Summary
Many recently discovered RNA functions rely on highly complex multi-step conformational transitions that occur in response to an array of cellular signals. These dynamics accompany and guide, for example, RNA co-transcriptional folding, ligand sensing and signaling, site-specific catalysis in ribozymes, and the hierarchically ordered assembly of ribonucleoproteins. RNA dynamics are encoded by both the inherent properties of RNA structure, spanning many motional modes with a large range of amplitudes and timescales, and external trigger factors, ranging from proteins, nucleic acids, metal ions, metabolites, and vitamins to temperature and even directional RNA biosynthesis itself. Here, we review recent advances in our understanding of RNA dynamics as highlighted by biophysical tools.
Introduction
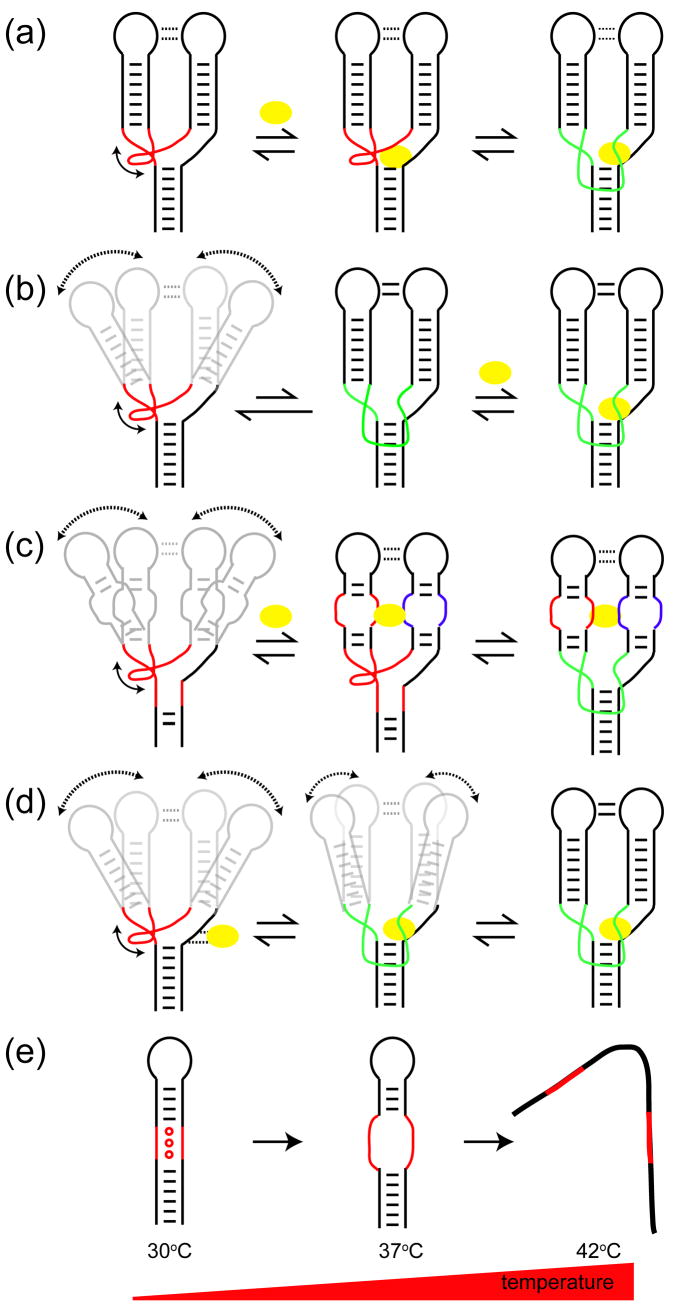
The ongoing discovery of a vast universe of non-coding RNAs that perform widespread roles in living organisms raises the fundamental question: How does a biopolymer composed of only four chemically similar building blocks realize such functional diversity? An emerging theme is that much of RNA’s functional complexity is rooted not only in the details of its intricate 3D structure, but equally in its ability to adaptively acquire very distinct conformations on its own or in response to specific cellular signals including the recognition of proteins, nucleic acids, metal ions, metabolites, vitamins, changes in temperature, and even RNA biosynthesis itself. These conformational transitions are spatially and temporally tuned to achieve a variety of functions (Figure 1). For example, they can guide folding pathways during RNA co-transcriptional folding (Figure 1a); enable sensing and signaling events that regulate gene expression in response to changes in environmental conditions (Figure 1b); allow ribozymes to dynamically meet the diverse structural requirements associated with their multi-step catalytic cycles (Figure 1c); and enable complex ribonucleoproteins to assemble in a hierarchical and sequentially ordered manner (Figure 1d).
Figure 1.
Role of RNA conformational transitions in (a) co-transcriptional folding (b) sensing and signaling transactions by riboswitches (c) catalysis (star, reaction chemistry; orange double arrow, global motions; green double arrow, local motions) and (d) hierarchical ribonucleoprotein assembly.
RNA conformational transitions occur through complex, often multi-layer RNA dynamics that comprise a combination of thermally activated internal motions and rearrangements induced by external co-factors. Motions in RNA range from rearrangements in secondary structure and large-scale collective bending and twisting of helical domains to more localized changes in base pairing and staking, sugar re-puckering, and fluctuations along the phosphodiester backbone, all of which occur over a range of timescales (Figure 2). Over the past few years, complementary biophysical tools have provided distinct cross-sectional views of RNA’s dazzling dynamical complexity (Figure 2), leading to new insights that are reviewed here with an emphasis on those derived from fluorescence and NMR spectroscopy.
Figure 2.
Time-chart of dynamic processes in RNA and corresponding biophysical techniques that can be applied towards their characterization.
Self-induced transitions during co-transcriptional folding
The RNA structural free energy landscape is highly rugged so that different folding pathways can lead to structurally distinct kinetically trapped intermediates. To facilitate folding on such a landscape, many RNAs have evolved to code for self-induced transitions involving short-lived non-native structural motifs that dynamically form during co-transcriptional folding (Figure 1a) [1]. Kinetic control over folding pathways becomes possible in the cell because the rate of transcription (as fast as ~10−3 sec/nt) is relatively slow compared to folding of RNA secondary structural elements (as fast as ~10−6 sec) (Figure 1). During 5′-to-3′ transcription both native and non-native secondary structure elements form efficiently, beginning from the 5′-end, and survive long enough to guide downstream folding along specific pathways (Figure 1a). Conversely, upstream structural elements are often still dynamic (short-lived) enough that competing downstream motifs or outside co-factors can efficiently refold the RNA into an alternate (native) structure (Figure 1a).
Studies are increasingly providing insights into the underlying code requirements and mechanisms for regulating co-transcriptional RNA folding via self-induced transitions. Xayaphoummine et al [2] recently showed how self-induced transitions involving non-native helices can be encoded in an RNA sequence based on the sequential order with which native helices of varying stability are transcribed. The authors demonstrated that a bistable RNA folds into either of two distinct conformations by simply reversing the order with which the sequence is transcribed.
Strategically positioned transcription pause sites can aid formation of fold-directing non-native structural elements by minimizing competition from alternative folds involving downstream regions [3]. This strategy is used to avoid kinetic traps when constructing structural elements from residues that are far apart in sequence. Pan and colleagues [4•] recently showed how the positioning of pause sites in between the strands of long helices in three distinct non-coding RNAs allows upstream portions of the helices to be sequestered into non-native structures that can subsequently transition into long native helices once downstream trigger strands are transcribed.
These studies underscore the importance of considering RNA folding dynamics in the cellular context of directional co-transcriptional folding.
Riboswitches
Riboswitches [5–7] beautifully illustrate how complex RNA dynamics can be used to achieve highly tunable and adaptable biological regulation (Figure 1b). Riboswitches are cis-acting mRNA elements that allow cells to adaptively change gene expression in response to their changing environment. Riboswitches are capable of sensing and quantifying diverse physiological parameters such as the concentration of metabolites, vitamins, Mg2+ and temperature and respond by transducing output signals once predefined thresholds are reached. The output signals serve to turn off and, more rarely, turn on gene expression, or modulate splicing in higher eukaryotes.
The sensing and signaling operations of riboswitches are made possible by highly orchestrated conformational transitions involving two RNA domains (Figure 1b). In a prototypical metabolite sensing riboswitch, a highly conserved aptamer domain binds to its metabolite target with exquisite selectivity and affinity (Figure 1b). In so doing, it undergoes a conformational change that is transduced into a change in the secondary structure of a downstream decision making expression platform. In turn, the platform modulates expression of metabolic pathway genes by forming a transcription (anti-)terminating helix (Figure 1b), sequestering the Shine-Dalgarno sequence and inhibiting translation, or activating catalytic self-cleavage and mRNA degradation [5,6].
Probing conformational changes in aptamer domains
Just like the X-ray structure of the first protein myoglobin begged the question of how CO2 reaches its active site, X-ray structures of several aptamer domains in complex with their metabolites [8] show no obvious path for ligands to reach their snug binding pockets buried deep within the elaborate and intricate aptamer architecture. The idea that ligand binding must be accompanied by a large conformational change was supported early on by in-line probing data [9] showing that aptamer domains are significantly less structured in the unbound state. Recent NMR studies have yielded detailed insights into the structure of the more disordered ligand free state of purine sensing aptamer domains[10-12]. These studies show that while the long-range loop-loop interactions that stabilize global structure are transiently preformed and reinforced by Mg2+ binding, the ligand binding pocket is largely disordered in the absence of ligands.
Several groups have directed their attention towards unraveling the detailed mechanism by which aptamer domains change capture ligands (Figure 3). The results so far reveal complex multi-step mechanisms that vary even across related riboswitches. Many of the mechanisms have been proposed based on real-time kinetics measurements employing fluorescent nucleobase ligands. Studies on a C74U variant of the guanine sensing aptamer domain from B. subtilis xpt-pbuX indicate that the unbound RNA has a well defined global structure but that local gating motions at the binding pocket allow backside ligand entry that in turn induces a lid closing motion [13] (Figure 3a). In contrast, studies on the Vibrio vulnificus adenosine deaminase (add) riboswitch suggest that the ligand shifts a preexisting equilibrium between free and ligand bound conformations, leading to regulation at the translational level [14] (Figure 3b). For the structurally distinct aptamer domain of the Escherichia coli thiamine pyrophosphate (TPP) riboswitch, the RNA was singly labeled with 2–aminopurine nucleobases at various strategically chosen positions [15]. Distinct kinetic rates were observed and interpreted in terms of a two-step mechanism in which one of the helices in a three-way junction is stabilized after two helices grip the ligand in the binding pocket (Figure 3c).
Figure 3.
Proposed multi-step mechanisms for the ligand-induced conformational transition in the aptamer domains of riboswitches. Shown are mechanisms based on fluorescence studies for (a) the C74U variant of the B. subtilis xpt-pbuX guanine riboswtich [13], (b) the Vibrio vulnificus adenosine deaminase riboswitch, and (c) the Escherichia coli thiamine pyrophosphate riboswitch [15], as well as NMR studies for (d) the B. subtilis xpt-pbuX guanine riboswitch [16••] and (e) the stem-loop IV thermosensor element from the repressor of heat-shock gene expression (ROSE).
New NMR methods that combine ultrafast experiments with laser-triggered release of ligands from photo-caged derivatives allowed the hypoxanthine-induced folding trajectory of the guanine-sensing riboswitch aptamer domain of the B. subtilis xpt-pbuX to be monitored with site-specific resolution [16••]. A three-step mechanism was proposed in which ligands initially non-specifically associate with pre-existing elements in the binding pocket, causing local conformational adaptation followed by long-range induced-fit stabilization of the loop-loop interactions between two helices (Figure 3d).
Aptamer domains do not necessarily undergo structural transitions between two well-defined conformations. For example, NMR studies show that an RNA thermosensor regulates expression of heat/cold shock genes by progressively undergoing conformational changes against a temperature gradient [17•] (Figure 3e). By contrast, the aptamer domain of the glmS catalytic riboswitch binds its target glucosamine-6-phosphate (GlcN6P) without undergoing a significant conformational change [18]. Here, GlcN6P acts as a co-enzyme in the cleavage reaction, and its binding contributes the missing chemical participants for self-cleavage as the signal that leads to mRNA degradation. Although most studies suggest that the ligand bound aptamer conformations of riboswitches are globally well-defined, recent kinetic fluorescence studies [19,20•] challenge this notion and suggest that adenine binding activates global helical motions in the adenine riboswitch. A detailed characterization of the dynamical properties of free and bound aptamer domains over a range of timescales will be important for understanding the molecular basis by which they sense transduce signals.
Transducing conformational changes to the expression platform
Relatively little is known about how changes in the aptamer domain are transduced into conformational changes in the decision-making expression platform and this will likely be focus of many future studies. Particularly for transcription terminating riboswitches, the signal has to be transduced efficiently during co-transcriptional folding before the decision-making expression platform is transcribed. The large conformational changes associated with aptamer binding unfavorably reduce the rate of complex formation to 104 –105 M−1sec−1, and thermodynamic equilibration between free and ligand bound forms may not be complete before the decision-making expression platform is transcribed [21]. Riboswitches can overcome this potential problem by incorporating transcription pause sites with durations ranging between 10 sec to 60 sec between the aptamer domain and expression platform [21].
Notably, prefolded full-length riboswitches commonly do not transduce the change in aptamer conformation efficiently into a change in the expression platform. This may not be surprising given that inter-conversion between the two secondary structure forms often involves high thermodynamic barriers associated with melting several base-pairs. It is therefore very likely that the process of co-transcriptional folding which is used to kinetically tune the threshold concentration of metabolite that activate riboswitches [21], also plays also an important role in the structural transitions underlying signal transduction. This highlights how RNA-based regulation in addition to folding has to be considered within the biological framework of co-transcriptional folding.
RNA catalysis
Enzymatic action by RNAs, called ribozymes, is an inherently dynamic process (Figure 1c). Just like protein enzymes, ribozymes likely exploit the dynamics of functional groups and domains to guide the catalytic process along a specific reaction coordinate. Given the local nature of the chemistry involved in most enzymatic reaction, the dynamics contributing directly to catalysis mostly entail vibrations, torsional librations, sugar re-puckering, and longitudinal and lateral motions of bases, which all take place at the tens of fs to low ns timescale (Figure 1c). However, global structural changes at the ns to min timescale are often required to properly position reaction participants in the catalytic core and may be coupled to the local dynamics (Figure 1c). Biophysical tools have recently begun to shed light on the dynamic processes involved in RNA catalysis (Figure 2).
A combination of single molecule FRET and fluorescence correlation spectroscopy was recently able to clock the transition time of the P4-P6 domain of the large Tetrahymena group I self-splicing intron from an extended to a compact docked structure at ~240 μs [22•]. This global folding transition time is much faster than the inherently long residence times of RNA in defined structural states before a transition so that the latter dominates the folding kinetics. This finding highlights the enhanced stability of intermediate structural states of RNA compared to protein and allows for the use of steady-state residence times for measuring RNA folding kinetics. A recent single molecule FRET study, for example, performed a set of sequential buffer exchanges that identified each intermediate on the reaction pathway of the hairpin ribozyme through a distinct time sequence of FRET signals [23••]. This kinetic “fingerprint” approach led to a full kinetic characterization of the reaction pathway of the catalytically competent enzyme-substrate complex. Clearly, slow global conformational dynamics significantly impact the overall catalytic rate constant [23••–25]. Single molecule FRET also allowed for the dissection of the metal-ion dependent multi-step folding pathways of an in vitro selected Diels-Alderase ribozyme [26] and a DNAzyme [27]. Sometimes FRET can demonstrate the lack of significant conformational dynamics of a ribozyme, for example of the glmS ribozyme upon cofactor binding [18]. Often a specific tertiary interaction of a larger ribozyme can be studied in isolation, which led, for example, to the FRET-based characterization of docking and undocking of the GAAA tetraloop and receptor as induced by metal ions and increased hydrostatic pressure, respectively [28,29].
A large ribozyme that has been extensively studied by single molecule probing is the ribosome. Labeling the A- and P-site tRNAs with a FRET fluorophore pair revealed the complexity of large-scale conformational dynamics along the ribosomal translation cycle. The use of inhibitors such as antibiotics known to impair specific steps in this cycle together with the postsynchronization of many individual single molecule FRET time traces facilitated the mechanistic dissection of initial selection, proofreading and translocation events [25,30••,31•]. Such dissection demonstrated, for example, that the ribosome uses rare large-scale thermal fluctuations to amplify slight positional differences into a 100-fold kinetic discrimination that favors a cognate over a non-cognate tRNA during initial selection [30••].
Biophysical tools to dissect the detailed catalytic core dynamics of ribozymes are still sparse. Molecular dynamics (MD) simulations in combination with fluorescence studies have highlighted the role of cross-linking structural water molecules in the catalytic core dynamics of the hairpin ribozyme [32•] and have described conformational dynamics in the hepatitis delta virus (HDV) ribozyme core, suggesting plausible reaction trajectories for catalysis [33,34] that now can be tested by emerging quantum mechanical tools [35,36]. Only recently have advanced NMR techniques been employed to elucidate the conformational dynamics of a core element of a ribozyme, specifically the catalytic domain 5 of a group II intron [37]. How exactly such local dynamics correlate and couple with global ribozyme dynamics to influence catalysis awaits further experimental scrutiny [24,38]. Computational tools such as correlation analyses of MD simulations promise to guide the experimentalist in search of such coupling modes [32•,36].
RNP assembly
Within the cell, most RNAs are part of RNA-protein (RNP) complexes wherein the protein component(s) assists RNA function and protects it from falling prey to chemical or enzymatic degradation. Intracellular binding of proteins begins concomitantly with RNA transcription and is often ordered and energetically driven by ATP (or GTP) consumption. In principle, RNA binding proteins behave like the metabolites that trigger riboswitches, but their multivalency yields additional binding free energy and thus can lead to more profound refolding of an RNA. The intricate interplay of protein binding and induced changes in RNA folding pathways and RNA dynamics are still ill-understood, but some key observations, derived with the help of biophysical tools, are highlighted here (Figure 1d).
A recent single molecule FRET study directly observed the assembly of Tetrahymena telomerase from its RNA and two protein components in vitro as a hierarchical pathway [39]. Binding of the first protein, p65, induces a conformational change (or, rather, favors a particular structure capture) that facilitates binding of telomerase reverse transcriptase. Similarly hierarchical protein binding has long been known from ribosomal subunit assembly and recently been fleshed out by pulse-chase experiments monitored by quantitative mass spectrometry [40]. While such in vitro studies recapitulate the essence of ordered RNP assembly, it is important to realize that intracellular RNP assembly is a much more controlled process that leads to superior yields of functional particles through compartmentalization and the targeted utilization of ATP hydrolysis. This notion is underscored by the fact that the in vitro assembly of the signal recognition particle (SRP) from its one RNA and two protein components is readily derailed by an incorrect temporal order of assembly [41]. Both correct and incorrect assembly may be facilitated by the fact that at least some RNA binding proteins are promiscuous in that they bind their RNA partner in both specific and non-specific modes [42]. Favorable electrostatic interactions have in some cases been shown to underlie a non-specific binding that increases the local protein concentration on the RNA surface and accelerates site-specific binding in form of a two-, rather than three-dimensional search [43•].
The multivalent binding of a protein often induces a complex series of conformational rearrangements in the RNA partner that can both rigidify and increase the flexibility of different segments of the RNA [44,45]. Such changes in RNA dynamics, as well as the associated changes in bsase accessibility, may help accelerate the strand exchange and annealing of complementary RNAs as induced by protein-based chaperones [46].
Intrinsic dynamics of RNA structures
Advances in biophysical techniques are allowing the resolution and visualization of intrinsic RNA motions that may potentiate specific functional transitions to take place. For example, a new NMR method allowed visualization of spatially structured motions between two helices in a bulge containing HIV-1 TAR that allow the ligand-free RNA to efficiently sample seven of its distinct ligand bound conformations (Figure 4) [47••]. Similarly, dynamics leading to the melting of Watson-Crick base-pairs near the internal loop of HIV-1 SL1 have been observed to recapitulate a secondary structural transition that occurs during viral maturational and is catalyzed by the NC protein [48]. Emerging 2H solid-state NMR techniques are uncovering fluctuations occurring over ns to μs, a timescale that so far has proven difficult to access experimentally in RNA [49•] (Figure 2). Likewise, ultrafast fluorescence techniques reveal RNA base-stacking motions over similar timescales in GNRA tetraloops that are known to undergo induced-fit interactions [50]. Together, these studies suggest that RNA sequences have evolved to code for structures with specific dynamical properties that can activate functional transitions.
Figure 4.
Spatially structured inter-helical motional trajectory in free HIV-1 TAR RNA visualized using NMR techniques [47••].
Outlook and challenges ahead
The biological functions of RNA depend on a dazzling assortment of dynamic structural changes (Figures 1 and 3) that occur at a range of timescales (Figure 2). Biophysical tools such as NMR and fluorescence spectroscopy, aided by computational approaches such as MD simulations, have just begun to shed light on the intricacies of RNA dynamics (Figure 2). Solving future challenges, including the visualization of RNA dynamical processes at atomic resolution under native conditions, will rely not only on expanding the capabilities of individual biophysical tools, but also on integrating them with one another to obtain a comprehensive picture of dynamics from femtoseconds to seconds and longer.
Two specific challenges on the road ahead are worth noting. One challenge arises from the often non-ergodic behavior of RNA; long observation of a single RNA molecule often does not reproduce a snapshot from an ensemble of the same molecules. Non-ergodicity underscores the deeply fluted nature of the RNA structural free energy landscape that can trap a molecule in multiple, non-(or slowly) exchanging folds [24,25,29]. This feature is best delineated using single molecule techniques and complicates interpretation of ensemble biophysical tools, necessitating a careful integration of the two approaches.
A second challenge derives from the strong influence that the solvent and metal ions have on RNA dynamics. While evidence is emerging that structural water molecules can mediate coupled molecular motions throughout a folded RNA core [32•] and that bound metal ions heavily modulate the electrostatic surface potential of RNA [33] and its conformational dynamics [48], the extent to which solvent dynamics couples with RNA dynamics at all timescales is still ill-understood. These open questions promise to yield many more surprising discoveries on RNA dynamics over years to come.
Acknowledgments
We thank Alex Hansen and Annette Casiano for help with preparation of Figures 1 and 2. Supported by the National Institutes of Health (RO1 AI066975-01 to H.M.A and RO1 GM062357, GM081025, and GM037006, to N.G.W), the National Science Foundation (MCB 0644278 to H.M.A and Chemical Bonding Center award 0533019.to N.G.W.), the American Chemical Society (43875-AC4 to N.G.W.), and the Camille and Henry Dreyfus Foundation (Teacher-Scholar Award to N.G.W.).
References
- 1.Nagel JH, Pleij CW. Self-induced structural switches in RNA. Biochimie. 2002;84:913–923. doi: 10.1016/s0300-9084(02)01448-7. [DOI] [PubMed] [Google Scholar]
- 2.Xayaphoummine A, Viasnoff V, Harlepp S, Isambert H. Encoding folding paths of RNA switches. Nucleic Acids Res. 2007;35:614–622. doi: 10.1093/nar/gkl1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landick R. The regulatory roles and mechanism of transcriptional pausing. Biochem Soc Trans. 2006;34:1062–1066. doi: 10.1042/BST0341062. [DOI] [PubMed] [Google Scholar]
- 4•.Wong TN, Sosnick TR, Pan T. Folding of noncoding RNAs during transcription facilitated by pausing-induced nonnative structures. Proc Natl Acad Sci U S A. 2007;104:17995–18000. doi: 10.1073/pnas.0705038104. This paper shows for three different non-coding RNAs that their sequences code for transcription pause sites that facilitate folding of long helices during co-transcriptional folding. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tucker BJ, Breaker RR. Riboswitches as versatile gene control elements. Curr Opin Struct Biol. 2005;15:342–348. doi: 10.1016/j.sbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Nudler E. Flipping riboswitches. Cell. 2006;126:19–22. doi: 10.1016/j.cell.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Narberhaus F, Waldminghaus T, Chowdhury S. RNA thermometers. FEMS Microbiol Rev. 2006;30:3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards TE, Klein DJ, Ferre-D’Amare AR. Riboswitches: small-molecule recognition by gene regulatory RNAs. Curr Opin Struct Biol. 2007;17:273–279. doi: 10.1016/j.sbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Winkler W, Nahvi A, Breaker RR. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature. 2002;419:952–956. doi: 10.1038/nature01145. [DOI] [PubMed] [Google Scholar]
- 10.Noeske J, Schwalbe H, Wohnert J. Metal-ion binding and metal-ion induced folding of the adenine-sensing riboswitch aptamer domain. Nucleic Acids Res. 2007;35:5262–5273. doi: 10.1093/nar/gkm565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noeske J, Buck J, Furtig B, Nasiri HR, Schwalbe H, Wohnert J. Interplay of ‘induced fit’ and preorganization in the ligand induced folding of the aptamer domain of the guanine binding riboswitch. Nucleic Acids Res. 2007;35:572–583. doi: 10.1093/nar/gkl1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottink OM, Rampersad SM, Tessari M, Zaman GJ, Heus HA, Wijmenga SS. Ligand-induced folding of the guanine-sensing riboswitch is controlled by a combined predetermined induced fit mechanism. Rna. 2007;13:2202–2212. doi: 10.1261/rna.635307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert SD, Stoddard CD, Wise SJ, Batey RT. Thermodynamic and kinetic characterization of ligand binding to the purine riboswitch aptamer domain. J Mol Biol. 2006;359:754–768. doi: 10.1016/j.jmb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Rieder R, Lang K, Graber D, Micura R. Ligand-induced folding of the adenosine deaminase A-riboswitch and implications on riboswitch translational control. Chembiochem. 2007;8:896–902. doi: 10.1002/cbic.200700057. [DOI] [PubMed] [Google Scholar]
- 15.Lang K, Rieder R, Micura R. Ligand-induced folding of the thiM TPP riboswitch investigated by a structure-based fluorescence spectroscopic approach. Nucleic Acids Res. 2007;35:5370–5378. doi: 10.1093/nar/gkm580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Buck J, Furtig B, Noeske J, Wohnert J, Schwalbe H. Time-resolved NMR methods resolving ligand-induced RNA folding at atomic resolution. Proc Natl Acad Sci U S A. 2007;104:15699–15704. doi: 10.1073/pnas.0703182104. A new method that combines photo-triggers with ultrafast NMR experiments allows the ligand induced transition in the guanine aptamer domain to be observed site-specifically in real-time. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Chowdhury S, Maris C, Allain FH, Narberhaus F. Molecular basis for temperature sensing by an RNA thermometer. EMBO J. 2006;25:2487–2497. doi: 10.1038/sj.emboj.7601128. The first 3D structure and detailed mechanism of action is reported for a functional RNA thermosensor domain by NMR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tinsley RA, Furchak JR, Walter NG. Trans-acting glmS catalytic riboswitch: locked and loaded. Rna. 2007;13:468–477. doi: 10.1261/rna.341807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemay JF, Penedo JC, Tremblay R, Lilley DM, Lafontaine DA. Folding of the adenine riboswitch. Chem Biol. 2006;13:857–868. doi: 10.1016/j.chembiol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 20•.Eskandari S, Prychyna O, Leung J, Avdic D, O’Neill MA. Ligand-directed dynamics of adenine riboswitch conformers. J Am Chem Soc. 2007;129:11308–11309. doi: 10.1021/ja073159l. This study along with [19] shows that the ligand bound adenine riboswitch aptamer domain is not a single highly ordered conformation in solution but rather exists as an ensemble of at least three distinct conformations. Ligand induced dynamics is also reported in [45]. [DOI] [PubMed] [Google Scholar]
- 21.Wickiser JK, Winkler WC, Breaker RR, Crothers DM. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol Cell. 2005;18:49h–60. doi: 10.1016/j.molcel.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 22•.Lee TH, Lapidus LJ, Zhao W, Travers KJ, Herschlag D, Chu S. Measuring the folding transition time of single RNA molecules. Biophys J. 2007;92:3275–3283. doi: 10.1529/biophysj.106.094623. A new photon correlation method is used to resolve the actual transition of a large ribozyme fragment between two RNA folds, going beyond the now standard method of measuring the residence time in each state to derive the transition kinetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Liu S, Bokinsky G, Walter NG, Zhuang X. Dissecting the multistep reaction pathway of an RNA enzyme by single-molecule kinetic “fingerprinting”. Proc Natl Acad Sci U S A. 2007;104:12634–12639. doi: 10.1073/pnas.0610597104. Distinct intermediates along a reaction pathway often give rise to a smaller number of degenerate FRET levels. To resolve the multi-step pathway of a catalytically active ribozyme-substrate complex, sequential buffer exchanges were used to identify each intermediate by a specific sequence of FRET values, rather than a single FRET level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rueda D, Bokinsky G, Rhodes MM, Rust MJ, Zhuang X, Walter NG. Single-molecule enzymology of RNA: essential functional groups impact catalysis from a distance. Proc Natl Acad Sci USA. 2004;101:10066–10071. doi: 10.1073/pnas.0403575101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ditzler MA, Aleman EA, Rueda D, Walter NG. Focus on function: single molecule RNA enzymology. Biopolymers. 2007;87:302–316. doi: 10.1002/bip.20819. [DOI] [PubMed] [Google Scholar]
- 26.Kobitski AY, Nierth A, Helm M, Jaschke A, Nienhaus GU. Mg2+-dependent folding of a Diels-Alderase ribozyme probed by single-molecule FRET analysis. Nucleic Acids Res. 2007;35:2047–2059. doi: 10.1093/nar/gkm072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK, Rasnik I, Liu J, Ha T, Lu Y. Dissecting metal ion-dependent folding and catalysis of a single DNAzyme. Nat Chem Biol. 2007;3:763–768. doi: 10.1038/nchembio.2007.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downey CD, Crisman RL, Randolph TW, Pardi A. Influence of hydrostatic pressure and cosolutes on RNA tertiary structure. J Am Chem Soc. 2007;129:9290–9291. doi: 10.1021/ja072179k. [DOI] [PubMed] [Google Scholar]
- 29.Downey CD, Fiore JL, Stoddard CD, Hodak JH, Nesbitt DJ, Pardi A. Metal ion dependence, thermodynamics, and kinetics for intramolecular docking of a GAAA tetraloop and receptor connected by a flexible linker. Biochemistry. 2006;45:3664–3673. doi: 10.1021/bi0520941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Lee TH, Blanchard SC, Kim HD, Puglisi JD, Chu S. The role of fluctuations in tRNA selection by the ribosome. Proc Natl Acad Sci U S A. 2007;104:13661–13665. doi: 10.1073/pnas.0705988104. Single molecule FRET shows how the induced fit during initial selection of a cognate tRNA leads not only to more stable binding, but also to a positioning of the ternary complex in a way that large-scale thermal fluctuations of the ribosome are more likely to lead to productive progress along the ribosomal translation cycle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Munro JB, Altman RB, O’Connor N, Blanchard SC. Identification of two distinct hybrid state intermediates on the ribosome. Mol Cell. 2007;25:505–517. doi: 10.1016/j.molcel.2007.01.022. Single molecule FRET at high time resolution resolved the dynamic exchange between three metastable tRNA configurations as translocation intermediates of the ribosome. The high activation barriers of these transitions suggest that they are coupled to large-scale conformational rearrangements of the ribosome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Rhodes MM, Reblova K, Sponer J, Walter NG. Trapped water molecules are essential to structural dynamics and function of a ribozyme. Proc Natl Acad Sci U S A. 2006;103:13380–13385. doi: 10.1073/pnas.0605090103. Explicit-solvent MD simulations in combination with single molecule FRET data support the notion that long-residency water molecules play an important role in the dynamic structural communication of local perturbations throughout the catalytic core of a ribozyme and possibly in general acid-base catalysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasovska MV, Sefcikova J, Reblova K, Schneider B, Walter NG, Sponer J. Cations and hydration in catalytic RNA: molecular dynamics of the hepatitis delta virus ribozyme. Biophys J. 2006;91:626–638. doi: 10.1529/biophysj.105.079368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sefcikova J, Krasovska MV, Sponer J, Walter NG. The genomic HDV ribozyme utilizes a previously unnoticed U-turn motif to accomplish fast site-specific catalysis. Nucleic Acids Res. 2007;35:1933–1946. doi: 10.1093/nar/gkl1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H, Robinet JJ, Ananvoranich S, Gauld JW. Density functional theory investigation on the mechanism of the hepatitis delta virus ribozyme. J Phys Chem B. 2007;111:439–445. doi: 10.1021/jp064292n. [DOI] [PubMed] [Google Scholar]
- 36.Radhakrishnan R. Coupling of fast and slow modes in the reaction pathway of the minimal hammerhead ribozyme cleavage. Biophys J. 2007;93:2391–2399. doi: 10.1529/biophysj.107.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eldho NV, Dayie KT. Internal bulge and tetraloop of the catalytic domain 5 of a group II intron ribozyme are flexible: implications for catalysis. J Mol Biol. 2007;365:930–944. doi: 10.1016/j.jmb.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Tinsley RA, Walter NG. Long-range impact of peripheral joining elements on structure and function of the hepatitis delta virus ribozyme. Biol Chem. 2007;388:705–715. doi: 10.1515/BC.2007.088. [DOI] [PubMed] [Google Scholar]
- 39.Stone MD, Mihalusova M, O’Connor CM, Prathapam R, Collins K, Zhuang X. Stepwise protein-mediated RNA folding directs assembly of telomerase ribonucleoprotein. Nature. 2007;446:458–461. doi: 10.1038/nature05600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talkington MW, Siuzdak G, Williamson JR. An assembly landscape for the 30S ribosomal subunit. Nature. 2005;438:628–632. doi: 10.1038/nature04261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maity TS, Weeks KM. A threefold RNA-protein interface in the signal recognition particle gates native complex assembly. J Mol Biol. 2007;369:512–524. doi: 10.1016/j.jmb.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bokinsky G, Nivon LG, Liu S, Chai G, Hong M, Weeks KM, Zhuang X. Two distinct binding modes of a protein cofactor with its target RNA. J Mol Biol. 2006;361:771–784. doi: 10.1016/j.jmb.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Korennykh AV, Piccirilli JA, Correll CC. The electrostatic character of the ribosomal surface enables extraordinarily rapid target location by ribotoxins. Nat Struct Mol Biol. 2006;13:436–443. doi: 10.1038/nsmb1082. A careful binding analysis reveals that the a-sarcin-like ribotoxin restrictocin binds the ribosomal surface at many sites through electrostatic interaction and rapidly diffuses within the ribosomal electrostatic field to its specific recognition site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shajani Z, Drobny G, Varani G. Binding of U1A protein changes RNA dynamics as observed by 13C NMR relaxation studies. Biochemistry. 2007;46:5875–5883. doi: 10.1021/bi602658x. [DOI] [PubMed] [Google Scholar]
- 45.Hansen AL, Al-Hashimi HM. Dynamics of large elongated RNA by NMR carbon relaxation. J Am Chem Soc. 2007;129:16072–16082. doi: 10.1021/ja0757982. [DOI] [PubMed] [Google Scholar]
- 46.Russell R. RNA misfolding and the action of chaperones. Front Biosci. 2008;13:1–20. doi: 10.2741/2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Zhang Q, Stelzer AC, Fisher CK, Al-Hashimi HM. Visualizing spatially correlated dynamics that directs RNA conformational transitions. Nature. 2007;450:1263–1267. doi: 10.1038/nature06389. This study reports the 3D visualization of spatially structured helical motions that allow an unbound RNA to efficiently sample seven of its distinct lignad bound conformations. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Zhang Q, Al-Hashimi HM. Resolving fast and slow motions in the internal loop containing stem-loop 1 of HIV-1 that are modulated by Mg2+ binding: role in the kissing-duplex structural transition. Nucleic Acids Res. 2007;35:1698–1713. doi: 10.1093/nar/gkm020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Olsen GL, Echodu DC, Shajani Z, Bardaro MF, Jr, Varani G, Drobny GP. Solid-State Deuterium NMR Studies Reveal mus-ns Motions in the HIV-1 Transactivation Response RNA Recognition Site. J Am Chem Soc. 2008 doi: 10.1021/ja0778803. This first modern application of solid-state NMR for site-specifically characterizing RNA conformational dynamics uncovers fluctuations at the ns-μs timescale. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L, Xia T. Direct revelation of multiple conformations in RNA by femtosecond dynamics. J Am Chem Soc. 2007;129:4118–4119. doi: 10.1021/ja068391q. [DOI] [PubMed] [Google Scholar]