Abstract
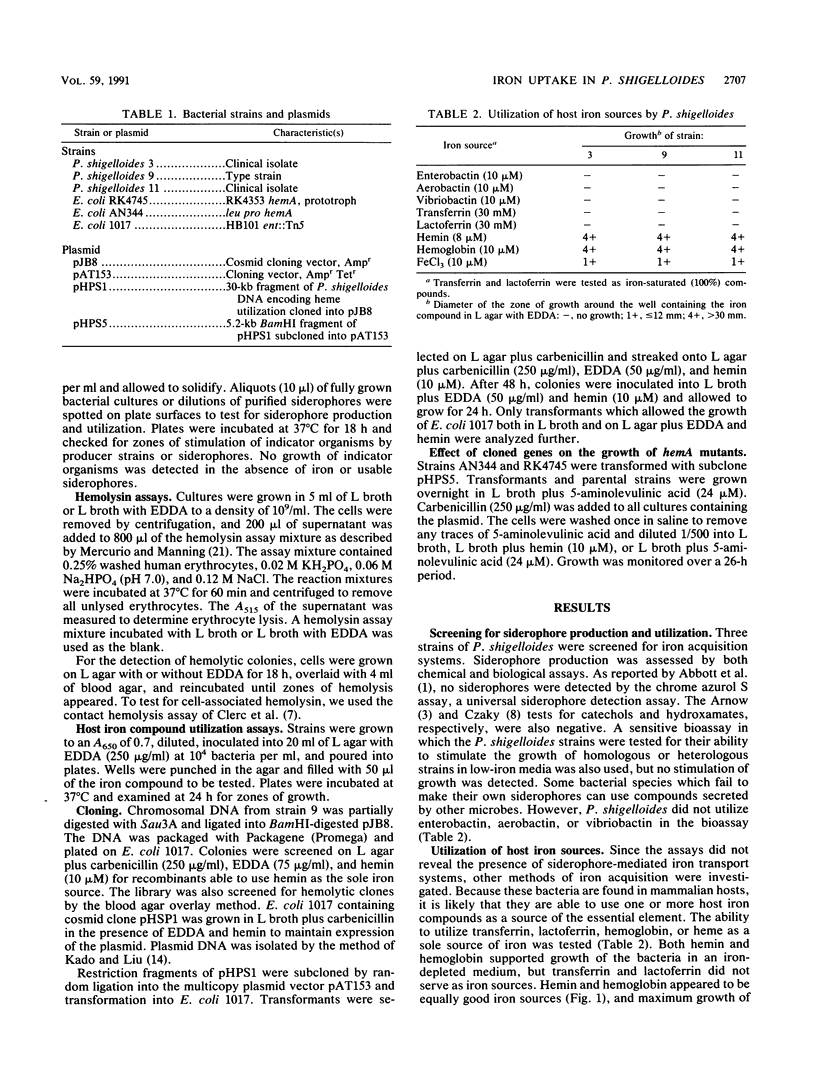
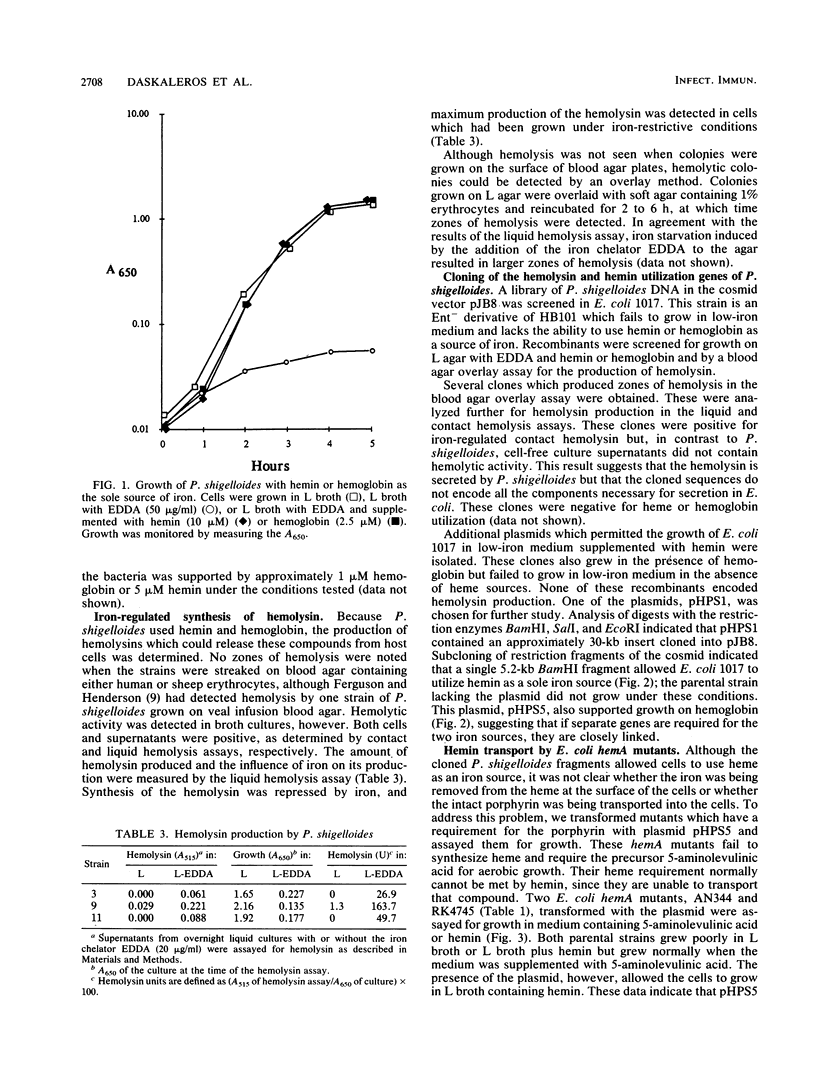
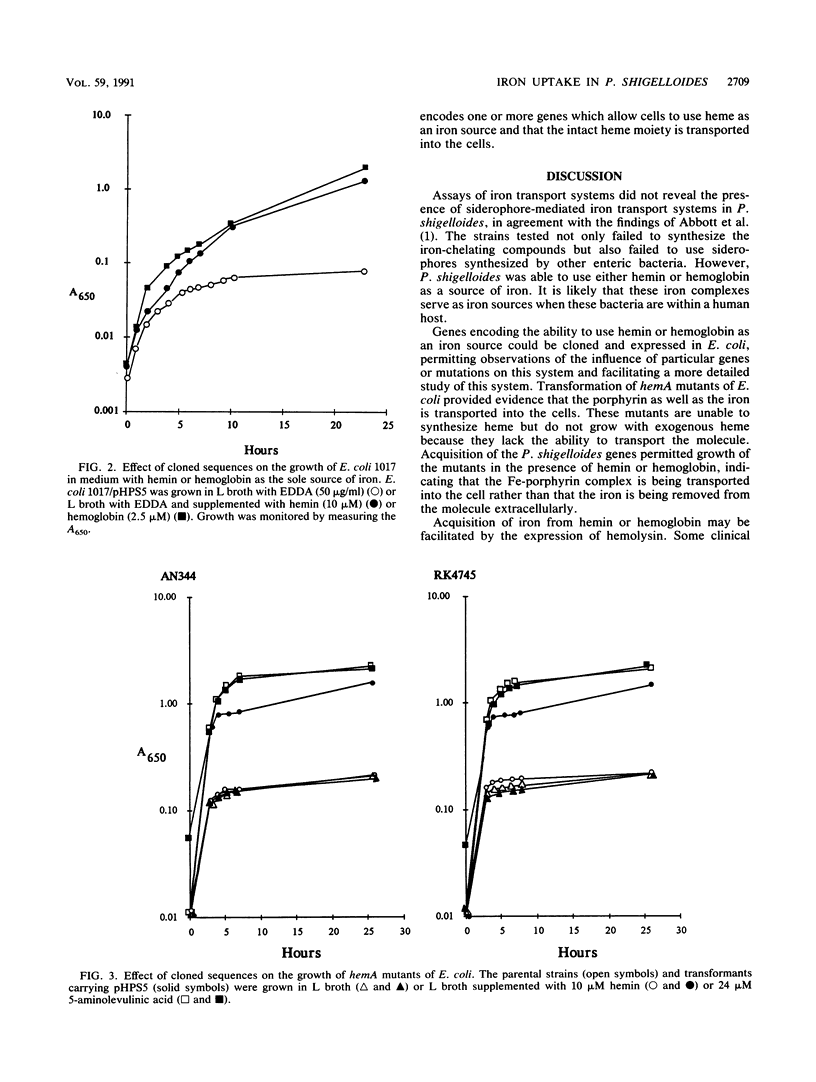
The iron uptake systems of Plesiomonas shigelloides strains were determined. Siderophore production was not detected by chemical or biological assays, and the strains tested were unable to use enterobactin, aerobactin, or vibriobactin for growth in low-iron media. Both hemin and hemoglobin supported full growth of the bacteria in media lacking other iron sources, but neither transferrin nor lactoferrin served as a source of iron. Hemolysin was detected, and the production of hemolysin was iron repressible. DNA sequences encoding hemolysin production and DNA sequences encoding the ability to use heme or hemoglobin as a sole source of iron were cloned from P. shigelloides and expressed in Escherichia coli. The abilities to use heme and hemoglobin as iron sources were closely linked, and the cloned sequences encoded the ability to transport the porphyrin, as well as iron, into the cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott S. L., Kokka R. P., Janda J. M. Laboratory investigations on the low pathogenic potential of Plesiomonas shigelloides. J Clin Microbiol. 1991 Jan;29(1):148–153. doi: 10.1128/jcm.29.1.148-153.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Neilands J. B., Phaff H. J. Rhodotorulic acid from species of Leucosporidium, Rhodosporidium, Rhodotorula, Sporidiobolus, and Sporobolomyces, and a new alanine-containing ferrichrome from Cryptococcus melibiosum. J Bacteriol. 1970 Sep;103(3):722–733. doi: 10.1128/jb.103.3.722-733.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenden R. A., Miller M. A., Janda J. M. Clinical disease spectrum and pathogenic factors associated with Plesiomonas shigelloides infections in humans. Rev Infect Dis. 1988 Mar-Apr;10(2):303–316. doi: 10.1093/clinids/10.2.303. [DOI] [PubMed] [Google Scholar]
- Clerc P., Baudry B., Sansonetti P. J. Plasmid-mediated contact haemolytic activity in Shigella species: correlation with penetration into HeLa cells. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):267–278. doi: 10.1016/s0769-2609(86)80033-3. [DOI] [PubMed] [Google Scholar]
- Ferguson W. W., Henderson N. D. Description of Strain C27: A Motile Organism with the Major Antigen of Shigella sonnei Phase I. J Bacteriol. 1947 Aug;54(2):179–181. doi: 10.1128/jb.54.2.179-181.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. E., Fowlston S. E., George W. L. In vitro production of cholera toxin-like activity by Plesiomonas shigelloides. J Infect Dis. 1987 Nov;156(5):720–722. doi: 10.1093/infdis/156.5.720. [DOI] [PubMed] [Google Scholar]
- Griffiths G. L., Sigel S. P., Payne S. M., Neilands J. B. Vibriobactin, a siderophore from Vibrio cholerae. J Biol Chem. 1984 Jan 10;259(1):383–385. [PubMed] [Google Scholar]
- Herrington D. A., Sparling P. F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Tzipori S., Robins-Browne R. M., Tall B. D., Levine M. M. In vitro and in vivo pathogenicity of Plesiomonas shigelloides. Infect Immun. 1987 Apr;55(4):979–985. doi: 10.1128/iai.55.4.979-985.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor K. M., Daskaleros P. A., Robinson R. E., Payne S. M. Virulence of iron transport mutants of Shigella flexneri and utilization of host iron compounds. Infect Immun. 1987 Mar;55(3):594–599. doi: 10.1128/iai.55.3.594-599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebek G., Gruenig H. M. Relation between the hemolytic property and iron metabolism in Escherichia coli. Infect Immun. 1985 Dec;50(3):682–686. doi: 10.1128/iai.50.3.682-686.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linggood M. A., Ingram P. L. The role of alpha haemolysin in the virulence of Escherichia coli for mice. J Med Microbiol. 1982 Feb;15(1):23–30. doi: 10.1099/00222615-15-1-23. [DOI] [PubMed] [Google Scholar]
- Mandal B. K., Whale K., Morson B. C. Acute colitis due to Plesiomonas shigelloides. Br Med J (Clin Res Ed) 1982 Nov 27;285(6354):1539–1540. doi: 10.1136/bmj.285.6354.1539-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. G., Douglas H., Guiney D. G. Production of a heat stable enterotoxin by Plesiomonas shigelloides. Microb Pathog. 1988 Sep;5(3):207–213. doi: 10.1016/0882-4010(88)90023-x. [DOI] [PubMed] [Google Scholar]
- McNeeley D., Ivy P., Craft J. C., Cohen I. Plesiomonas: biology of the organism and diseases in children. Pediatr Infect Dis. 1984 Mar-Apr;3(2):176–181. [PubMed] [Google Scholar]
- Mercurio A., Manning P. A. Cellular localization and export of the soluble haemolysin of Vibrio cholerae El Tor. Mol Gen Genet. 1985;200(3):472–475. doi: 10.1007/BF00425733. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Iron and virulence in the family Enterobacteriaceae. Crit Rev Microbiol. 1988;16(2):81–111. doi: 10.3109/10408418809104468. [DOI] [PubMed] [Google Scholar]
- Payne S. M. Synthesis and utilization of siderophores by Shigella flexneri. J Bacteriol. 1980 Sep;143(3):1420–1424. doi: 10.1128/jb.143.3.1420-1424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. H., TESSMAN I. THYMIDINE-REQUIRING MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1963 Sep;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S. C., Saraswathi B., Sharma P. Enteropathogenicity of Plesiomonas shigelloides. J Med Microbiol. 1980 Aug;13(3):401–409. doi: 10.1099/00222615-13-3-401. [DOI] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull T. L. Protein sources of heme for Haemophilus influenzae. Infect Immun. 1987 Jan;55(1):148–153. doi: 10.1128/iai.55.1.148-153.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto T., Kinoshita Y., Shimada T., Sakazaki R. Two epidemics of diarrhoeal disease possibly caused by Plesiomonas shigelloides. J Hyg (Lond) 1978 Apr;80(2):275–280. doi: 10.1017/s0022172400053638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., MacLaren D. M., de Graaff J. In vivo function of hemolysin in the nephropathogenicity of Escherichia coli. Infect Immun. 1983 Oct;42(1):245–249. doi: 10.1128/iai.42.1.245-249.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]



