Periodontitis is a group of inflammatory diseases that affect the connective tissue attachment and supporting bone around the teeth. It is widely accepted that the initiation and the progression of periodontitis are dependent on the presence of virulent microorganisms capable of causing disease. Although the bacteria are initiating agents in periodontitis, the host response to the pathogenic infection is critical to disease progression [1–3]. After its initiation, the disease progresses with the loss of collagen fibers and attachment to the cemental surface, apical migration of the junctional epithelium, formation of deepened periodontal pockets, and resorption of alveolar bone [4]. If left untreated, the disease continues with progressive bone destruction, leading to tooth mobility and subsequent tooth loss. Periodontal disease afflicts over 50% of the adult population in the United States, with approximately 10% displaying severe disease concomitant with early tooth loss [5].
A goal of periodontal diagnostic procedures is to provide useful information to the clinician regarding the present periodontal disease type, location, and severity. These findings serve as a basis for treatment planning and provide essential data during periodontal maintenance and disease-monitoring phases of treatment.
Traditional periodontal diagnostic parameters used clinically include probing depths, bleeding on probing, clinical attachment levels, plaque index, and radiographs assessing alveolar bone level [6]. The strengths of these traditional tools are their ease of use, their cost-effectiveness, and that they are relatively noninvasive. Traditional diagnostic procedures are inherently limited, in that only disease history, not current disease status, can be assessed. Clinical attachment loss readings by the periodontal probe and radiographic evaluations of alveolar bone loss measure damage from past episodes of destruction and require a 2- to 3-mm threshold change before a site can be identified as having experienced a significant anatomic event [7]. Advances in oral and periodontal disease diagnostic research are moving toward methods whereby periodontal risk can be identified and quantified by objective measures such as biomarkers (Table 1).
Table 1.
Diagnostic tools to measure periodontal disease at the molecular, cellular, tissue, and clinical levels
| Level | Example of process | Example of diagnostic tools |
|---|---|---|
| Molecular | Activation of receptors for endotoxin: CD-14; Toll-like receptors |
Polymerase chain reaction; DNA- DNA hybridization; laser-capture microdissection |
| Cellular | Inflammatory cell activation such as neutrophils; osteoclast activation |
ELISA; immunohistochemistry |
| Tissue | Downgrowth of junctional epithelium; bone and connective tissue loss |
Histomorphometry; immunohistochemistry |
| Clinical | Attachment loss | Periodontal probing |
| Bone loss | Radiographs |
There are several key questions regarding current clinical decision making: How can clinicians assess risk for periodontal disease? What are the useful laboratory and clinical methods for periodontal risk assessment? and What can be achieved by controlling periodontal disease using a risk profile?[8–11]. Risk factors are considered modifiers of disease activity. In association with host susceptibility and a variety of local and systemic conditions, they influence the initiation and progression of periodontitis and successive changes on biomarkers [12–14]. Biomarkers of disease in succession play an important role in life sciences and have begun to assume a greater role in diagnosis, monitoring of therapy outcomes, and drug discovery. The challenge for biomarkers is to allow earlier detection of disease evolution and more robust therapy efficacy measurements. For biomarkers to assume their rightful role in routine practice, it is essential that their relation to the mechanism of disease progression and therapeutic intervention be more fully understood (Table 2) [13].
Table 2.
Predictors for periodontal diseases
| Term | Definition |
|---|---|
| Risk marker | An attribute or event that is associated with increased probability of disease but is not necessarily a causal factor |
| Risk indicator | An event that is associated with an outcome only in cross- sectional studies |
| Risk factor | An action or event that is related statistically in some way to an outcome and is truly causal |
| Risk determinant | An attribute or event that increases the probability of occurrence of disease |
| Biomarker | A substance that is measured objectively and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention |
There is a need for the development of new diagnostic tests that can detect the presence of active disease, predict future disease progression, and evaluate the response to periodontal therapy, thereby improving the clinical management of periodontal patients. The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease represent challenges for clinical investigators and practitioners [15]. This article highlights recent advances in the use of biomarker-based disease diagnostics that focus on the identification of active periodontal disease from plaque biofilms [16], gingival crevicular fluid (GCF) [17], and saliva [18]. Mediators that are released into GCF and saliva as biomarkers of disease are shown in Fig. 1. The authors also present an overview of well-studied mediators associated with microbial identification, host response factors, and bone resorptive mediators.
Fig. 1.
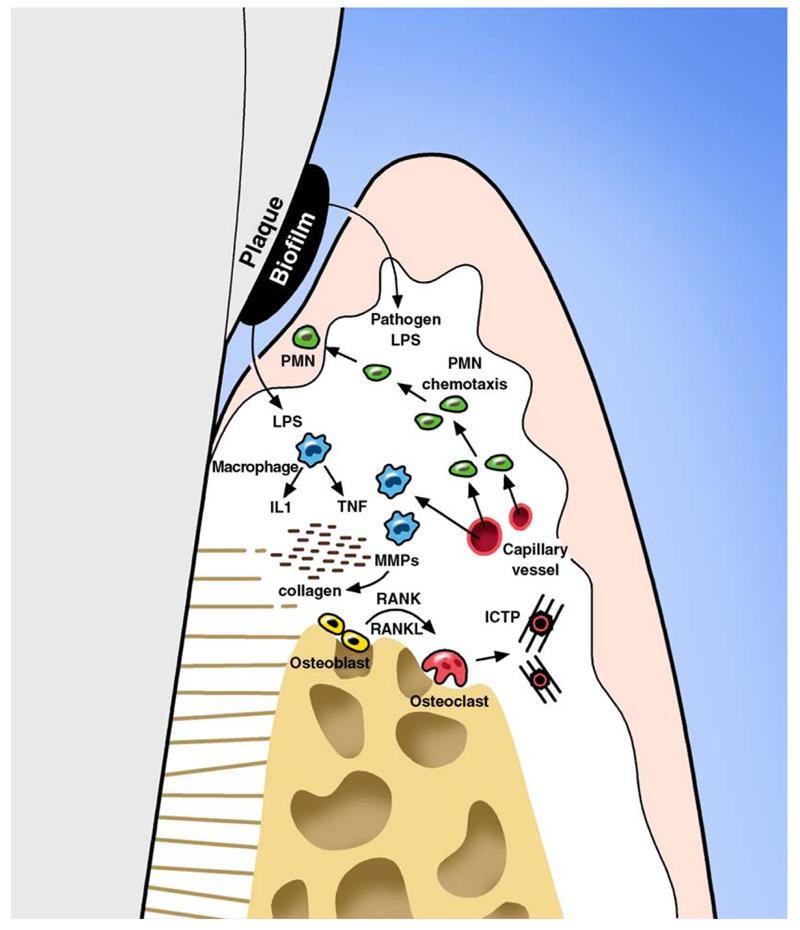
Schematic representation of the initial events triggered by lipopolysaccharide (LPS) from plaque biofilms on periodontal tissues. Pathogens present in plaque biofilm activate chemotaxis of polymorphonuclear leucocytes (PMN) as a first line of defense against infection. Monocytes and activated macrophages respond to endotoxin by releasing cytokines (tumor necrosis factor [TNF] and interleukin 1 [IL1]) that are mediators for bone resorption. Matrix metalloproteinases (MMPs) released by fibroblast and PMNs are potent collagen destruction enzymes. TNF, IL-1, and receptor activator of NF-κB ligand (RANKL) are elevated in disease sites and play an important role in osteoclastogenesis and bone resorption. Tissue degradation molecules such as pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) that are specific to bone resorption are released into the GCF and can be used as biomarkers for periodontal disease and the previously mentioned cytokines and enzymes. RANK, receptor activator of NF-κB.
Microbial factors for the diagnosis of periodontal diseases
Of the more than 600 bacterial species that have been identified from subgingival plaque, only a small number have been suggested to play a causal role in the pathogenesis of destructive periodontal diseases in the susceptible host [16]. Furthermore, technologic advances in methodologies such as analysis of 16S ribosomal RNA bacterial genes indicate that as many as several hundred additional species of not-yet-identified bacteria may exist [19]. The presence of bacteria adjacent to the gingival crevice and the intimate contact of bacterial lipopolysaccharide with the host cells trigger monocytes, polymorphonuclear leukocytes (neutrophils), macrophages, and other cells to release inflammatory mediators such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and prostaglandin E2[3]. The role of host response factors derived from GCF and saliva is discussed later.
A number of specific periodontal pathogens have been implicated in periodontal diseases, including Tanerella forsythensis, Porphyromonas gingivalis, and Treponema denticola. These three organisms are members of the “red complex” of bacteria (and exhibit benzoyl-DL-arginine-naphthylamide, or BANA, activity) [20,21] that are highly implicated in the progression of periodontal diseases [22]. Actinobacillus actinomycetemcomitans has been linked with early-onset forms of periodontal disease and aggressive periodontitis, whereas red complex bacteria are associated with chronic periodontitis [23]. The rationale for the use of microbial analysis for periodontitis monitoring is to target pathogens implicated in disease to (1) identify specific periodontal diseases, (2) identify antibiotic susceptibility of infecting organisms colonizing diseased sites, and (3) predict disease activity. Thus, the goal of microbiologic monitoring is twofold (disease monitoring and disease treatment guidance); however, microbial tests (eg, BANA test, DNA probe analysis, or culturing) have failed to predict future disease progression [24]. These findings can be explained by the fact that the presence of specific periodontal pathogens is necessary to initiate periodontal disease but not suffcient to cause disease in the nonsusceptible host[25].
A recent systematic review of the topic asked the focused question, “In patients with periodontal diseases, does microbial identification influence patient management compared with treatment prescribed without this information?” [24]. The answer to this question is central for the clinical utility of microbial-based diagnostic tests for oral and periodontal diseases. If tests do not influence clinical decision making, then in essence, there is no clinical value for the test. Of the 24 studies (a total of 835 subjects) noted in the review, 13 reported microbial identification as an aid in treatment planning [26-38]. The investigators concluded that the literature lacks studies with a high evidence rating; the most-pertinent studies were case reports or case series without controls [24]. Furthermore, although some practitioners consider microbial identification a valuable adjunct to the management of patients with periodontal diseases, there is a lack of strong evidence to support this practice. It is also possible that as-yet-unidentified, uncultivable microbial species are essential to disease initiation and progression. If so, microbial-based tests for these species are obviously unavailable. Future studies are needed in this area to justify the use of microbial testing to predict progression of periodontal diseases [24]. New strategies that combine microbial identification with host response or tissue breakdown factors using discriminant analysis may better improve the ability of microbial analysis to predict future periodontal disease around teeth and dental implants [39,40].
Host response and inflammatory mediators as potential biomarkers
Periodontal inflammation occurs in the gingival tissue in response to plaque bacteria biofilms [3,41]. Gingivitis is characterized by an initial increase in blood flow, enhanced vascular permeability, and the influx of cells (neutrophils and monocyte-macrophages) from the peripheral blood to the gingival crevice [42]. Subsequently, T cells and B cells appear at the infection site. After they appear at the lesion, these cells produce a myriad of cytokines such as IL-1β, IL-6, TNF-α, and immunoglubulins as an antigen-specific response [4]. Initially, tissue degradation is limited to epithelial cells and collagen fibers from the connective tissue. Later on, the inflammatory process may reach periodontal supportive tissue, leading to bone resorption (see Fig. 1) [43].
GCF has been extensively investigated for the release of host response factors. It includes a mixture of molecules from blood, host tissue, and plaque biofilms, such as electrolytes, small molecules, proteins, cytokines, antibodies, bacterial antigens, and enzymes [44–60]. Host cell—derived enzymes such as matrix metalloproteinases (MMPs) are an important group of neutral proteinases implicated in the destructive process of periodontal disease that can be measured in GCF [44,61–67]. The neutrophils are the major cells responsible for MMP release at the infected site, specifically MMP-8 (collagenase-2) and MMP-9 (gelatinase-B) [65]. Although MMP-8 is able to potently degrade interstitial collagens, MMP-9 degrades several extracellular matrix proteins [68–71]. Kinane et al [64] and Mantyla et al [65] presented the use of a rapid chairside test based on the immunologic detection of elevated MMP-8 in GCF to diagnose and monitor the course and treatment of periodontitis. With a threshold of 1 mg/L MMP-8 activity, the test provided a sensitivity of 0.83 and specificity of 0.96, demonstrating value as a potential tool to differentiate periodontitis from gingivitis and healthy sites and to monitor treatment of periodontitis.
Macrophages and polymorphonuclear leukocytes, in response to the chemoattractant effect of bacterial lipopolysaccharide [72], are activated to produce important inflammatory mediators—notably, TNF-α, IL-1, IL-6, and other cytokines [73] related to the host response and tissue destruction [72]. Holmlund et al [74] investigated bone resorption activity, IL-1α, IL-1β, and IL-1 receptor antagonist levels in GCF in sites having no signs of periodontal disease and in sites having horizontal or angular periodontal bone loss. The amounts of IL-1α, IL-1β, and IL-1 receptor antagonist from GCF were quantified by ELISA. It was observed that levels of bone resorption activity, IL-1α, IL-1β, and IL-1 receptor antagonist were significantly higher in GCF from diseased sites compared with healthy sites but did not relate to defect morphology [74].
The severity of periodontitis is associated with local (GCF or tissue) increases in IL-1β, TNF-α, prostaglandins such as prostaglandin E2[4], and MMPs [61,67,71], whereas inhibition of these substances produces substantial reductions in periodontal disease. Specifically for IL-1 and TNF, local protein blockade in a monkey model of periodontitis produced significant reductions in bone loss [75,76], highlighting the important role of these mediators in periodontal disease.
Advanced stages of periodontal lesions are populated by a large proportion of B lymphocytes and plasma cells [77–79] and increased levels of immunoglobulins in GCF [54,80–82]. Plombas et al [83] investigated GCF and whole saliva from periodontitis patients and periodontally healthy adults for the presence of IgA and IgG antibodies to Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum. Compared with healthy patients, the GCF of periodontitis patients contained significantly higher levels of IgA and IgG antibodies to the four microorganisms tested.
Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans are examples of well-studied gram-negative pathogens implicated in the immune and inflammatory host response in periodontal disease [84]. Porphyromonas gingivalis produces by far the greatest proteolytic activity through peptidases, elastases, trypsinlike proteases, and collagenases [85] that can be monitored by GCF analysis [86,87]. Figueredo et al [62] compared elastase and collagenase activities in GCF before and after nonsurgical periodontal treatment. Improvement in clinical parameters after therapy was accompanied by a significant reduction in the values of total elastase activity, free elastase, MMP-8, and collagenolytic activity in gingivitis and periodontitis sites.
Aspartate aminotransferase, a tissue destruction biomarker released from necrotic cells in GCF, is associated with periodontitis severity [88,89]. Aspartate aminotransferase—positive sites are positively correlated with higher prevalence of Porphyromonas gingivalis, Streptococcus intermedius, Peptostreptococcus micros, Campylobacter concisus, Bacteroides forsythus, Camplobacter gracilis, Campylobacter rectus, and Selenomonas sputigena [90]. Moreover, to evaluate the relationship between aspartate aminotransferase and periodontal disease, periodontitis subjects were monitored for 12 months using a chairside assay. After nonsurgical therapy, the percentage of sites exhibiting higher levels of aspartate aminotransferase and bleeding on probing was significantly lower at 6 and 12 months compared with baseline. Elevated levels of aspartate aminotransferase, however, were present at sites that did not subsequently exhibit disease progression [89]. Therefore, the biomarker does not discriminate between progressive sites and sites that are stable but inflamed.
In summary, GCF carries multiple molecular factors derived from the host response and is considered a significant protective mechanism in periodontal infection (Table 3) [91]. These host response factors represent important mediators that can aid in the development of periodontal diagnostics.
Table 3.
Examples of biomarkers of periodontal disease identified from plaque biofilm, gingival crevicular fluid, or saliva
| Category mediator | Examples |
|---|---|
| Microbial factors | DNA probes or culturing of putative periodontal pathogens (eg, Porphyromonas gingivalis, Tanerella forsythensis, Treponema denticola) |
| Host response factors | IL-1β; TNF-α; aspartate aminotransferase; elastase |
| Connective tissue breakdown products |
Collagen telopeptides; osteocalcin; proteoglycans; fibronection fragments |
Bone-specific markers of tissue destruction for periodontal diagnosis
Of the 50 or more different components in GCF and saliva evaluated to date for periodontal diagnosis, most lack specificity to alveolar bone destruction and essentially constitute soft tissue inflammatory events [92]. When examining the destruction of alveolar bone that is preceded by a microbial infection and inflammatory response, the measurement of connective tissue—derived molecules may lead to a more accurate assessment of tissue breakdown due to the tremendous variability of the host response among individuals [92].
Advances in bone cell biology over the past decade have resulted in several new biochemical markers for the measurement of bone homeostasis. With mounting evidence for a relationship between osteoporosis and oral bone loss, investigators have sought to develop better biologic markers to determine and predict oral bone loss [93]. Two of the more well studied mediators (bone collagen fragments and osteocalcin) are presented in the following text.
Pyridinoline cross-linked carboxyterminal telopeptide of type I collagen
Type I collagen composes 90% of the organic matrix of bone and is the most abundant collagen in osseous tissue [94]. Collagen degradation products have emerged as valuable markers of bone turnover in a multitude of bone resorptive and metabolic diseases [95]. Pyridinoline cross-links represent a class of collagen degradative molecules that include pyridinoline, deoxypyridinoline, N-telopeptides, and C-telopeptides [96]. Pyridinoline and deoxypyridinoline are mature intermolecular cross-links of collagen. Subsequent to osteoclastic bone resorption and collagen matrix degradation, pyridinoline, deoxypyridinoline, and amino- and carboxyterminal cross-linked telopeptides of type I collagen are released into the circulation. Because the cross-linked telopeptides result from post-translational modification of collagen molecules, they cannot be reused during collagen synthesis and are therefore considered specific biomarkers for bone resorption [97].In addition, the value of pyridinoline cross-links as potential markers of bone turnover relates to their specificity for bone. In skin and other soft tissues, histidine cross-links are the predominant form and no pyridinoline-like structures exist. Recently, a degradation fragment originating from thehelical part of type I collagen and consisting of the 620–633 sequence of the α1 chain has been identified to correlate highly with amino- and carboxyterminal telopeptides associated with bone resorption [98].
The pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a 12- to 20-kd fragment of bone type I collagen released by digestion with trypsin or bacterial collagenase [99]. Elevated serum ICTP and other pyridinoline cross-linked components have been shown to be correlated with the bone resorptive rate in several bone metabolic diseases including osteoporosis [100], rheumatoid arthritis [101], and Paget’s disease[102]. Furthermore, pyridinoline cross-links demonstrated significant decreases in postmenopausal osteoporotic subjects after bisphosphonate [103] or estrogen [104] therapy.
Given their specificity for bone resorption, pyridinoline cross-links represent a potentially valuable diagnostic aid in periodontics because biochemical markers specific for bone degradation may be useful in differentiating between the presence of gingival inflammation and active periodontal or peri-implant bone destruction [105]. Several investigations have explored the ability of pyridinoline cross-links to detect bone resorption in periodontitis lesions [39,63,106–108], in peri-implantitis [40], and in response to periodontal therapy [40,61,66,107,109–111].
Palys et al [39] related ICTP levels to the subgingival microflora of various disease states on GCF. Subjects were divided into groups representing health, gingivitis, and chronic periodontitis, and GCF and plaque samples were collected from each subject. The samples were analyzed for ICTP levels and the presence of 40 subgingival species using checkerboard DNA-DNA hybridization techniques. ICTP levels differed significantly between health, gingivitis, and periodontitis subjects, and related modestly to several clinical disease parameters. ICTP levels were also strongly correlated with whole-subject levels of several periodontal pathogens including Tanerella forsythensis, Porphyromonas gingivalis, Prevotella intermedia, and Treponema denticola. Oringer et al [40] examined the relationship between ICTP levels and subgingival species around implants and teeth in 20 partially edentulous and 2 fully edentulous patients. No significant differences were found among ICTP levels and subgingival plaque composition between implants and teeth. Strong correlations were found between elevated ICTP levels at implant sites and colonization with organisms associated with failing implants such as Prevotella intermedia, Fusobacterim nucleatum subsp vincentii, and Streptococcus gordonii [40].
Diagnostic tools have also been applied to evaluate the response to active periodontal therapy. Golub et al [63] found that treatment of chronic periodontitis patients with scaling and root planing (SRP) and an MMP inhibitor (subantimicrobial doxycycline hyclate) resulted in a 70% reduction in GCF ICTP levels after 1 month, concomitant with a 30% reduction in collagenase levels. An investigation of periodontitis patients treated with SRP also demonstrated significant correlations between GCF ICTP levels and clinical periodontal disease parameters, including attachment loss, pocket depth, and bleeding on probing [93]. In addition, elevated GCF ICTP levels at baseline, especially at shallow sites, were found to be predictive for future attachment loss as early as 1 month after sampling. Furthermore, treatment of a group of periodontitis subjects by SRP and locally delivered minocycline led to rapid reductions in GCF ICTP levels [63].
Studies assessing the role of GCF ICTP levels as a diagnostic marker of periodontal disease activity have produced promising results to date. ICTP has been shown to be a good predictor of future alveolar bone and attachment loss, was strongly correlated with clinical parameters and putative periodontal pathogens, and demonstrated significant reductions after periodontal therapy [92]. Controlled human longitudinal trials are needed to fully establish the role of ICTP as a predictor of periodontal tissue destruction, disease activity, and response to therapy in periodontal patients.
Osteocalcin
Osteocalcin is a calcium-binding protein of bone and is the most abundant noncollagenous protein in mineralized tissues [112]. Osteocalcin is synthesized predominantly by osteoblasts [113] and has an important role in bone formation and turnover [114,115]. Osteocalcin exhibits chemoattractive activity for osteoclast progenitor cells and monocytes [116–118], and its synthesis in vitro is stimulated by 1,25-dihydroxyvitamin D3. It has also been shown to promote bone resorption, and stimulate differentiation of osteoclast progenitor cells [112,119]. Elevated serum osteocalcin levels have been shown during periods of rapid bone turnover (eg, osteoporosis, multiple myeloma, and fracture repair) [120,121]. Serum osteocalcin is presently a valid marker of bone turnover when resorption and formation are coupled and is a specific marker of bone formation when formation and resorption are uncoupled [115,120,122,123].
Several studies have investigated the relationship between GCF osteocalcin levels and periodontal disease [49,63,106,124–126]. Kunimatsu et al [124] reported a positive correlation between GCF osteocalcin aminoterminal peptide levels and clinical parameters in a cross-sectional study of periodontitis and gingivitis patients. The investigators also reported that osteocalcin could not be detected in patients with gingivitis. In contrast, Nakashima et al [126] reported significant GCF osteocalcin levels from periodontitis and gingivitis patients. Osteocalcin levels were also significantly correlated with pocket depth, gingival index scores, and GCF levels of alkaline phosphatase and prostaglandin E2. In a longitudinal study of untreated periodontitis patients with ≥1.5 mm attachment loss during the monitoring period, GCF osteocalcin levels alone were unable to distinguish between active and inactive sites [49]. When a combination of the biochemical markers osteocalcin, collagenase, prostaglandin E2, α2-macro-globulin, elastase, and alkaline phosphatase was evaluated, however, increased diagnostic sensitivity and specificity values of 80% and 91%, respectively, were reported [49].
A longitudinal study using an experimental periodontitis model in beagle dogs reported a strong correlation between GCF osteocalcin levels and active bone turnover as assessed by bone-seeking radio pharmaceutical uptake [106]. Osteocalcin, however, was shown to possess only modest predictive value for future bone loss measured by computer-assisted digitizing radiography. Moreover, treatment of chronic periodontitis patients with subantimicrobial doxycycline failed to reduce GCF osteocalcin levels [63], and a cross-sectional study of periodontitis patients reported no differences in GCF osteocalcin levels between deep and shallow sites in the same patients [125]. In addition, osteocalcin levels in the GCF during orthodontic tooth movement were highly variable between subjects and lacked a consistent pattern related to the stages of tooth movement [127]. Taken together, the results of these studies show a potential role for intact osteocalcin as a bone-specific marker of bone turnover but not as a predictive indicator for periodontal disease. Greater promise appears to be in the detection of aminoterminal osteocalcin fragments for periodontal disease detection. Additional longitudinal studies may be warranted to more fully elucidate the utility of osteocalcin as a periodontal disease activity diagnostic aid.
Role of oral fluid biomarkers in periodontal diagnosis
A biomarker or biologic marker, according to the most recent definition[128], is a substance that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. Because saliva and GCF are fluids easily collected and contain locally and systemically derived markers of periodontal disease, they may offer the basis for a patient-specific biomarker assessment for periodontitis and other systemic diseases [18,129].
Due to the noninvasive and simple nature of their collection, analysis of saliva and GCF may be especially beneficial in the determination of current periodontal status and a means of monitoring response to treatment[130,131]. Many studies have shown that the determination of inflammatory mediator levels in biologic fluids is a good indicator of inflammatory activity. Therefore, studies related to the pathogenesis of periodontal diseases usually examine whether biochemical and immunologic markers in saliva or GCF might reflect the extent of periodontal destruction and possibly predict future disease progression [18,129]. Oral fluid biomarkers that have been studied for periodontal diagnosis include proteins of hostorigin (ie, enzymes and immunoglobulins), phenotypic markers, host cells, hormones, bacteria and bacterial products, ions, and volatile compounds[18,132–135]. Table 3 lists a sample of compounds obtained by diagnostic screening of saliva or GCF.
Future directions
There is a plethora of possibilities for the future use of oral fluids in biotechnology and health care applications, especially in the field of diagnostics. A tremendous amount of research activity is currently under way to explore the role of oral fluids as a possible medium in a variety of applications.
Recent advances in HIV diagnosis have been made using oral fluids. A commercially available kit (OraSure, OraSure Technologies, Bethlehem, Pennsylvania) has an oral specimen collection device that is placed between the buccal mucosa and buccal gingiva for 2 to 5 minutes to collect HIV-1 antibodies (not the virus) from the tissues of the cheek and gingiva. OraSure HIV-1 does not collect saliva but rather a sample called oral mucosal transudate. For different fluids (oral fluid, finger-stick or venipuncture whole blood or plasma specimens), the alternative test OraQuick (OraSure Technologies) provides accurate results for HIV-1 and HIV-2 in 20 minutes. The collector pad is placed in a vial with preservative and sent to a clinical laboratory for testing with an initial “screening” assay (ELISA). If necessary, a supplementary test (Western blot assay) is performed to verify the results of the screening assay. This process is referred to as the OraSure testing algorithm [136].
Several researchers have focused on genetic single nucleotide polymorphisms in the study of periodontitis. There is a genetic susceptibility test currently available for severe chronic periodontitis (Interleukin Genetics, Waltham, Massachusetts). This system works by detection of two types of IL-1 genetic alleles, IL-1α +4845 and IL-1β +3954 [137]. Individuals identified as “genotype positive,” or found to have both of these alleles, are more likely to have the phenotype of overexpression of this gene. The increased GCF and salivary IL-1 predisposes the patient to the severe form of chronic periodontitis by way of a hyperinflammatory response to bacterial challenge. In this way, genomics has been found to be applicable in the prediction of predisposition to periodontitis in certain patient populations [138]. Socransky et al [139] took a different approach in researching IL-1 gene polymorphisms in periodontitis patients. These investigators linked previous findings regarding the association of IL-1 polymorphisms and severity of adult periodontitis with microbial species found in IL-1 genotype-negative versus IL-1 genotype-positive patients. These researchers concluded that those who were IL-1 genotype positive tended to have higher levels of the more damaging microbial species (redand orange complex organisms) associated with periodontal inflammation[139].
Li et al [140] investigated the potential use of genomics in the development of salivary diagnostics. They performed microarray testing of cell-free saliva for RNA profiling. RNA was isolated from unstimulated saliva that was collected from healthy subjects. After analysis by microarray and quantitative polymerase chain reaction, they found that it was possible to profile messenger RNAs, of which there were thousands present in the saliva. More recently, the group demonstrated the potential of salivary IL-8 levels to predict patients afficted with squamous cell carcinoma [141].
Salivary immunocomponents have also been studied at length in oral health, including immunoglobulin subclass, immunoglobulin isotypes, and antibody levels [142–148]. Other salivary constituents that have been investigated for diagnostic uses include epithelial keratins [149], occult blood [150], salivary ions such as calcium and phosphates [151,152], and serum markers such as cortisol [153–155].
Summary
Researchers in the biotechnology and medical realm are currently investigating the use of oral fluids for the diagnosis of oral and systemic diseases and for drug development. In the pharmaceutical industry, the use of biomarkers is avidly being developed for use in tailored dosing and drug metabolism studies. Professionals in seemingly unrelated arenas such as the insurance industry, the Environment Protection Agency, and Homeland Security are interested in the possible use of oral fluids to monitor biomarkers. Under investigation are possible uses of GCF and saliva in the preliminary screening for biological/chemical warfare agent exposure, environmental toxin detection, and screening for metabolites of drugs of abuse.
In the field of oral disease diagnosis, there has been a steady growing trend during the last 2 decades to develop tools to monitor periodontitis. From physical measurements such as periodontal probing to sophisticated genetic susceptibility analysis and molecular assays for the detection of biomarkers on the different stages of the disease, substantial improvements have been made on the understanding of the mediators implicated on the initiation and progression of periodontitis. At the same time, this evolutionary process has promoted the discovery of new biomarkers and the development of new therapeutic approaches mainly using host modulation. Moreover, new diagnostic technologies such as nucleic acid and protein microarrays and microfluidics are under development for risk assessment and comprehensive screening of biomarkers. These recent advances are leading to the development of more powerful diagnostic tools for practitioners to optimize their treatment predictability (Fig. 2).
Fig. 2.
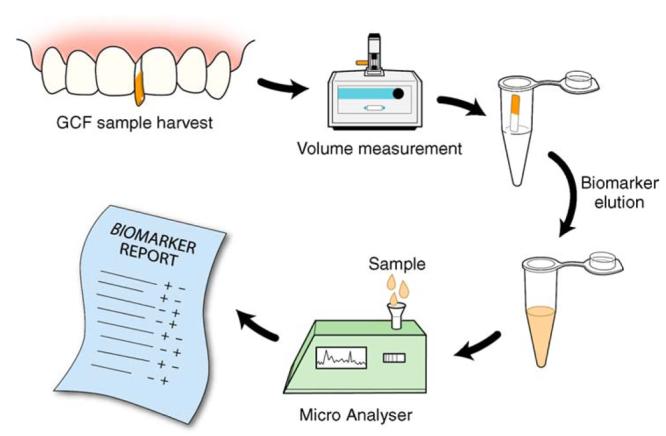
Futuristic chairside diagnostic test based on GCF sampling. Considering the GCF fluid as a potential analyte for the screening of multiple biomarkers, a rapid, chairside diagnostic tool (represented in the figure as a Micro Analyser) or a “mini-lab” could be used by clinicians for risk assessment and decision making on treatment planning. The advantages of such a tool would be enhanced predictability of clinical outcomes and well-informed patients regarding personalized treatment needs. As shown, a simple clinical procedure for GCF collection could be used, followed by extraction of analytes from the test strip. The fluid present on the test strip would be subjected to volumetric quantification. After an elution procedure to “wash” and retrieve the compounds from the fluid, the sample would be analyzed. An immediate comprehensive risk report profile and biomarkers screening would enable evidence-based decision making.
Acknowledgments
This work was supported by NIDCR grants U01-DE14961 and R43-DE14810 to W.V. Giannobile.
References
- [1].Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(4 Suppl):322–31. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- [2].Genco RJ. Host responses in periodontal diseases: current concepts. J Periodontol. 1992;63(Suppl 4):338–55. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- [3].Kirkwood KL, Taba M, Jr, Rossa C, et al. Molecular biology of the host-microbe interaction in periodontal diseases. Selected topics: molecular signaling aspects of pathogen-mediated bone destruction in periodontal diseases. In: Newman M, Takei H, editors. Carranza’s periodontology. 10th Elsevier; St. Louis (MO): ; in press.
- [4].Offenbacher S. Periodontal diseases: pathogenesis. Ann Periodontol. 1996;1(1):821–78. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- [5].Albandar JM. Periodontal diseases in North America. Periodontol 2000. 2002;29:31–69. doi: 10.1034/j.1600-0757.2002.290103.x. [DOI] [PubMed] [Google Scholar]
- [6].Armitage GC. The complete periodontal examination. Periodontol 2000. 2004;34:22–33. doi: 10.1046/j.0906-6713.2002.003422.x. [DOI] [PubMed] [Google Scholar]
- [7].Goodson JM. Conduct of multicenter trials to test agents for treatment of periodontitis. J Periodontol. 1992;63(Suppl 12):1058–63. doi: 10.1902/jop.1992.63.12s.1058. [DOI] [PubMed] [Google Scholar]
- [8].Page RC, Martin J, Krall EA, et al. Longitudinal validation of a risk calculator for periodontal disease. J Clin Periodontol. 2003;30(9):819–27. doi: 10.1034/j.1600-051x.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- [9].Genco RJ. Assessment of risk of periodontal disease. Compend Suppl. 1994;18:678–83. [PubMed] [Google Scholar]
- [10].Lamster IB. Current concepts and future trends for periodontal disease and periodontal therapy, part 2: classification, diagnosis, and nonsurgical and surgical therapy. Dent Today. 2001;20(3):86–91. [PubMed] [Google Scholar]
- [11].Lang NP, Tonetti MS.Periodontal diagnosis in treated periodontitis. Why, when and how to use clinical parameters J Clin Periodontol 1996233 Pt 2):240–50. [DOI] [PubMed] [Google Scholar]
- [12].Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67(Suppl 10):1041–9. doi: 10.1902/jop.1996.67.10s.1041. [DOI] [PubMed] [Google Scholar]
- [13].Colburn WA. Biomarkers in drug discovery and development: from target identification through drug marketing. J Clin Pharmacol. 2003;43(4):329–41. doi: 10.1177/0091270003252480. [DOI] [PubMed] [Google Scholar]
- [14].Kamma JJ, Giannopoulou C, Vasdekis VG, et al. Cytokine profile in gingival crevicular fluid of aggressive periodontitis: influence of smoking and stress. J Clin Periodontol. 2004;31(10):894–902. doi: 10.1111/j.1600-051X.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- [15].Souza SL, Taba M., Jr Cross-sectional evaluation of clinical parameters to select high prevalence populations for periodontal disease: the site comparative severity methodology. Braz Dent J. 2004;15(1):46–53. doi: 10.1590/s0103-64402004000100009. [DOI] [PubMed] [Google Scholar]
- [16].Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- [17].Uitto VJ. Gingival crevice fluid—an introduction. Periodontol 2000. 2003;31:9–11. doi: 10.1034/j.1600-0757.2003.03101.x. [DOI] [PubMed] [Google Scholar]
- [18].Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis—a review. J Clin Periodontol. 2000;27(7):453–65. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- [19].Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Loesche WJ, Lopatin DE, Giordano J, et al. Comparison of the benzoyl-DL-argininenaphthylamide (BANA) test, DNA probes, and immunological reagents for ability to detect anaerobic periodontal infections due to Porphyromonas gingivalis, Treponema denticola, and Bacteroides forsythus. J Clin Microbiol. 1992;30(2):427–33. doi: 10.1128/jcm.30.2.427-433.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Loesche WJ, Kazor CE, Taylor GW. The optimization of the BANA test as a screening instrument for gingivitis among subjects seeking dental treatment. J Clin Periodontol. 1997;24(10):718–26. doi: 10.1111/j.1600-051x.1997.tb00188.x. [DOI] [PubMed] [Google Scholar]
- [22].Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25(2):134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- [23].Zambon JJ. Periodontal diseases: microbial factors. Ann Periodontol. 1996;1(1):879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- [24].Listgarten MA, Loomer PM. Microbial identification in the management of periodontal diseases. A systematic review. Ann Periodontol. 2003;8(1):182–92. doi: 10.1902/annals.2003.8.1.182. [DOI] [PubMed] [Google Scholar]
- [25].Offenbacher S, Jared HL, O’Reilly PG, et al. Potential pathogenic mechanisms of periodontitis associated pregnancy complications. Ann Periodontol. 1998;3(1):233–50. doi: 10.1902/annals.1998.3.1.233. [DOI] [PubMed] [Google Scholar]
- [26].Muller HP, Streletz E, Muller RF, et al. Microbiologic diagnosis and treatment of periodontally involved, “hopeless” teeth. Int J Periodontics Restorative Dent. 1991;11(5):376–86. [PubMed] [Google Scholar]
- [27].Levy D, Csima A, Birek P, et al. Impact of microbiological consultation on clinical decision making: a case-control study of clinical management of recurrent periodontitis. J Periodontol. 1993;64(11):1029–39. doi: 10.1902/jop.1993.64.11.1029. [DOI] [PubMed] [Google Scholar]
- [28].Rosenberg ES, Torosian JP, Hammond BF, et al. Routine anaerobic bacterial culture and systemic antibiotic usage in the treatment of adult periodontitis: a 6-year longitudinal study. Int J Periodontics Restorative Dent. 1993;13(3):213–43. [PubMed] [Google Scholar]
- [29].Fine DH. Microbial identification and antibiotic sensitivity testing, an aid for patients refractory to periodontal therapy. A report of 3 cases. J Clin Periodontol. 1994;21(2):98–106. doi: 10.1111/j.1600-051x.1994.tb00286.x. [DOI] [PubMed] [Google Scholar]
- [30].Ishikawa I, Umeda M, Laosrisin N. Clinical, bacteriological, and immunological examinations and the treatment process of two Papillon-Lefevre syndrome patients. J Periodontol. 1994;65(4):364–71. doi: 10.1902/jop.1994.65.4.364. [DOI] [PubMed] [Google Scholar]
- [31].Ishikawa I, Kawashima Y, Oda S, et al. Three case reports of aggressive periodontitis associated with Porphyromonas gingivalis in younger patients. J Periodontal Res. 2002;37(5):324–32. doi: 10.1034/j.1600-0765.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- [32].Renvert S, Dahlen G, Wikstrom M. Treatment of periodontal disease based on microbiological diagnosis. Relation between microbiological and clinical parameters during 5 years. J Periodontol. 1996;67(6):562–71. doi: 10.1902/jop.1996.67.6.562. [DOI] [PubMed] [Google Scholar]
- [33].Worch KP, Listgarten MA. Treatment considerations in rapidly progressive periodontitis: a case report. Compend Contin Educ Dent. 1998;19(12):1203–6. [PubMed] [Google Scholar]
- [34].Worch KP, Listgarten MA, Korostoff JM. A multidisciplinary approach to the diagnosis and treatment of early-onset periodontitis: a case report. J Periodontol. 2001;72(1):96–106. doi: 10.1902/jop.2001.72.1.96. [DOI] [PubMed] [Google Scholar]
- [35].Kamma JJ, Lygidakis NA, Nakou M. Subgingival microflora and treatment in prepubertal periodontitis associated with chronic idiopathic neutropenia. J Clin Periodontol. 1998;25(9):759–65. doi: 10.1111/j.1600-051x.1998.tb02518.x. [DOI] [PubMed] [Google Scholar]
- [36].De Vree H, Steenackers K, De Boever JA. Periodontal treatment of rapid progressive periodontitis in 2 siblings with Papillon-Lefevre syndrome: 15-year follow-up. J Clin Periodontol. 2000;27(5):354–60. doi: 10.1034/j.1600-051x.2000.027005354.x. [DOI] [PubMed] [Google Scholar]
- [37].Eickholz P, Kugel B, Pohl S, et al. Combined mechanical and antibiotic periodontal therapy in a case of Papillon-Lefevre syndrome. J Periodontol. 2001;72(4):542–9. doi: 10.1902/jop.2001.72.4.542. [DOI] [PubMed] [Google Scholar]
- [38].Pacheco JJ, Coelho C, Salazar F, et al. Treatment of Papillon-Lefevre syndrome periodontitis. J Clin Periodontol. 2002;29(4):370–4. doi: 10.1034/j.1600-051x.2002.290414.x. [DOI] [PubMed] [Google Scholar]
- [39].Palys MD, Haffajee AD, Socransky SS, et al. Relationship between C-telopeptide pyridinoline cross-links (ICTP) and putative periodontal pathogens in periodontitis J Clin Periodontol 19982511 Pt 1):865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oringer RJ, Palys MD, Iranmanesh A, et al. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res. 1998;9(6):365–73. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Novaes AB, Junior, Souza SL, Taba M, Jr, et al. Control of gingival inflammation in a teenager population using ultrasonic prophylaxis. Braz Dent J. 2004;15(1):41–5. doi: 10.1590/s0103-64402004000100008. [DOI] [PubMed] [Google Scholar]
- [42].Madianos PN, Papapanou PN, Sandros J. Porphyromonas gingivalis infection of oral epithelium inhibits neutrophil transepithelial migration. Infect Immun. 1997;65(10):3983–90. doi: 10.1128/iai.65.10.3983-3990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34(3):235–49. [PubMed] [Google Scholar]
- [44].Soder B, Jin LJ, Wickholm S. Granulocyte elastase, matrix metalloproteinase-8 and prostaglandin E2 in gingival crevicular fluid in matched clinical sites in smokers and non-smokers with persistent periodontitis. J Clin Periodontol. 2002;29(5):384–91. doi: 10.1034/j.1600-051x.2002.290502.x. [DOI] [PubMed] [Google Scholar]
- [45].Golub LM, Kleinberg I. Gingival crevicular fluid: a new diagnostic aid in managing the periodontal patient. Oral Sci Rev. 1976;8:49–61. [PMC free article] [PubMed] [Google Scholar]
- [46].Golub LM, McNamara TF, Ryan ME, et al. Adjunctive treatment with subantimicrobial doses of doxycycline: effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J Clin Periodontol. 2001;28(2):146–56. doi: 10.1034/j.1600-051x.2001.028002146.x. [DOI] [PubMed] [Google Scholar]
- [47].Cimasoni G. Crevicular fluid updated. Monogr Oral Sci. 1983;12(IIIVII):1–152. [PubMed] [Google Scholar]
- [48].Estreicher A, Broggiato A, Duroux P, et al. Low molecular-weight proteins in human gingival crevicular fluid. Arch Oral Biol. 1996;41(8–9):733–8. doi: 10.1016/s0003-9969(96)00076-3. [DOI] [PubMed] [Google Scholar]
- [49].Nakashima K, Giannopoulou C, Andersen E, et al. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23(9):832–8. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- [50].Kojima T, Andersen E, Sanchez JC, et al. Human gingival crevicular fluid contains MRP8 (S100A8) and MRP14 (S100A9), two calcium-binding proteins of the S100 family. J Dent Res. 2000;79(2):740–7. doi: 10.1177/00220345000790020701. [DOI] [PubMed] [Google Scholar]
- [51].Engebretson SP, Hey-Hadavi J, Ehrhardt FJ, et al. Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol. 2004;75(9):1203–8. doi: 10.1902/jop.2004.75.9.1203. [DOI] [PubMed] [Google Scholar]
- [52].Lamster IB, Kaufman E, Grbic JT, et al. Beta-glucuronidase activity in saliva: relationship to clinical periodontal parameters. J Periodontol. 2003;74(3):353–9. doi: 10.1902/jop.2003.74.3.353. [DOI] [PubMed] [Google Scholar]
- [53].Engebretson SP, Grbic JT, Singer R, et al. GCF IL-1beta profiles in periodontal disease. J Clin Periodontol. 2002;29(1):48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- [54].Grbic JT, Lamster IB, Fine JB, et al. Changes in gingival crevicular fluid levels of immunoglobulin A following therapy: association with attachment loss. J Periodontol. 1999;70(10):1221–7. doi: 10.1902/jop.1999.70.10.1221. [DOI] [PubMed] [Google Scholar]
- [55].Grbic JT, Lamster IB, Mitchell-Lewis D. Inflammatory and immune mediators in crevicular fluid from HIV-infected injecting drug users. J Periodontol. 1997;68(3):249–55. doi: 10.1902/jop.1997.68.3.249. [DOI] [PubMed] [Google Scholar]
- [56].Gustafsson A, Asman B, Bergstrom K. Elastase and lactoferrin in gingival crevicular fluid: possible indicators of a granulocyte-associated specific host response. J Periodontal Res. 1994;29(4):276–82. doi: 10.1111/j.1600-0765.1994.tb01222.x. [DOI] [PubMed] [Google Scholar]
- [57].Lerner UH, Modeer T, Krekmanova L, et al. Gingival crevicular fluid from patients with periodontitis contains bone resorbing activity. Eur J Oral Sci. 1998;106(3):778–87. doi: 10.1046/j.0909-8836.1998.eos106304.x. [DOI] [PubMed] [Google Scholar]
- [58].Rasmussen L, Hanstrom L, Lerner UH. Characterization of bone resorbing activity in gingival crevicular fluid from patients with periodontitis. J Clin Periodontol. 2000;27(1):41–52. doi: 10.1034/j.1600-051x.2000.027001041.x. [DOI] [PubMed] [Google Scholar]
- [59].Shapiro L, Goldman H, Bloom A. Sulcular exudate flow in gingival inflammation. J Periodontol. 1979;50(6):301–4. doi: 10.1902/jop.1979.50.6.301. [DOI] [PubMed] [Google Scholar]
- [60].Novaes AB, Jr, Shapiro L, Fillios LC, et al. Gingival fluid fucose to protein ratios as indicators of the severity of periodontal disease. J Periodontol. 1980;51(2):88–94. doi: 10.1902/jop.1980.51.2.88. [DOI] [PubMed] [Google Scholar]
- [61].Gapski R, Barr JL, Sarment DP, et al. Effect of systemic matrix metalloproteinase inhibition on periodontal wound repair: a proof of concept trial. J Periodontol. 2004;75(3):441–52. doi: 10.1902/jop.2004.75.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Figueredo CM, Areas A, Miranda LA, et al. The short-term effectiveness of non-surgical treatment in reducing protease activity in gingival crevicular fluid from chronic periodontitis patients. J Clin Periodontol. 2004;31(8):615–9. doi: 10.1111/j.1600-051X.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- [63].Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46(8):310–9. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]
- [64].Kinane DF, Darby IB, Said S, et al. Changes in gingival crevicular fluid matrix metalloproteinase-8 levels during periodontal treatment and maintenance. J Periodontal Res. 2003;38(4):400–4. doi: 10.1034/j.1600-0765.2003.00663.x. [DOI] [PubMed] [Google Scholar]
- [65].Mantyla P, Stenman M, Kinane DF, et al. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. J Periodontal Res. 2003;38(4):436–9. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- [66].Oringer RJ, Al-Shammari KF, Aldredge WA, et al. Effect of locally delivered minocycline microspheres on markers of bone resorption. J Periodontol. 2002;73(8):835–42. doi: 10.1902/jop.2002.73.8.835. [DOI] [PubMed] [Google Scholar]
- [67].Ryan ME, Ramamurthy S, Golub LM. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol. 1996;3:85–96. [PubMed] [Google Scholar]
- [68].Ingman T, Tervahartiala T, Ding Y, et al. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23(12):1127–32. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- [69].Mellanen L, Ingman T, Lahdevirta J, et al. Matrix metalloproteinases-1, -3 and -8 and myeloperoxidase in saliva of patients with human immunodeficiency virus infection. Oral Dis. 1996;2(4):263–71. doi: 10.1111/j.1601-0825.1996.tb00236.x. [DOI] [PubMed] [Google Scholar]
- [70].Teronen O, Konttinen YT, Lindqvist C, et al. Inhibition of matrix metalloproteinase-1 by dichloromethylene bisphosphonate (clodronate) Calcif Tissue Int. 1997;61(1):59–61. doi: 10.1007/s002239900295. [DOI] [PubMed] [Google Scholar]
- [71].Makela M, Salo T, Uitto VJ, et al. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994;73(8):1397–406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- [72].Amar S, Oyaisu K, Li L, et al. Moesin: a potential LPS receptor on human monocytes. J Endotoxin Res. 2001;7(4):281–6. [PubMed] [Google Scholar]
- [73].Mogi M, Otogoto J, Ota N, et al. Interleukin 1 beta, interleukin 6, beta 2-microglobulin, and transforming growth factor-alpha in gingival crevicular fluid from human periodontal disease. Arch Oral Biol. 1999;44(6):535–9. doi: 10.1016/s0003-9969(99)00020-5. [DOI] [PubMed] [Google Scholar]
- [74].Holmlund A, Hanstrom L, Lerner UH. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. J Clin Periodontol. 2004;31(6):475–82. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- [75].Assuma R, Oates T, Cochran D, et al. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160(1):403–9. [PubMed] [Google Scholar]
- [76].Graves DT, Delima AJ, Assuma R, et al. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontol. 1998;69(12):1419–25. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- [77].Ranney RR.Immunologic mechanisms of pathogenesis in periodontal diseases: an assessment J Periodontal Res 1991263 Pt 2):243–54. [DOI] [PubMed] [Google Scholar]
- [78].Seymour GJ, Cole KL, Powell RN. Analysis of lymphocyte populations extracted from chronically inflamed human periodontal tissues. I. Identification. J Periodontal Res. 1985;20(1):47–57. doi: 10.1111/j.1600-0765.1985.tb00410.x. [DOI] [PubMed] [Google Scholar]
- [79].Seymour GJ, Cole KL, Powell RN. Analysis of lymphocyte populations extracted from chronically inflamed human periodontal tissues. II. Blastogenic response. J Periodontal Res. 1985;20(6):571–9. doi: 10.1111/j.1600-0765.1985.tb00841.x. [DOI] [PubMed] [Google Scholar]
- [80].Condorelli F, Scalia G, Cali G, et al. Isolation of Porphyromonas gingivalis and detection of immunoglobulin A specific to fimbrial antigen in gingival crevicular fluid. J Clin Microbiol. 1998;36(8):2322–5. doi: 10.1128/jcm.36.8.2322-2325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kinane DF, Takahashi K, Mooney J.Crevicular fluid and serum IgG subclasses and corresponding mRNA expressing plasma cells in periodontitis lesions J Periodontal Res 1997321 Pt 2):176–8. [DOI] [PubMed] [Google Scholar]
- [82].Dibart S, Eftimiadi C, Socransky S, et al. Rapid evaluation of serum and gingival crevicular fluid immunoglobulin G subclass antibody levels in patients with early-onset periodontitis using checkerboard immunoblotting. Oral Microbiol Immunol. 1998;13(3):166–72. doi: 10.1111/j.1399-302x.1998.tb00728.x. [DOI] [PubMed] [Google Scholar]
- [83].Plombas M, Gobert B, De March AK, et al. Isotypic antibody response to plaque anaerobes in periodontal disease. J Periodontol. 2002;73(12):1507–11. doi: 10.1902/jop.2002.73.12.1507. [DOI] [PubMed] [Google Scholar]
- [84].Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- [85].Courant PR, Bader H. Bacteroides melaninogenicus and its products in the gingiva of man. Periodontics. 1966;4(3):131–6. [PubMed] [Google Scholar]
- [86].Eley BM, Cox SW. Bacterial proteases in gingival crevicular fluid before and after periodontal treatment. Br Dent J. 1995;178(4):133–9. doi: 10.1038/sj.bdj.4808681. [DOI] [PubMed] [Google Scholar]
- [87].Eley BM, Cox SW. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV-like activities in gingival crevicular fluid: correlation with clinical parameters in untreated chronic periodontitis patients. J Periodontal Res. 1992;27(1):62–9. doi: 10.1111/j.1600-0765.1992.tb02087.x. [DOI] [PubMed] [Google Scholar]
- [88].Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2(1):123–37. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- [89].Oringer RJ, Howell TH, Nevins ML, et al. Relationship between crevicular aspartate aminotransferase levels and periodontal disease progression. J Periodontol. 2001;72(1):17–24. doi: 10.1902/jop.2001.72.1.17. [DOI] [PubMed] [Google Scholar]
- [90].Kamma JJ, Nakou M, Persson RG. Association of early onset periodontitis microbiota with aspartate aminotransferase activity in gingival crevicular fluid. J Clin Periodontol. 2001;28(12):1096–105. doi: 10.1034/j.1600-051x.2001.281203.x. [DOI] [PubMed] [Google Scholar]
- [91].Takahashi K, Mooney J, Frandsen EV, et al. IgG and IgA subclass mRNA-bearing plasma cells in periodontitis gingival tissue and immunoglobulin levels in the gingival crevicular fluid. Clin Exp Immunol. 1997;107(1):158–65. doi: 10.1046/j.1365-2249.1997.d01-891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Giannobile WV. C-telopeptide pyridinoline cross-links. Sensitive indicators of periodontal tissue destruction. Ann N Y Acad Sci. 1999;878:404–12. doi: 10.1111/j.1749-6632.1999.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontol 2000. 2003;31:125–34. doi: 10.1034/j.1600-0757.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- [94].Narayanan AS, Page RC. Connective tissues of the periodontium: a summary of current work. Coll Relat Res. 1983;3(1):33–64. [PubMed] [Google Scholar]
- [95].Johnell O, Oden A, De Laet C, et al. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002;13(7):523–6. doi: 10.1007/s001980200068. [DOI] [PubMed] [Google Scholar]
- [96].Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17(4):333–68. doi: 10.1210/edrv-17-4-333. [DOI] [PubMed] [Google Scholar]
- [97].Eriksen EF, Charles P, Melsen F, et al. Serum markers of type I collagen formation and degradation in metabolic bone disease: correlation with bone histomorphometry. J Bone Miner Res. 1993;8(2):127–32. doi: 10.1002/jbmr.5650080202. [DOI] [PubMed] [Google Scholar]
- [98].Garnero P, Delmas PD. An immunoassay for type I collagen alpha 1 helicoidal peptide 620–633, a new marker of bone resorption in osteoporosis. Bone. 2003;32(1):20–6. doi: 10.1016/s8756-3282(02)00922-5. [DOI] [PubMed] [Google Scholar]
- [99].Risteli J, Elomaa I, Niemi S, et al. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: a new serum marker of bone collagen degradation. Clin Chem. 1993;39(4):635–40. [PubMed] [Google Scholar]
- [100].Colwell A, Russell RG, Eastell R. Factors affecting the assay of urinary 3-hydroxy pyridinium crosslinks of collagen as markers of bone resorption. Eur J Clin Invest. 1993;23(6):341–9. doi: 10.1111/j.1365-2362.1993.tb02034.x. [DOI] [PubMed] [Google Scholar]
- [101].Black D, Marabani M, Sturrock RD, et al. Urinary excretion of the hydroxypyridinium cross links of collagen in patients with rheumatoid arthritis. Ann Rheum Dis. 1989;48(8):641–4. doi: 10.1136/ard.48.8.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Uebelhart D, Gineyts E, Chapuy MC, et al. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone disease. Bone Miner. 1990;8(1):87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- [103].Garnero P, Gineyts E, Riou JP, et al. Assessment of bone resorption with a new marker of collagen degradation in patients with metabolic bone disease. J Clin Endocrinol Metab. 1994;79(3):780–5. doi: 10.1210/jcem.79.3.8077361. [DOI] [PubMed] [Google Scholar]
- [104].Yasumizu T, Hoshi K, Iijima S, et al. Serum concentration of the pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) is a useful indicator of decline and recovery of bone mineral density in lumbar spine: analysis in Japanese postmenopausal women with or without hormone replacement. Endocr J. 1998;45(1):45–51. doi: 10.1507/endocrj.45.45. [DOI] [PubMed] [Google Scholar]
- [105].Giannobile WV. Crevicular fluid biomarkers of oral bone loss. Curr Opin Periodontol. 1997;4:11–20. [PubMed] [Google Scholar]
- [106].Giannobile WV, Lynch SE, Denmark RG, et al. Crevicular fluid osteocalcin and pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) as markers of rapid bone turnover in periodontitis. A pilot study in beagle dogs. J Clin Periodontol. 1995;22(12):903–10. doi: 10.1111/j.1600-051x.1995.tb01793.x. [DOI] [PubMed] [Google Scholar]
- [107].Shibutani T, Murahashi Y, Tsukada E, et al. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. J Periodontol. 1997;68(4):385–91. doi: 10.1902/jop.1997.68.4.385. [DOI] [PubMed] [Google Scholar]
- [108].Talonpoika JT, Hamalainen MM. Type I collagen carboxyterminal telopeptide in human gingival crevicular fluid in different clinical conditions and after periodontal treatment. J Clin Periodontol. 1994;21(5):320–6. doi: 10.1111/j.1600-051x.1994.tb00720.x. [DOI] [PubMed] [Google Scholar]
- [109].Perez LA, Al-Shammari KF, Giannobile WV, et al. Treatment of periodontal disease in a patient with Ehlers-Danlos syndrome. A case report and literature review. J Periodontol. 2002;73(5):564–70. doi: 10.1902/jop.2002.73.5.564. [DOI] [PubMed] [Google Scholar]
- [110].Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72(11):1535–44. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- [111].Al-Shammari KF, Giannobile WV, Aldredge WA, et al. Effect of non-surgical periodontal therapy on C-telopeptide pyridinoline cross-links (ICTP) and interleukin-1 levels. J Periodontol. 2001;72(8):1045–51. doi: 10.1902/jop.2001.72.8.1045. [DOI] [PubMed] [Google Scholar]
- [112].Lian JB, Gundberg CM. Osteocalcin. Biochemical considerations and clinical applications. Clin Orthop. 1988;226:267–91. [PubMed] [Google Scholar]
- [113].Bronckers AL, Gay S, Dimuzio MT, et al. Immunolocalization of gamma-carboxyglutamic acid containing proteins in developing rat bones. Coll Relat Res. 1985;5(3):273–81. doi: 10.1016/s0174-173x(85)80017-0. [DOI] [PubMed] [Google Scholar]
- [114].Ducy P, Geoffroy V, Karsenty G. Study of osteoblast-specific expression of one mouse osteocalcin gene: characterization of the factor binding to OSE2. Connect Tissue Res. 1996;35(1–4):7–14. doi: 10.3109/03008209609029169. [DOI] [PubMed] [Google Scholar]
- [115].Garnero P, Delmas PD. Biochemical markers of bone turnover. Applications for osteoporosis. Endocrinol Metab Clin N Am. 1998;27(2):303–23. doi: 10.1016/s0889-8529(05)70007-4. [DOI] [PubMed] [Google Scholar]
- [116].Chenu C, Colucci S, Grano M, et al. Osteocalcin induces chemotaxis, secretion of matrix proteins, and calcium-mediated intracellular signaling in human osteoclast-like cells. J Cell Biol. 1994;127(4):1149–58. doi: 10.1083/jcb.127.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Glowacki J, Lian JB. Impaired recruitment and differentiation of osteoclast progenitors by osteocalcin-deplete bone implants. Cell Differ. 1987;21(4):247–54. doi: 10.1016/0045-6039(87)90479-9. [DOI] [PubMed] [Google Scholar]
- [118].Mundy GR, Poser JW. Chemotactic activity of the gamma-carboxyglutamic acid containing protein in bone. Calcif Tissue Int. 1983;35(2):164–8. doi: 10.1007/BF02405025. [DOI] [PubMed] [Google Scholar]
- [119].Canalis E. Effect of growth factors on bone cell replication and differentiation. Clin Orthop. 1985;193:246–63. [PubMed] [Google Scholar]
- [120].Bataille R, Delmas P, Sany J. Serum bone gla-protein in multiple myeloma. Cancer. 1987;59(2):329–34. doi: 10.1002/1097-0142(19870115)59:2<329::aid-cncr2820590227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [121].Slovik DM, Gundberg CM, Neer RM, et al. Clinical evaluation of bone turnover by serum osteocalcin measurements in a hospital setting. J Clin Endocrinol Metab. 1984;59(2):228–30. doi: 10.1210/jcem-59-2-228. [DOI] [PubMed] [Google Scholar]
- [122].Brown JP, Delmas PD, Malaval L, et al. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984;1(8386):1091–3. doi: 10.1016/s0140-6736(84)92506-6. [DOI] [PubMed] [Google Scholar]
- [123].Delmas PD, Chatelain P, Malaval L, et al. Serum bone GLA-protein in growth hormone deficient children. J Bone Miner Res. 1986;1(4):333–8. doi: 10.1002/jbmr.5650010406. [DOI] [PubMed] [Google Scholar]
- [124].Kunimatsu K, Mataki S, Tanaka H, et al. A cross-sectional study on osteocalcin levels in gingival crevicular fluid from periodontal patients. J Periodontol. 1993;64(9):865–9. doi: 10.1902/jop.1993.64.9.865. [DOI] [PubMed] [Google Scholar]
- [125].Lee AJ, Walsh TF, Hodges SJ, et al. Gingival crevicular fluid osteocalcin in adult periodontitis. J Clin Periodontol. 1999;26(4):252–6. doi: 10.1034/j.1600-051x.1999.260409.x. [DOI] [PubMed] [Google Scholar]
- [126].Nakashima K, Roehrich N, Cimasoni G. Osteocalcin, prostaglandin E2 and alkaline phosphatase in gingival crevicular fluid: their relations to periodontal status. J Clin Periodontol. 1994;21(5):327–33. doi: 10.1111/j.1600-051x.1994.tb00721.x. [DOI] [PubMed] [Google Scholar]
- [127].Griffiths GS, Moulson AM, Petrie A, et al. Evaluation of osteocalcin and pyridinium crosslinks of bone collagen as markers of bone turnover in gingival crevicular fluid during different stages of orthodontic treatment. J Clin Periodontol. 1998;25(6):492–8. doi: 10.1111/j.1600-051x.1998.tb02478.x. [DOI] [PubMed] [Google Scholar]
- [128].Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- [129].Ozmeric N. Advances in periodontal disease markers. Clin Chim Acta. 2004;343(1–2):1–16. doi: 10.1016/j.cccn.2004.01.022. [DOI] [PubMed] [Google Scholar]
- [130].Zambon JJ, Nakamura M, Slots J. Effect of periodontal therapy on salivary enzymatic activity. J Periodontal Res. 1985;20(6):652–9. doi: 10.1111/j.1600-0765.1985.tb00850.x. [DOI] [PubMed] [Google Scholar]
- [131].Hayakawa H, Yamashita K, Ohwaki K, et al. Collagenase activity and tissue inhibitor of metalloproteinases-1 (TIMP-1) content in human whole saliva from clinically healthy and periodontally diseased subjects. J Periodontal Res. 1994;29(5):305–8. doi: 10.1111/j.1600-0765.1994.tb01226.x. [DOI] [PubMed] [Google Scholar]
- [132].Ferguson DB. Current diagnostic uses of saliva. J Dent Res. 1987;66(2):420–4. doi: 10.1177/00220345870660020601. [DOI] [PubMed] [Google Scholar]
- [133].Lamster IB, Grbic JT. Diagnosis of periodontal disease based on analysis of the host response. Periodontol 2000. 1995;7:83–99. doi: 10.1111/j.1600-0757.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]
- [134].Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19(3):119–25. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- [135].Nakamura M, Slots J. Salivary enzymes. Origin and relationship to periodontal disease. J Periodontal Res. 1983;18(6):559–69. doi: 10.1111/j.1600-0765.1983.tb00393.x. [DOI] [PubMed] [Google Scholar]
- [136].Reynolds SJ, Muwonga J. OraQuick ADVANCE Rapid HIV-1/2 antibody test. Expert Rev Mol Diagn. 2004;4(5):587–91. doi: 10.1586/14737159.4.5.587. [DOI] [PubMed] [Google Scholar]
- [137].Kornman KS, Crane A, Wang HY, et al. The interleukin-1 genotype as a severity factor in adult periodontal disease. J Clin Periodontol. 1997;24(1):72–7. doi: 10.1111/j.1600-051x.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- [138].Greenstein G, Hart TC. Clinical utility of a genetic susceptibility test for severe chronic periodontitis: a critical evaluation. J Am Dent Assoc. 2002;133(4):452–9. doi: 10.14219/jada.archive.2002.0203.[quiz: 492–3].
- [139].Socransky SS, Haffajee AD, Smith C, et al. Microbiological parameters associated with IL-1 gene polymorphisms in periodontitis patients. J Clin Periodontol. 2000;27(11):810–8. doi: 10.1034/j.1600-051x.2000.027011810.x. [DOI] [PubMed] [Google Scholar]
- [140].Li Y, Zhou X, John MA, et al. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004;83(3):199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- [141].John MA, Li Y, Zhou X, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(8):929–35. doi: 10.1001/archotol.130.8.929. [DOI] [PubMed] [Google Scholar]
- [142].Aufricht C, Tenner W, Salzer HR, et al. Salivary IgA concentration is influenced by the saliva collection method. Eur J Clin Chem Clin Biochem. 1992;30(2):81–3. [PubMed] [Google Scholar]
- [143].Wilton JM, Curtis MA, Gillett IR, et al. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers from analysis of saliva. J Clin Periodontol. 1989;16(8):475–83. doi: 10.1111/j.1600-051x.1989.tb02323.x. [DOI] [PubMed] [Google Scholar]
- [144].Bokor M. Immunoglobulin A levels in the saliva in patients with periodontal disease. Med Pregl. 1997;50(1–2):9–11. [PubMed] [Google Scholar]
- [145].Anil S, Remani P, Beena VT, et al. Immunoglobulins in the saliva of diabetic patients with periodontitis. Ann Dent. 1995;54(1–2):30–3. [PubMed] [Google Scholar]
- [146].Schenck K, Poppelsdorf D, Denis C, et al. Levels of salivary IgA antibodies reactive with bacteria from dental plaque are associated with susceptibility to experimental gingivitis. J Clin Periodontol. 1993;20(6):411–7. doi: 10.1111/j.1600-051x.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- [147].Sandholm L, Tolo K, Olsen I. Salivary IgG, a parameter of periodontal disease activity? High responders to Actinobacillus actinomycetemcomitans Y4 in juvenile and adult periodontitis. J Clin Periodontol. 1987;14(5):289–94. doi: 10.1111/j.1600-051x.1987.tb01535.x. [DOI] [PubMed] [Google Scholar]
- [148].Nieminen A, Kari K, Saxen L. Specific antibodies against Actinobacillus actinomycetemcomitans in serum and saliva of patients with advanced periodontitis. Scand J Dent Res. 1993;101(4):196–201. doi: 10.1111/j.1600-0722.1993.tb01104.x. [DOI] [PubMed] [Google Scholar]
- [149].McLaughlin WS, Kirkham J, Kowolik MJ, et al. Human gingival crevicular fluid keratin at healthy, chronic gingivitis and chronic adult periodontitis sites. J Clin Periodontol. 1996;23(4):331–5. doi: 10.1111/j.1600-051x.1996.tb00554.x. [DOI] [PubMed] [Google Scholar]
- [150].Kopczyk RA, Graham R, Abrams H, et al. The feasibility and reliability of using a home screening test to detect gingival inflammation. J Periodontol. 1995;66(1):52–4. doi: 10.1902/jop.1995.66.1.52. [DOI] [PubMed] [Google Scholar]
- [151].Sewon L, Makela M. A study of the possible correlation of high salivary calcium levels with periodontal and dental conditions in young adults. Arch Oral Biol. 1990;35(Suppl):211–2. doi: 10.1016/0003-9969(90)90160-c. [DOI] [PubMed] [Google Scholar]
- [152].Sewon LA, Karjalainen SM, Sainio M, et al. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J Clin Periodontol. 1995;22(4):267–70. doi: 10.1111/j.1600-051x.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- [153].Genco RJ, Ho AW, Kopman J, et al. Models to evaluate the role of stress in periodontal disease. Ann Periodontol. 1998;3(1):288–302. doi: 10.1902/annals.1998.3.1.288. [DOI] [PubMed] [Google Scholar]
- [154].Beck JD. Risk revisited. Community Dent Oral Epidemiol. 1998;26(4):220–5. doi: 10.1111/j.1600-0528.1998.tb01954.x. [DOI] [PubMed] [Google Scholar]
- [155].Burt BA. Risk factors, risk markers, and risk indicators. Community Dent Oral Epidemiol. 1998;26(4):219. doi: 10.1111/j.1600-0528.1998.tb01957.x. [DOI] [PubMed] [Google Scholar]


