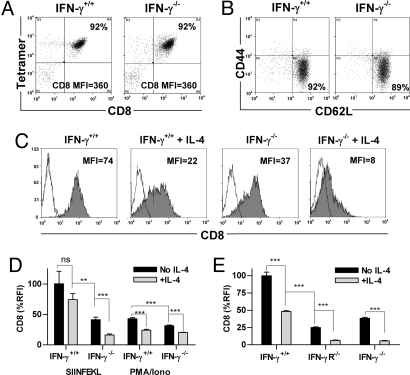
Approximately 90% of peripheral CD8+ T cells from OT-I mice carry the transgenic TCR specific for the OVA257–264 epitope SIINFEKL and bind SIINFEKL/H-2Kb tetramers in a CD8-dependent manner (17). Fig. 1A shows that CD8+ cells freshly isolated from pooled lymph nodes (LN) and spleen of OT-I and OT-I x IFN-γ−/− mice bound anti-CD8 Ab at similar levels when co-stained with SIINFEKL tetramers. Most cells of both genotypes displayed a naïve (CD44low CD62Lhigh) phenotype (Fig. 1B). No consistent differences were detected in surface CD8 levels (median fluorescence intensity, MFI) or in the frequencies or absolute numbers of CD4− CD8+, CD4+ CD8+ or CD4− CD8− TCRβ+ cells in thymus, LN or spleen between C57BL/6 and C57BL/6 x IFN-γ−/− mice (data not shown). The data indicate that disruption of the IFN-γ pathway does not alter CD8 expression in naïve CD8+ T cells, consistent with earlier reports (18).
Fig. 1.
CD8 expression by activated CD8+ T cells is reduced in the presence of IL-4 and/or the absence of IFN-γ. CD8+ cells from OT-I (IFN-γ+/+) and OT-I x IFN-γ−/− (IFN-γ−/−) mice were stained with (A) anti-CD8α Ab and SIINFEKL tetramer or (B) Ab to CD44 and CD62L. The CD8 MFI and the percentage of double-positive cells (A), and the percentage of cells with a naïve (CD44low CD62Lhigh) phenotype (B), are shown within the frames. (C) The two CD8+ populations were cultured with anti-receptor Ab and IL-2 with or without IL-4 for 6 days then stained with anti-CD8α Ab (filled histograms) or isotype control Ab (open histograms). (D) The same CD8+ populations were cultured with SIINFEKL-coated APC or PMA and ionomycin, and IL-2 with or without IL-4. CD8 expression is shown as relative fluorescence intensity, obtained by normalizing the MFI to the highest sample in the experiment (100%). (E) CD8 expression by CD8+ cells from C57BL/6 WT (IFN-γ+/+), IFN-γR−/− or IFN-γ−/− mice was measured after culture with anti-receptor Ab, IL-2 and with or without IL-4 for 10 days. Groups were compared by unpaired t test (see Materials and Methods).
We have previously shown that activation of naïve CD8+ T cells in the presence of IL-4 led to progressive loss of surface CD8 expression over 7–10 days (13, 14). As IL-4 and IFN-γ exert reciprocal effects on some T cell functions, the effect of IL-4 was compared in purified intact and IFN-γ-deficient OT-I CD8+ T cells activated with immobilized Ab to CD3, CD8 and CD11a (anti-receptor Ab) and IL-2 for 6 days. These conditions activate 85–95% of WT CD8+ T cells to proliferate and give rise to effector cells that secrete IFN-γ and display perforin-dependent cytolytic activity (13, 14). CD8 levels were highest in cells from OT-I mice activated in the absence of IL-4, reduced in IFN-γ−/− cells, or in IFN-γ+/+ cells in the presence of IL-4, and lowest in IFN-γ−/− cells activated in the presence of IL-4 (Fig. 1C). Similar observations were made when intact and IFN-γ−/− OT-I CD8+ T cells were activated with SIINFEKL-coated APC or PMA and ionomycin (Fig. 1D) and when intact, IFN-γR−/− and IFN-γ−/− C57BL/6 CD8+ T cells were activated with anti-receptor Ab and IL-2 (Fig. 1E). These data indicate that the effects of IL-4 addition and IFN-γ deficiency on CD8 levels do not depend on TCR engagement, are not limited to the anti-receptor Ab activation system or to OT-I cells, but do depend on IFN-γR-mediated signaling.
CD8 Expression Is Determined by the Balance Between IFN-γ and IL-4 During Primary Activation.
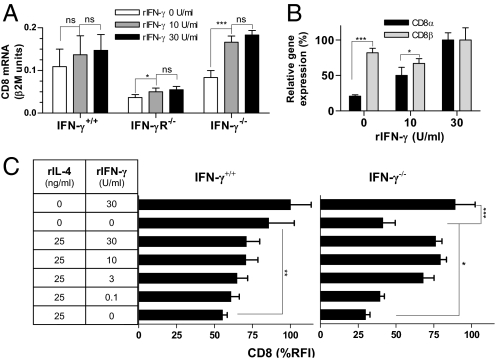
We have previously found that IL-4 decreased expression of the α-chain of the CD8αβ heterodimer at the mRNA level (13). To determine whether exogenous IFN-γ could overcome the effects of IFN-γ deficiency on CD8 expression, CD8α mRNA levels were measured in CD8+ T cells from WT, IFN-γR−/− and IFN-γ−/− mice following activation in the presence and absence of IFN-γ (Fig. 2A). Exogenous IFN-γ did not increase CD8α mRNA expression by WT cells, presumably because these cells secreted IFN-γ in response to activation. A small but significant effect was observed on IFN-γR−/− cells. In IFN-γ−/− cells, however, IFN-γ exposure restored expression to WT levels. By comparison, IFN-γ deficiency and replacement exerted only minor effects on CD8β mRNA levels (Fig. 2B). Fig. 2C shows that mixing of exogenous IFN-γ and IL-4 in varying concentrations modulated surface CD8 levels, particularly in the absence of endogenous IFN-γ. The data demonstrate the striking sensitivity of CD8+ T cells to cytokine-dependent regulation of surface CD8 levels during primary activation and show that these effects are mediated mainly by controlling expression of the α-chain of the CD8 heterodimer at the mRNA level. As CD8α is required for surface expression of CD8β (19), regulation of the α-chain determines levels of surface expression of both components of the heterodimer. The down-regulation of CD8 expression was maintained for at least 21 days in vitro [supporting information (SI) Fig. S1], indicating that the effect was not a transient response to activation.
Fig. 2.
IL-4 and IFN-γ exert opposing dose-dependent effects on CD8 expression. (A and B) CD8+ cells from C57BL/6 WT (IFN-γ+/+), IFN-γR−/−, and IFN-γ−/− mice were cultured with anti-receptor Ab, IL-2 and the indicated concentrations of IFN-γ for 5 days. CD8α mRNA levels are expressed as β2M units (mean and SD, n = 3) in A, and as relative CD8α and CD8β mRNA expression (mean and SD, n = 4) in B. (C) In an independent experiment, CD8+ cells from C57BL/6 WT (IFN-γ+/+) and IFN-γ−/− mice were cultured with anti-receptor Ab, IL-2 and the indicated concentrations of IL-4 and IFN-γ for 5 days. Surface CD8 expression was analyzed by flow cytometry and expressed as relative fluorescence intensity (see legend to Fig. 1) (mean and SD, n = 5).
CD8 Expression Is Reciprocally Regulated by IL-4 and IFN-γ in Vivo.
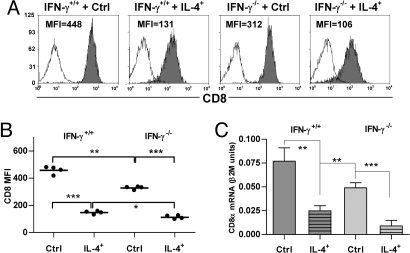
To test the ability of IL-4 and IFN-γ to regulate CD8 expression in vivo, Vα2+ CD8+ cells from OT-I or OT-I x IFN-γ−/− mice were adoptively transferred into RAG-2−/−γc−/− hosts and activated with OVA-expressing tumor cells transfected with an IL-4-expressing or a control vector. Donor (Vα2+) cells exhibited a similar hierarchy of CD8 expression levels to that observed in vitro, with maximal down-regulation in cells from IFN-γ−/− mice that received the IL-4-secreting tumor (Fig. 3A). Median CD8 levels differed significantly between all groups (Fig. 3B). The down-regulation of CD8 expression observed in this protocol apparently depended on T cell activation as CFSE-labeled CD8+ T cells from OT-I and OT-I x IFN-γ−/− mice proliferated in vivo but maintained their surface CD8 levels when recovered 5 days after s.c. injection into RAG-2−/−γc−/− hosts in the absence of the tumor (Fig. S2). Fig. 3C shows that the hierarchy of CD8 expression observed ex vivo was maintained at the level of CD8α mRNA when the cells from the four groups were restimulated in vitro with anti-receptor Ab. The data demonstrate that the IL-4-dependent down-regulation of CD8 expression we and others previously observed in vitro (12–14) can also occur in vivo but is normally dampened by endogenous IFN-γ.
Fig. 3.
IL-4 and IFN-γ modulate CD8 expression in vivo. Vα2+ CD8+ cells from OT-I (IFN-γ+/+) or OT-I x IFN-γ−/− (IFN-γ−/−) mice were co-injected with EG7-IL-4+ (IL-4+) or EG7-IL-4− (Ctrl) tumor cells into RAG-2−/−γc−/− mice. After 9 days, splenic Vα2+ cells were isolated and analyzed for surface CD8 expression by flow cytometry. (A) Representative data from individual mice are shown. (B) Data from all mice in a representative experiment are shown. Each point represents one mouse; horizontal lines indicate the mean of each group. (C) CD8α mRNA expression was measured after restimulation in vitro with anti-receptor Ab for 6 days.
Cytokine-Mediated Changes in CD8 Levels Affect CD8+ T cell Function.
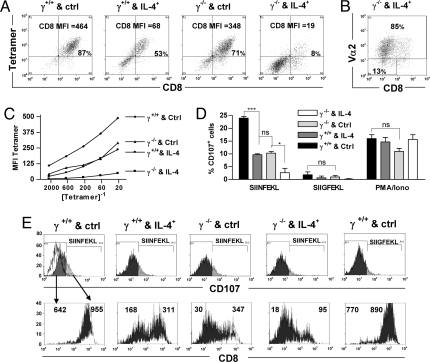
To assess the functional consequences of CD8 down-regulation, tetramer binding and peptide/MHC-dependent degranulation were measured in IFN-γ+/+ and IFN-γ−/− cell populations activated in vivo with IL-4-secreting or control tumor cells and restimulated in vitro. Binding of SIINFEKL/H-2Kb tetramers was strongly correlated with surface CD8 levels when co-stained (Fig. 4A), consistent with earlier evidence that CD8 is required for binding of this and some other peptide/class I multimers (17). Differences in CD8 levels between the populations were more pronounced than those observed ex vivo but followed the same hierarchy. Down-regulation of the TCR was modest: Vα2 was detected on 85% of cells from IFN-γ−/− mice that received the IL-4-secreting tumor (Fig. 4B) and at least 96% of cells in the other three populations (Fig. S3). These findings suggest that, despite continued TCR expression, most antigen-specific cells that had down-regulated CD8 would not be detected by tetramer binding. When the tetramer was titrated on its own to avoid the enhancement of tetramer binding that can occur with CD8 co-staining (17), binding was ≈10-fold more efficient to IFN-γ+/+/control cells than to IFN-γ+/+/IL-4 or IFN-γ−/−/control cells, and >100-fold more efficient than to IFN-γ−/−/IL-4 cells (Fig. 4C).
Fig. 4.
Modulation of CD8 levels by IL-4 and IFN-γ affects T cell function. The restimulated cell populations in Fig. 3C were co-stained with anti-CD8α Ab and (A) SIINFEKL tetramers at a dilution of 16.7 × 10−3 or (B) anti-Vα2 Ab. Values within the frames indicate the percentage of cells in that region. (C) The tetramer MFI is shown for each population following staining with the indicated tetramer dilutions in the absence of anti-CD8α Ab. (D) The restimulated cell populations were rested for 6 days then cultured with SIINFEKL or SIIGFEKL peptide-coated APC or with PMA, ionomycin and IL-2, in the presence of anti-CD107 Ab and monensin. (E) Upper frames show CD107 profiles of the indicated populations from (D); the open histogram in the left frame shows CD107 Ab binding to cells cultured with APC without peptide. Lower frames show corresponding CD8 profiles and CD8 MFI following gating on CD107− (dark) and CD107+ (light) cells.
The ability of the same four cell populations to degranulate following peptide/MHC recognition was determined based on surface exposure of the granule membrane proteins CD107a and CD107b, which correlates with granule-mediated cytolytic function (20). After resting, cells were stimulated with congenic CD45.1+ APC coated with SIINFEKL (whose complex with H-2Kb has high affinity for the OT-I TCR) or SIIGFEKL (low affinity) peptides (21) and stained with anti-CD107 Ab. The percentage of CD107+ CD45.2+ cells in each population followed the same hierarchy described above for CD8 expression (Fig. 4D). The observation that CD107 exposure in response to SIIGFEKL was lower than to SIINFEKL but followed the same pattern, whereas the four populations displayed similar CD107 levels in response to PMA and ionomycin, suggests that degranulation was impaired because of reduced signaling at the level of the TCR, rather than a distal effect on signaling or an intrinsic cell defect. Gating of the populations based on CD107 levels showed that median CD8 levels were lower on the CD107− fraction than the CD107+ fraction (Fig. 4E), indicating that cells with higher surface CD8 responded preferentially to high and low affinity peptide/MHC complexes.
Discussion
We have identified a mechanism of reciprocal CD8 modulation in which IFN-γ up-regulates and IL-4 down-regulates CD8 expression during effector CD8+ T cell development both in vitro and in vivo. We report that IFN-γ not only counteracts the down-regulatory effect of IL-4 on CD8α mRNA and CD8αβ surface protein levels but also contributes to their maintenance in the absence of exogenous IL-4.
Several recent reports have shown that modulation of CD8 expression, or tuning, can alter the signaling threshold through the TCR, with even moderate changes in CD8 levels markedly affecting TCR-dependent effector function (7, 9, 10). Here we show that IFN-γ- and IL-4-dependent modulation of CD8 levels correlated with changes in peptide/MHC tetramer binding and in degranulation in response to specific TCR stimulation. These data are consistent with the work of others showing that reduced CD8 expression correlated with a reduction in tetramer binding (9, 10) and our earlier observations that CD8+ T cells that down-regulated CD8 during activation in the presence of IL-4 exhibited lower perforin-dependent cytolytic activity in 51Cr-release assays than their CD8high counterparts (13). In preliminary experiments, we have also found that IFN-γ−/− donor T cells recovered from host mice injected with IL-4-producing tumor cells (in the protocol used in Fig. 3) not only expressed lower levels of CD8 but also showed reduced ability to control EG7 tumor cells when co-injected into RAG-1−/− recipients, compared with IFN-γ−/− cells recovered from mice injected with control tumor cells (unpublished data).
A number of earlier studies have shown that IL-4 induces both the de novo production of IL-4 and down-regulation of CD8 expression in activated CD8+ T cells (12–14, 22). Important clinical correlates have been reported in HIV infection and chronic B cell lymphocytic leukemia, in which IL-4-expressing CD8+ T cells with reduced CD8 expression have been described and may be associated with disease progression (23–25). The data in HIV have recently been supported experimentally by the observation that therapy of simian HIV-infected macaques with antisense IL-4 DNA was associated with an increase in CD8 expression by CD8 T cells (26).
In our earlier studies, we found that IL-4 induced the down-regulation of CD8 expression in essentially every conventional CD8+ T cell during activation in vitro. However, the extent of CD8 down-regulation was variable in vitro (14) and exposure of WT CD8+ T cells to IL-4-expressing tumor cells in vivo affected their IL-4 and granzyme mRNA levels but did not lead to a significant reduction in CD8 expression (27). The findings reported here offer an explanation for these results, namely that endogenous or host-derived IFN-γ might counteract the effects of IL-4 on CD8 levels, inhibiting the emergence of CD8low effector cells. An alternative explanation might be that CD8 is down-modulated in the absence of IFN-γ because lower MHC Class I expression leads to sub-threshold T cell priming. We think this is unlikely as we recovered similar numbers of IFN-γ+/+ and IFN-γ−/− cells following adoptive transfer with tumor cells in the experiment shown in Fig. 3 and all displayed a CD44high phenotype (unpublished observations), suggesting comparable activation efficiency. Our data in vitro also argue against an association of CD8 down-regulation with sub-threshold priming as cells progressively lost CD8 over time and many cell divisions in the presence of optimal concentrations of immobilized anti-receptor Ab (14).
The finding that IFN-γ contributes to the maintenance of CD8 expression following TCR-dependent activation in the absence of IL-4 was unexpected. One implication is that “normal” CD8 levels on effector cells are determined, at least in part, by ambient IFN-γ concentrations and are therefore subject to tuning in response to changes in the levels both of this cytokine and of its receptor. It is notable that IFN-γR1 levels on CD8+ T cells varied over the course of one viral infection (28) and that IFN-γ exposure altered the expansion, immunodominance and death of antigen-specific CD8+ T cells during the course of infection in other models (29, 30), although a direct link between altered CD8 levels, TCR avidity and immunodominance remains to be established.
In our hands, cytokine-induced alterations in CD8 expression are maintained over several weeks in cultures of proliferating CD8+ T cells, suggesting heritable epigenetic changes in the expression machinery. The molecular mechanisms by which IFN-γ and IL-4 modulate CD8 levels are not yet known. Several transcription factors have been implicated in the control of CD8α gene expression, including STAT5 and STAT6 for which a putative binding site has been identified within the CD8 enhancer element E8I (7), MAZR which binds enhancer E8II and negatively regulates CD8 expression (31), cKrox which binds E8I and negatively regulates both CD8 and IFN-γ expression while up-regulating GATA-3 (32), and GATA-3 for which binding sites have been identified in both mouse and human CD8 enhancer regions (33, 34). Two observations suggest that GATA-3 may have a role as a negative regulator: first, in the thymus, GATA-3 promotes CD4 lineage development while inhibiting CD8 lineage development (35); second, GATA-3 expression is induced in CD8+ T cells exposed to IL-4 (36) or transduced with cKrox which, as noted above, down-regulates CD8 expression (32).
Selective regulation of CD8α was recently reported by Park et al. (7) who found that the γc cytokines IL-2, IL-4, IL-7, and IL-15 increased CD8α (not β) mRNA and protein levels in LN CD8+ T cells in the absence of TCR signaling; IL-4 and IL-7 acted by increasing the CD8α transcription rate, at least in part via the E8I enhancer. Their data are consistent with our observation that IFN-γ and IL-4 act preferentially on CD8α mRNA levels and suggest that the effects we observed might be mediated via one or more of the CD8 enhancer elements, most of which are located within the CD8 locus and lie downstream of the CD8β and upstream of the CD8α genes (37). On the other hand, their observation that IL-4 enhanced CD8 expression on T cells in the absence of TCR signaling contrasts with our finding that IL-4 inhibits CD8 expression following TCR-dependent activation. It will now be interesting to investigate the immunological significance of this shift in CD8 responsiveness to IL-4 following primary activation.
In summary, IFN-γ and IL-4 reciprocally modulate CD8 levels on CD8+ T cells activated with various stimuli in vitro and in response to tumor cells in vivo, acting at the level of CD8α mRNA to alter surface CD8αβ protein levels. These changes in CD8 expression are correlated with functional alterations in tetramer binding and antigen-specific degranulation, reflecting their potential to exert profound effects on the sensitivity of CD8+ T cells to peptide/MHC levels and the resultant response to pathogens.
Materials and Methods
Mice.
Specific pathogen-free C57BL/6 (CD45.2) and B6.SJL/J-Ptprca (CD45.1) mice (Animal Resources Centre) were used at 6–9 weeks of age. RAG-2−/−γc−/− [provided by Dr. Alberto Pinzon-Charry, Queensland Institute of Medical Research (QIMR)], OT-I (243.2) (Dr. William Health, The Walter and Eliza Hall Institute of Medical Research) and OT-I x IFN-γ−/−mice (Dr. Guna Karupiah, John Curtin School of Medical Research) were bred at QIMR. IFN-γ−/− and IFN-γR−/− mice (Dr. Geoff Hill, QIMR) were bred at The Herston Medical Research Centre. All animal studies were approved by the QIMR Animal Ethics Committee.
Naive CD8+ T Cell Preparation.
CD8+ cells were enriched by positive selection from pooled cell suspensions from spleen and brachial, axillary, inguinal and lumbar LN of untreated mice using MACS CD8 Microbeads (Miltenyi Biotec); >85% of purified CD8+ cells displayed a naive (CD44low CD62Lhigh) phenotype.
Activation of CD8+ T Cells in Vitro.
(1) Anti-receptor Ab. Naive CD8+ T cells were cultured at 125 × 103/well in 6-well plates coated with purified Ab to CD3ε (145–2C11; 10 μg/ml), CD8α (53.6; 10 μg/ml) and CD11a (I21/7.7; 5 μg/ml) (38) in 8 ml growth medium (modified DMEM, 50 μM 2-mercaptoethanol and 216 mg/l l-glutamine), 10% heat-inactivated FCS and 20 IU/ml human rIL-2 (National Institutes of Health AIDS Research & Reference Reagent Program) (39) with or without 25 ng/ml mouse rIL-4 and the indicated concentrations of mouse rIFN-γ (both from ProSpec-Tany TechnoGene, Rehovot, Israel) (2). PMA/ionomycin. Naïve CD8+ cells were cultured at 24 × 103/well with 10 μg/ml PMA and 250 ng/ml ionomycin (Sigma) in 12-well plates with 4 ml growth medium supplemented as above. (3) Peptide activation. Naïve CD8+ cells were cultured at 24 × 103/well with 5 × 106 γ-irradiated congenic CD45.1+ splenocytes previously incubated with 10 μg/ml SIINFEKL peptide (Mimotopes) for 1 h and γ-irradiated with 3000 cGy, in 12-well plates with 4 ml growth medium supplemented as above.
Activation of CD8+ T Cells in Vivo with IL-4-Expressing or Control Tumor Cells.
Cells of the OVA-expressing EL4 thymoma line EG7 were electroporated with control or IL-4-expressing EBO-pLPP vectors (27). Transfected cells were selected by limiting dilution and maintained with 0.3 mg/ml hygromycin B (Roche Diagnostics). The IL-4 content in the supernatant after culture at 2 × 105 cells/ml for 48 h was 24 U/ml by ELISA for EG7-IL-4+ cells (line D9) and <0.03 U/ml for control vector-containing EG7-IL-4− cells (line G11). Naive CD8+ T cells were activated in vivo by co-injection of 1.6 × 106 cells s.c. with 2.5 × 107 EG7-IL-4+ or EG7-IL-4− cells at the base of the tail in 200 μl saline.
Fluorescence-Activated Cell Sorting and Analysis.
Cells were incubated on ice with combinations of fluorochrome-conjugated reagents: Ab to CD8α (53–6.7), CD8β (CD8β), CD62L (MEL-14) and CD45.2 (104) and isotype controls (BioLegend); Ab to CD44 (IM7), CD107a (1D4B) and CD107b (H4B4) and isotype controls (BD Biosciences); Ab to Vα2 (B20.1) and isotype control (eBioscience); SIINFEKL/H-2Kb tetramers (provided by Dr. Stephen Turner, University of Melbourne). Cells were washed and resuspended with 1 μg/ml propidium iodide (PI; Calbiochem). CD8+ T cells were purified by using a MoFlo cytometer (DakoCytomation) with exclusion of dead cells based on forward scatter and PI uptake. For analysis without sorting, a FACSCalibur was used with CellQuest version 3.1f software (BD Biosciences). Post acquisition data analyses were performed by using Summit Software V4.3 (Dako).
In Vitro Restimulation of in Vivo-Activated T Cells.
Donor Vα2+ CD8+ T cells were enriched from spleen (9 days after injection) by fluorescence-activated cell sorting and cultured at 2 × 104/well with anti-receptor Ab under the same conditions as naive T cells except that plates were coated with a reduced concentration of anti-CD3 Ab (1 μg/ml).
RNA Preparation and Real-Time PCR Analysis.
RNA was extracted by Nonidet P-40 hypotonic lysis of 3 × 103 cells and cDNA was prepared as described (13). Triplicate cDNA samples were prepared from each culture and each cDNA sample was assayed in duplicate. cDNA was quantified by real-time PCR using the following primers and probes for β2-microglobulin (β2M) (5′: 5′-TCTTTCTGGTGCTTGTCTCAC-3′; 3′: 5′-GTTCGGCTTCCCATTCTC-3′; probe: 5′-JOE-CGGCCTGTATGCTATCCAGAAAACC-BHQ-1–3′), CD8α (5′: 5′d-GCCAGTCCTTCAGAAAGTGA-3′; 3′: 5′d-CCGACAATCTTCTGGTCTCT-3′; probe: 5′-FAMd-CTACCAAGCCAGTGCTGCGAACTC-BHQ-1–3′) and CD8β (kit ID number Mm00438116_m1, Applied Biosystems). Samples were amplified in a Corbett Rotor-Gene 3000 (Corbett Research) for 40 cycles at 95°C for 2 min, 95°C for 5 s, and 60°C for 30 s.
All results except those comparing CD8α and CD8β are reported in β2M units (target gene copy number/β2M copy number) and represent the mean of triplicate cDNA samples. Relative CD8α and CD8β mRNA expression was determined by comparison to a standard curve obtained by titrating CD8+ T cell cDNA containing known CD8α and unknown CD8β cDNA copy numbers; the derived values of the test samples were then divided by the β2M copy number and expressed as a percentage of the highest mean value of the test samples.
CD107 Mobilization Assay.
After restimulation CD8+ T cells were rested in 20 IU/ml human rIL-2 for 6 days. Live cells were then isolated on Ficoll-Paque and cultured at 5 × 104 cells/well with either 105 APC (CD45.1+ splenocytes incubated with or without 10 μg/ml SIINFEKL or SIIGFEKL peptide at 37°C for 1 h) or 40 ng/ml PMA and 1.5 μg/ml ionomycin in 96-well V-bottom plates (Sarstedt) in 100 μl growth medium and 5% FCS and either CD107a and CD107b Ab or isotype control Ab (10 μl/ml), with 0.67 μl/ml monensin (all from BD Biosciences) for 5 h at 37°C (20). Cells were then washed and analyzed for CD45.2 and CD8 expression by using a FACSCalibur with exclusion of dead cells by PI. The percentage of CD107a/b+ cells was determined by subtraction of the unstimulated control from the test sample using Summit Software V4.3 (Dako).
Statistical Analyses.
Data were evaluated by unpaired t test (Prism 4.02 software package, GraphPad Software1). P values are indicated in the figures by the symbols: >0.05, ns; 0.01–0.05, *; 0.001–0.01, **; <0.001, ***.
Supplementary Material
Acknowledgments.
We thank Drs. William Heath, Geoff Hill, Guna Karupiah, Alberto Pinzon-Charry, and Stephen Turner for their generous gifts of mice and reagents; Professor Stuart Kellie for valuable advice; Grace Chojnowski and Paula Hall for assistance with cell sorting; Suzanne Cassidy and staff from the QIMR animal facility for animal husbandry; and Stuart Olver, Kathy Buttigieg, and Kelly Kenney for technical contributions. The human rIL-2 (from Dr. Maurice Gately, Hoffmann - La Roche Inc.) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health. This work was supported by grants from Cooperative Research Centre for Vaccine Technology (established and supported under the Australian Government's Cooperative Research Centres Program), National Health and Medical Research Council of Australia, and The Cancer Council Queensland. S.H.A. was supported by the Basil Shaw Fellowship of the Australian Rotary Health Research Fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809549105/DCSupplemental.
References
- 1.Garcia KC, et al. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 2.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 3.Bosselut R, et al. Association of the adaptor molecule LAT with CD4 and CD8 coreceptors identifies a new coreceptor function in T cell receptor signal transduction. J Exp Med. 1999;190:1517–1526. doi: 10.1084/jem.190.10.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcaro A, et al. CD8β endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56lck complexes. J Exp Med. 2001;194:1485–1495. doi: 10.1084/jem.194.10.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil D, Schrum AG, Daniels MA, Palmer E. A role for CD8 in the developmental tuning of antigen recognition and CD3 conformational change. J Immunol. 2008;180:3900–3909. doi: 10.4049/jimmunol.180.6.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 8.Maile R, et al. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J Immunol. 2001;167:3708–3714. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204:2667–2677. doi: 10.1084/jem.20062376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maile R, et al. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 11.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 12.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8−CD4− cells that make TH2 cytokines and help B cells. Science. 1993;260:1802–1805. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 13.Kienzle N, et al. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J Immunol. 2002;168:1672–1681. doi: 10.4049/jimmunol.168.4.1672. [DOI] [PubMed] [Google Scholar]
- 14.Kienzle N, et al. Progressive differentiation and commitment of CD8+ T cells to a poorly cytolytic CD8low phenotype in the presence of IL-4. J Immunol. 2005;174:2021–2029. doi: 10.4049/jimmunol.174.4.2021. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 16.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 17.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalton DK, et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 19.Gorman SD, Sun YH, Zamoyska R, Parnes JR. Molecular linkage of the Ly-3 and Ly-2 genes. Requirement of Ly-2 for Ly-3 surface expression. J Immunol. 1988;140:3646–3653. [PubMed] [Google Scholar]
- 20.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 21.Jameson SC, Carbone FR, Bevan MJ. Clone-specific T cell receptor antagonists of major histocompatibility complex class I-restricted cytotoxic T cells. J Exp Med. 1993;177:1541–1550. doi: 10.1084/jem.177.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemp RA, Backstrom BT, Ronchese F. The phenotype of type 1 and type 2 CD8+ T cells activated in vitro is affected by culture conditions and correlates with effector activity. Immunology. 2005;115:315–324. doi: 10.1111/j.1365-2567.2005.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggi E, et al. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180:489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dianzani U, et al. Expansion of T cells expressing low CD4 or CD8 levels in B-cell chronic lymphocytic leukemia: correlation with disease status and neoplastic phenotype. Blood. 1994;83:2198–2205. [PubMed] [Google Scholar]
- 25.Schmitz JE, et al. Expression of the CD8αβ-heterodimer on CD8+ T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus− and human immunodeficiency virus+ individuals. Blood. 1998;92:198–206. [PubMed] [Google Scholar]
- 26.Dhillon NK, et al. Therapy of “SHIV” infected macaques with liposomes delivering antisense interleukin-4 DNA. AIDS. 2006;20:1125–1130. doi: 10.1097/01.aids.0000226952.49353.36. [DOI] [PubMed] [Google Scholar]
- 27.Olver S, et al. Tumor-derived interleukin-4 reduces tumor clearance and deviates the cytokine and granzyme profile of tumor-induced CD8+ T cells. Cancer Res. 2006;66:571–580. doi: 10.1158/0008-5472.CAN-05-1362. [DOI] [PubMed] [Google Scholar]
- 28.Whitmire JK, Tan JT, Whitton JL. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science. 2000;290:1354–1358. doi: 10.1126/science.290.5495.1354. [DOI] [PubMed] [Google Scholar]
- 30.Turner SJ, et al. Disregulated influenza A virus-specific CD8+ T cell homeostasis in the absence of IFN-γ signaling. J Immunol. 2007;178:7616–7622. doi: 10.4049/jimmunol.178.12.7616. [DOI] [PubMed] [Google Scholar]
- 31.Bilic I, et al. Negative regulation of CD8 expression via CD8 enhancer-mediated recruitment of the zinc finger protein MAZR. Nat Immunol. 2006;7:392–400. doi: 10.1038/ni1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson SR, et al. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4-CD8 lineage differentiation. J Exp Med. 2007;204:267–272. doi: 10.1084/jem.20061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieffer LJ, et al. Identification of a candidate regulatory region in the human CD8 gene complex by colocalization of DNase I hypersensitive sites and matrix attachment regions which bind SATB1 and GATA-3. J Immunol. 2002;168:3915–3922. doi: 10.4049/jimmunol.168.8.3915. [DOI] [PubMed] [Google Scholar]
- 34.Landry DB, Engel JD, Sen R. Functional GATA-3 binding sites within murine CD8α upstream regulatory sequences. J Exp Med. 1993;178:941–949. doi: 10.1084/jem.178.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Omori M, et al. CD8 T cell-specific downregulation of histone hyperacetylation and gene activation of the IL-4 gene locus by ROG, repressor of GATA. Immunity. 2003;19:281–294. doi: 10.1016/s1074-7613(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 36.Chtanova T, et al. Gene microarrays reveal extensive differential gene expression in both CD4+ and CD8+ type 1 and type 2 T cells. J Immunol. 2001;167:3057–3063. doi: 10.4049/jimmunol.167.6.3057. [DOI] [PubMed] [Google Scholar]
- 37.Bosselut R. CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol. 2004;4:529–540. doi: 10.1038/nri1392. [DOI] [PubMed] [Google Scholar]
- 38.Maraskovsky E, Troutt AB, Kelso A. Co-engagement of CD3 with LFA-1 or ICAM-1 adhesion molecules enhances the frequency of activation of single murine CD4+ and CD8+ T cells and induces synthesis of IL-3 and IFN-γ but not IL-4 or IL-6. Int Immunol. 1992;4:475–485. doi: 10.1093/intimm/4.4.475. [DOI] [PubMed] [Google Scholar]
- 39.Lahm HW, Stein S. Characterization of recombinant human interleukin-2 with micromethods. J Chromatogr. 1985;326:357–361. doi: 10.1016/s0021-9673(01)87461-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.