Abstract
Human Bartter syndrome IV is an autosomal recessive disorder characterized by congenital deafness and severe renal salt and fluid loss. It is caused by mutations in BSND, which encodes barttin, a β-subunit of ClC-Ka and ClC-Kb chloride channels. Inner-ear-specific disruption of Bsnd in mice now reveals that the positive potential, but not the high potassium concentration, of the scala media depends on the presence of these channels in the epithelium of the stria vascularis. The reduced driving force for K+-entry through mechanosensitive channels into sensory hair cells entails a profound congenital hearing loss and subtle vestibular symptoms. Although retaining all cell types and intact tight junctions, the thickness of the stria is reduced early on. Cochlear outer hair cells degenerate over several months. A collapse of endolymphatic space was seen when mice had additionally renal salt and fluid loss due to partial barttin deletion in the kidney. Bsnd−/− mice thus demonstrate a novel function of Cl− channels in generating the endocochlear potential and reveal the mechanism leading to deafness in human Bartter syndrome IV.
Keywords: anion transport, inner ear, otoacoustic emission, potassium recycling, Sox10
Introduction
During evolution, the cochlea has been tuned to be exquisitely sensitive to small changes in sound pressure that are transformed into vibrations of the organ of Corti by the mechanic properties of the middle ear and the cochlea (Forge and Wright, 2002; Brown et al, 2008). At the level of the sensory organ of Corti, hearing depends on two types of mechanosensitive cells (Figure 1A). Inner hair cells (IHCs) generate electrical signals that are conveyed to neurons of the spiral ganglion and then on to the brain. By contrast, the electrical response of outer hair cells (OHCs) primarily serves to drive the motor protein prestin in their lateral membrane. This leads to active contractions of OHCs that increase the mechanical vibrations in the organ of Corti in a positive feedback loop, enhancing the sensitivity of hearing by about 50 dB. Both types of hair cells respond to movements of their apical hair bundles by a modulation of cation influx through mechanosensitive channels. If this depolarizing influx were carried by Na+ as in nerve and muscle, Na+ would have to be extruded continuously by the energy-consuming Na,K-ATPase. This would require a vascularization of the organ of Corti that might interfere with its mechanical properties. Nature has chosen a different solution (Hibino and Kurachi, 2006; Wangemann, 2006): the depolarizing current across the apical membrane of hair cells is carried by K+, which can leave hair cells by diffusion through basal K+ channels including KCNQ4 (Kv 7.4) (Kubisch et al, 1999; Kharkovets et al, 2000, 2006) and BK channels (Rüttiger et al, 2004). K+ is then taken up by the closely apposed supporting cells, probably by the K–Cl cotransporters KCC4 (Boettger et al, 2002) and KCC3 (Boettger et al, 2003), and diffuses away through an epithelial gap junction system (Kikuchi et al, 2000). Neither of these transport steps requires a direct input of metabolic energy as the ions move passively along their (combined) electrochemical gradients.
Figure 1.
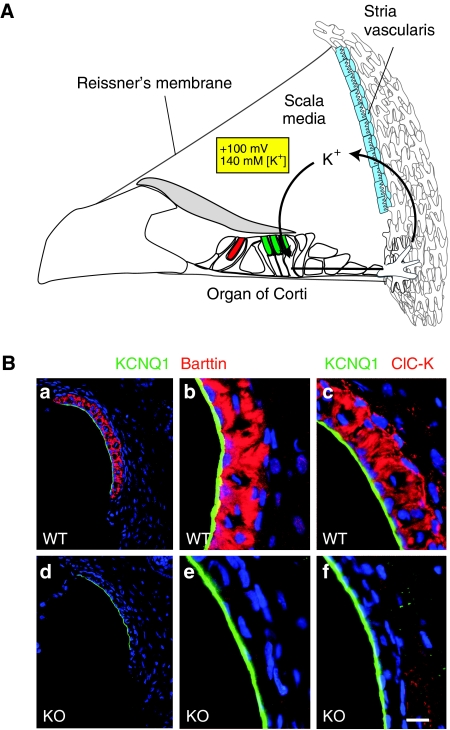
ClC-K and its β-subunit barttin in the cochlea. (A) Model for potassium recycling in the inner ear. The stria vascularis (blue) establishes the high K+ concentration of 140 mM and the positive potential of +100 mV of the endolymph that fills the cavity of the scala media. Both properties are important for the depolarizing K+ current through apical mechanosensitive channels of inner (red) and outer (green) hair cells. The apical membrane of these sensory cells contacts the endolymph, whereas their basolateral membrane (separated by tight junctions) is surrounded by perilymph that displays the usual low potassium concentration and zero potential of normal extracellular space. In the K+-recycling model, K+ is transported back to the stria vascularis through a gap junction system (Wangemann, 2006). (B) Immunohistochemistry on stria vascularis from WT (a–c) and Bsndlox/lox Sox10::Cre (d–f) mice with antibodies against barttin (a, b, d, e; red) and ClC-K (c, f; red). Apical membranes of marginal cells are stained with KCNQ1 (green). Nuclei are stained with TO-PRO-3 (blue). Scale bar: 65 μm (a, d) and 15 μm (b, c, e, f).
To enable a depolarizing influx of K+ through apical mechanosensitive cation channels of hair cells, the endolymph that fills the cavity of the scala media (and that contacts apical, but not basolateral, membranes of hair cells) is unusually rich in K+ (∼140 mM) and is held at a positive potential of +80 to +100 mV with respect to normal extracellular space (Hibino and Kurachi, 2006; Wangemann, 2006) (Figure 1A). Both properties are owed to the transport activity of the multilayered epithelium of the stria vascularis that is located in the lateral wall of the scala media. Nature has thereby put the ‘battery' that powers sound sensation at a convenient distance from the organ of Corti, where its high degree of vascularization cannot interfere with cochlear micromechanics.
Accordingly, impaired ion transport across the stria vascularis might cause deafness. Mutational loss of either the KCNQ1 or the KCNE1 subunit of the apical K+ channel of marginal cells causes deafness in mice and men (Vetter et al, 1996; Neyroud et al, 1997; Schulze-Bahr et al, 1997; Lee et al, 2000), as does the disruption of their basolateral NaK2Cl cotransporter in mice (Delpire et al, 1999). In all three cases, an impairment of strial K+ secretion entails a collapse of Reissner's membrane that separates the fluid space of the scala media from the scala vestibuli (Vetter et al, 1996; Delpire et al, 1999; Lee et al, 2000). More recently, mutations in the ClC-K Cl−-channel β-subunit barttin (Estévez et al, 2001) have been associated with Bartter syndrome type IV (Birkenhäger et al, 2001). This inherited disorder combines severe renal loss of salt and fluid with congenital sensorineural deafness. In the cochlea, ClC-K/barttin Cl− channels localize exclusively to the basolateral membrane of marginal cells of the stria (Estévez et al, 2001). We therefore suspected that they are needed for the secretion of K+ and fluid into the scala media by recycling Cl− that is taken up by the basolateral Nkcc1.
We now generated a mouse model in which barttin is deleted in the inner ear, but not in kidney. Similar to patients with Bartter IV, these mice display congenital deafness. Unlike mice with disruptions of either Kcnq1, Kcne1 or Nkcc1, these mice show neither a collapse of Reissner's membrane nor a circling behaviour indicative of a strong vestibular phenotype. Although sufficient levels of K+ and fluid secretion are maintained in the absence of ClC-K/barttin Cl− channels, their disruption leads to a drastic decrease in endocochlear potential (EP). This decrease is sufficient to cause a severe hearing loss and may also be responsible for subtle vestibular symptoms.
Results
Generation of conditional Bsnd knockout mice
We generated a conditional Bsnd knockout (KO) mouse (named Bsndlox/lox) by flanking exon 2 with loxP sites. Cre-mediated excision of this exon leads to a stop codon at position 74, deleting the functionally important second transmembrane domain and the cytoplasmic carboxy terminus (Estévez et al, 2001; Scholl et al, 2006). Constitutive KO mice were obtained by crossing these mice with Cre-deleter mice (Schwenk et al, 1995). Consistent with the symptoms of Bartter syndrome IV and with the proposed function of ClC-K/barttin Cl− channels in renal salt and fluid reabsorption (Estévez et al, 2001), these mice (the renal phenotype of which will be described elsewhere) were severely dehydrated. In contrast to properly treated infants with Bartter IV, these constitutive KO mice could not be kept alive for more than a few days after birth. To explore the function of barttin in the inner ear, it was therefore essential to identify transgenic mice that express the Cre-recombinase in the inner ear, but not in kidney. Attempts with several Cre lines (Nestin-cre (Tronche et al, 1999), FoxG1-Cre (Hébert and McConnell, 2000), Otog-Cre (Cohen-Salmon et al, 2002)) failed due to either a lack of deletion in the stria or a deletion of barttin in the kidney. For instance, when mating Bsndlox/lox mice with FoxG1::Cre mice that express the Cre-recombinase in the telencephalon, otic vesicle and other developing head structures (Hébert and McConnell, 2000), we observed a complete deletion of barttin in the stria. Unexpectedly, however, partial deletion occurred also in the kidney (data not shown). Although the renal salt and water loss of Bsndlox/lox FoxG1::Cre mice was more moderate than in the total KO mice, these mice did not survive till adulthood. From a large breeding colony, we were able to analyse 11 Bsndlox/lox FoxG1::Cre mice between postnatal day 6 (P6) and P15. As mice begin to hear at roughly 2 weeks of age, these mice are not suited to investigate the impact of barttin disruption on hearing.
We finally identified the Sox10-Cre line (Matsuoka et al, 2005) as deleting in the inner ear without causing a renal phenotype. Although barttin and ClC-K are expressed in the basolateral membranes of strial marginal cells in the WT (Estévez et al, 2001; Sage and Marcus, 2001) (Figure 1Ba–c), the staining for barttin was completely abolished in all marginal cells of Bsndlox/lox Sox10::Cre mice (Figure 1Bd and e). Importantly, these mice were not dehydrated, survived normally and had no immediately visible phenotype. The renal expression of barttin was examined by immunohistochemistry and appeared to be normal (data not shown). Furthermore, hearing was not affected in Sox::Cre mice carrying WT Bsnd alleles. Thus, Sox10::Cre mice are well suited to specifically delete barttin in the inner ear.
Immunohistochemistry revealed that loss of the β-subunit barttin led to a drastic reduction of ion-conducting ClC-K α-subunits (Figure 1Bf). Our new ClC-K antibody as well as the previously reported one (Kieferle et al, 1994; Estévez et al, 2001) cannot distinguish the highly related ClC-K1 and ClC-K2 isoforms (which correspond to ClC-Ka and ClC-Kb, respectively, in humans). RT–PCR and the phenotypic effects of gene deletions, however, strongly suggest that both isoforms are expressed in marginal cells (Estévez et al, 2001; Schlingmann et al, 2004). The lack of ClC-K immunostaining in the barttin KO therefore suggests that both α-subunits are unstable in the absence of their β-subunit and hence predicts an almost complete loss of associated Cl− currents.
Hearing loss of Bsndlox/lox Sox10::Cre mice
Auditory brain stem responses (ABR) in response to clicks were used to assess the hearing of Bsndlox/lox Sox10::Cre mice (Figure 2A). They displayed a hearing loss of about 60 dB when compared with control littermates. This hearing loss was already observed at the youngest age investigated (3 weeks, i.e., 1 week after the onset of hearing), remained stable over time and affected all frequency regions (data not shown). This hearing loss was less pronounced than in mice lacking Nkcc1 (Pace et al, 2000) (Figure 2A), the basolateral NaK2Cl cotransporter believed to need ClC-K/barttin channels for Cl− recycling (Estévez et al, 2001).
Figure 2.
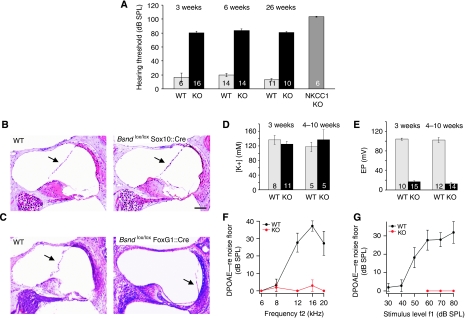
Hearing loss and endolymph of conditional barttin KO mice. (A) Hearing thresholds in 3-, 6- and 26-week-old WT and inner-ear-specific barttin KO mice (Bsndlox/lox Sox10::Cre mice) and 30-week-old Nkcc1−/− mice measured by auditory brainstem responses (ABR). Numbers in columns: number of measured ears; error bars: s.e.m. (B) Unchanged position of Reissner's membrane (arrows) in 1-year-old WT and Bsndlox/lox Sox10::Cre cochleae (HE-stained). At all ages investigated (7 days to 1 year), there was no collapse in Bsndlox/lox Sox10::Cre mice. Scale bar: 100 μm (B and C). (C) HE-stained cochleae from 12-day-old WT and Bsndlox/lox FoxG1::Cre mice (displaying renal salt and fluid loss). Reissner's membrane had collapsed in all 11 Bsndlox/lox FoxG1::Cre mice analysed between P6 and P15. (D) Endolymphatic K+ concentrations and (E) endocochlear potential (EP) of 3 and 4- to 10-week-old WT and Bsndlox/lox Sox10::Cre mice (labelled as ‘KO'). The total number of mice is indicated in each column. (F) Amplitudes of DPOAE (otoacoustic emissions) in 3-week-old Bsndlox/lox Sox10::Cre mice (red, n=4–6) and WT littermates (black, n=6) in response to stimuli at varying frequencies (f1) at 60 dB (WT) or 80 dB (KO) or (G) to 10/12 kHz stimuli at varying levels. Error bars: s.e.m.
Consequences of barttin deletion for the endolymph
If ClC-K/barttin channels were essential for strial K+-secretion, the concomitant impairment of fluid secretion should lead to a collapse of Reissner's membrane. However, the position of Reissner's membrane was unchanged in Bsndlox/lox Sox10::Cre mice at all ages that were examined (Figure 2B). In agreement with this finding, measurements with double-barreled K+-selective microelectrodes in anaesthetized mice revealed no difference in the endocochlear K+ concentration, neither at 3 weeks of age nor later (Figure 2D). By contrast, the EP was drastically reduced (from 104.1±2.1 mV (s.e.m., n=10) WT to 16.7±2.1 mV (s.e.m., n=15), both measured between 20 and 30 days of age) in the inner-ear-specific conditional KO (Figure 2E).
In a surprising contrast to the normal position of Reissner's membrane in the inner-ear-specific KO of barttin in Bsndlox/lox Sox10::Cre mice (Figure 2B), we observed a collapse of Reissner's membrane in all Bsndlox/lox FoxG1::Cre mice that could be investigated between P6 and P15 (Figure 2C). The collapse of Reissner's membrane precluded measurements of the potential and [K+] of the endolymph. However, the collapse indicates that strial K+ and fluid secretion have fallen below the critical threshold for maintaining a normal endolymph volume when mice face the renal salt and fluid loss that is observed in Bsndlox/lox FoxG1::Cre mice.
Impact of changed EP on cochlear hair cells
We next explored whether the strong decrease in EP, which severely reduces the driving force for K+ entry into hair cells, suffices to impair OHC function. We measured distortion product otoacoustic emissions (DPOAEs), tones that are generated within the inner ear when active cochlear amplification interacts in two different frequency regions of the cochlea. DPOAEs depend on intact OHC function and were abolished in Bsndlox/lox Sox10::Cre mice at the age of 3–4 weeks (Figure 2F and G). As staining for the motor protein prestin appeared normal in OHCs at the same age (Figure 3Ab), these results strongly suggest that the drop in EP, by reducing the K+ transduction current, severely impairs the ability of OHCs to respond electromechanically to sound. The presence of ABR responses above 80 dB and the steep suprathreshold ABR peak I amplitude growth function (data not shown) are consistent with depolarization of IHCs by the remaining voltage difference and a recruitment of auditory nerve fibres with broadened tuning curves.
Figure 3.
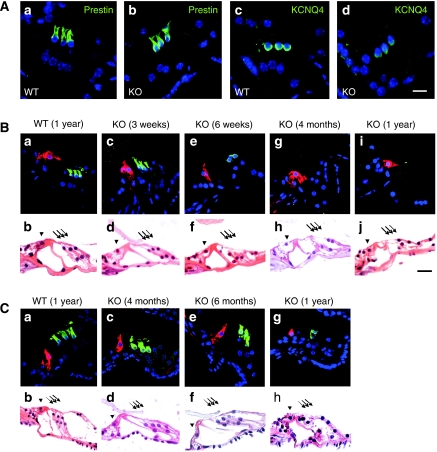
Degeneration of sensory hair cells in the organ of Corti. (A) Immunohistochemistry of hair cells of 3-week-old WT (a, c) and inner-ear-specific barttin KO (b, d) mice (Bsndlox/lox Sox10::Cre). OHCs were stained with antibodies against the motor protein prestin (a, b) and the K+ channel KCNQ4 (c–d). (B) Basal and (C) apical turns of cochleae from WT and barttin KO mice at ages stated above were stained either with an antibody against prestin (green; staining OHCs) and calretinin (red; staining IHCs) or with HE. Scale bar: 15 μm.
Although both IHCs and OHCs appeared normal at 3 weeks, the time point when we had measured DPOAEs and when the hearing loss was fully apparent, OHCs in the basal turn began to degenerate a few weeks afterwards (Figure 3B). Figure 3Be shows a basal turn in which green prestin staining surrounding nuclei identifies disintegrating OHCs, whereas red calretinin staining identifies intact IHCs. Deiter's cells, the supporting cells below OHCs, appeared normal. Within a few months, OHCs were completely lost in the basal high frequency turn, whereas they were preserved for a longer time in apical regions of the cochlea. They eventually degenerated also in those low-frequency turns, as shown in Figure 3Cg and h for a 1-year-old KO mouse. IHCs, by contrast, remained intact even in old mice. Interestingly, staining cochleae for the K+-channel KCNQ4 (Kharkovets et al, 2000, 2006) revealed that its expression in OHCs of the basal turn was decreased (Figure 3Ac and d). This occurred already at 3 weeks of age, before OHC degeneration became visible in paraffin sections or by prestin immunostaining.
Morphology of barttin-less stria vascularis
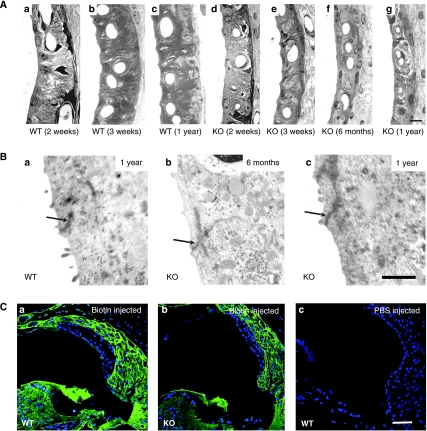
Semithin sections of the stria vascularis (Figure 4A) revealed that its width was reduced by 30–50% in Bsndlox/lox Sox10::Cre mice already at 2 weeks of age (Figure 4Aa and d). Although the extensive interdigitations between marginal and intermediate cells of the WT stria were partially preserved in the KO mice at that early age (Figure 4Ad and e), they were lost later through progressive degeneration (Figure 4Af and g). Nonetheless, all cell types of the stria were retained. This was evident by immunohistochemical staining of marginal cells for the apical K+ channel KCNQ1 (Estévez et al, 2001) (Figure 1Bd–f) and for the basolateral Na,K-ATPase (McGuirt and Schulte, 1994) and H,K-ATPase (Shibata et al, 2006) (Supplementary Figure S1A–D), of intermediate cells for the K+ channel Kir4.1 (Ando and Takeuchi, 1999) (Supplementary Figure S1E and F), and of strial capillaries for the glucose transporter Glut-1 (Supplementary Figure S1G and H). Consistent with the morphological changes revealed by the semithin sections, the staining for basolateral proteins of marginal cells and of the apical Kir4.1 of intermediate cells was less pronounced in barttin-deficient stria (Supplementary Figure S1B, D and F). Electron microscopy revealed that the degeneration of the stria did not lead to a loss of tight junctions (Figure 4B). The overall integrity of strial barriers was assessed by intracochlear injections of reactive biotin, which was labelled by avidin after about 10 min (Kitajiri et al, 2004). Although biotin had reached the scala media as indicated by the staining of the tectorial membrane and the apical surface of marginal cells, and had extensively stained the fibrocyte system underlying the stria, it was excluded from the stria in both WT and Bsndlox/lox Sox10::Cre mice (Figure 4C). Thus, tight junctions continue to form a barrier between the apical parts of marginal cells, as well as basal cells.
Figure 4.
Degeneration of stria vascularis. (A) Semithin sections of stria vascularis from WT (a–c) and inner-ear-specific barttin KO (d–g) mice at different ages. Scale bar: 7 μm (a–g). (B) Electron microscopy of marginal cell tight junctions (arrows) from 6-month-old and 1-year-old WT and barttin KO mice. Scale bar: 1 μm (a–c). (C) Biotin tracer permeability assay. Biotin injected into endolymph and perilymph fails to penetrate the stria vascularis of 3-month-old WT (a) and KO (b) mice. PBS was injected as control (c). Scale bar: 70 μm (a–c). KO always refers to Bsndlox/lox Sox10::Cre mice.
Impact of barttin loss on the vestibular system
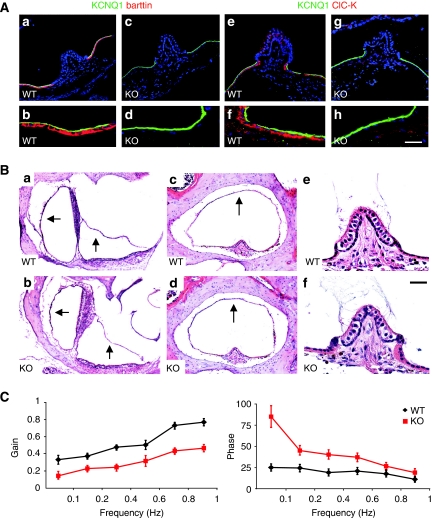
ClC-K/barttin Cl− channels are also expressed in K+-secreting dark cells of the vestibular organ (Estévez et al, 2001). Barttin was completely deleted in those cells in Bsndlox/lox Sox10::Cre mice, which was again associated with a loss of ClC-K protein (Figure 5A). The lack of ClC-K/barttin did not cause a collapse of the vestibular wall (Figure 5Ba-d). Contrasting with the degeneration of cochlear OHCs, we did not observe vestibular hair cell degeneration even in 1-year-old KO mice (Figure 5Be and f). Vestibular hair cells also maintained normal KCNQ4 expression (Supplementary Figure S2). Bsndlox/lox Sox10::Cre mice lacked a classic shaker/waltzer behaviour indicative of a strong vestibular phenotype, which was, for example, observed with the deletion of Nkcc1 (Delpire et al, 1999). They also behaved normally in swimming and rotarod tests of motor coordination and equilibrium (data not shown). However, more sophisticated tests revealed an impairment of the vestibular system. Mice were subjected to vestibular stimulation to investigate the amplitude (gain) and timing (phase) of their angular vestibulo ocular reflexes (VORs). These measure a compensatory ocular movement in response to head movements that generate eye movements in the opposite direction. Both the gain and phase of their angular VOR was significantly changed in the dark (Figure 5C). When a similar test was performed in the light, however, these VVOR (visually enhanced VOR) measurements did not show differences between the genotypes (Supplementary Figure S3). Neither there were differences in the optokinetic reflex that measures the eye movements following a moving optical stimulus. Hence, Bsndlox/lox Sox10::Cre mice have a subtle vestibular phenotype that can be compensated for by optical cues.
Figure 5.
The vestibular organ of inner-ear-specific barttin KO mice. (A) Staining of vestibular dark cells form WT (a, b, e, f) and barttin KO (c, d, g, h) mice with antibodies against barttin (a–d; red) and ClC-K (e-h; red). Apical membranes of dark cells are labelled with KCNQ1 (green). Scale bar: 50 μm (a, c, e, g) and 18 μm (b, d, f, h). (B) HE staining of vestibular organs from WT (a, c, e) and KO (b, d, f) mice. Position of vestibular wall above sacculus and utriculus (a, b) and surrounding the crista ampullaris (c, d) is not different between WT and KO. Hair cells in the crista ampullaris of 1-year-old KO mice had not degenerated (e, f). Scale bar: 150 μm (a, b), 100 μm (c, d) and 20 μm (e, f). (C) Vestibulo ocular reflex (VOR) in the dark of 12- to 20-week-old Bsndox/lox Sox10::Cre and WT mice. The amplitude (gain) (left panel) and timing (phase) (right panel) of eye movements is shown as a function of the rotation frequency of the turntable. Mice lacking barttin showed lower gains than the WT (P<0.001; two-way ANOVA; left). Following this turntable stimulation at 0.1–1 Hz, phase leads of Bsndox/lox Sox10::Cre mice were larger than the WT (P<0.001 ; two-way ANOVA; right). KO always refers to Bsndlox/lox Sox10::Cre mice. Values are obtained from seven mice per genotype.
Discussion
Using Sox10::Cre mice to specifically delete the Cl−-channel β-subunit barttin in Bsndlox/lox mice in the inner ear, but not in the kidney, we have investigated the mechanism leading to congenital deafness in human Bartter syndrome type IV. Our work has revealed that ClC-K/barttin Cl− channels are necessary for the generation of the EP. However, their absence does not impair strial K+ and fluid secretion sufficiently to change the high K+ concentration and pressure in the scala media. The large drop in EP apparently suffices to cause deafness by severely reducing the driving force for K+ entry through mechanosensitive channels of outer and IHCs. An almost complete functional impairment of OHCs is a major factor in Bartter IV deafness, with no further impairment of hearing when these cells are lost by degeneration.
Barttin, a small protein with two transmembrane domains, has been identified as being mutated in Bartter syndrome type IV (Birkenhäger et al, 2001) and has subsequently been shown to be a β-subunit of the ClC-Ka and ClC-Kb Cl− channels that are highly related to each other (Estévez et al, 2001). In heterologous expression, either ClC-K isoform does not yield currents without co-expression of barttin (Kieferle et al, 1994; Estévez et al, 2001). This is mainly because of a lack of plasma membrane expression in the absence of barttin (Estévez et al, 2001; Waldegger et al, 2002). We found here that ClC-K proteins cannot be detected by immunohistochemistry in cells lacking barttin, suggesting that both α-subunits are unstable without their cognate β-subunit, just as ClC-7 is unstable without its β-subunit Ostm1 (Lange et al, 2006). This loss of ClC-K α-subunits is important because, as an exception, rodent ClC-K1 (the orthologue of human ClC-Ka) yields small currents even without barttin in heterologous expression (Uchida et al, 1993; Waldegger and Jentsch, 2000; Waldegger et al, 2002; Scholl et al, 2006). We therefore conclude that the KO of barttin leads to an almost complete loss of ClC-K chloride currents.
ClC-K/barttin channels and the EP
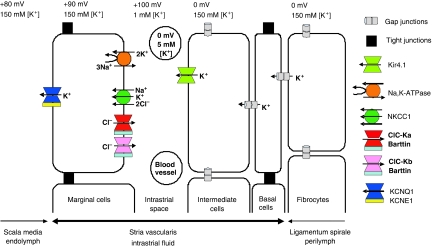
How does the loss of barttin entail the drastic decrease in EP? The stria vascularis, which generates the positive potential and high potassium concentration of the endolymph that bathes the apical membranes of sensory hair cells (Figure 1A), is a highly unusual, multilayered epithelium (Figure 6) (Wangemann, 2006; Nin et al, 2008). It displays two diffusion barriers formed by tight junctions, one between the apical poles of marginal cells and the other between basal cells. Basal cells are connected by gap junctions to both the underlying fibrocyte system and epithelial intermediate cells. The intrastrial space between intermediate cells and marginal cells is perforated by capillaries, hence the name stria vascularis. Potassium ions are actively taken up from the intrastrial space into the marginal cells through the Na,K-ATPase and the NaK2Cl cotransporter Nkcc1 and are then secreted into the endolymph through apical KCNQ1/KCNE1 K+ channels. This uptake creates an unusually low K+ concentration in the intrastrial space (Salt et al, 1987; Ikeda and Morizono, 1989; Nin et al, 2008), creating a large K+-diffusion potential across the apical membrane of intermediate cells that prominently express the K+ channel Kir4.1 (Salt et al, 1987; Takeuchi et al, 2000; Marcus et al, 2002; Nin et al, 2008). As unknown ionic mechanisms keep the intracellular potential of intermediate cells close to zero, this diffusion potential clamps the intrastrial space to about +100 mV, a value almost equal to the EP. This model is strongly supported by the virtually complete breakdown of the EP in mice lacking Kir4.1 (Marcus et al, 2002).
Figure 6.
Schematic model of cochlear potassium secretion across the stria vascularis and of the formation of endocochlear potential (EP). The stria is an unusual multilayered epithelium in which tight junctions between marginal cells, which face directly the endolymph, as well as tight junctions between basal cells isolate an intrastrial space that has a low K+ concentration. The EP is largely generated by a K+-diffusion potential through the Kir4.1 K+ channel across the apical membrane of intermediate cells. The loss of barttin abolishes currents through both ClC-Ka and ClC-Kb, indirectly impairing basolateral K+ uptake by the NaK2Cl cotransporter and the Na,K-ATPase. This loss of K+-uptake raises [K+] in the intrastrial space and thereby interferes with the generation of the EP by K+ exiting intermediate cells through Kir4.1. The lack of depolarizing Cl− efflux over the basolateral membrane of marginal cells should further decrease EP, as could a decrease of [K+] in marginal cells. Published values of EP vary, with the higher values reported here (+100 mV) being less likely affected by leaks introduced by microelectrode measurements (for depicted ion concentrations, see Salt et al, 1987; Ikeda and Morizono, 1989; Nin et al, 2008). The basolateral H,K-ATPase of marginal cells (Supplementary Figure S1) has not been depicted, as its function is rather unclear.
In our model (Figure 6), ClC-K/barttin channels are needed to recycle chloride ions that are taken up by the NaK2Cl cotransporter Nkcc1 across the basolateral membrane of marginal cells (Estévez et al, 2001). By inhibiting indirectly the operation of Nkcc1 and the Na,K-ATPase (which is deprived of its intracellular substrate Na+ that is no longer accumulated by Nkcc1), the disruption of barttin is expected to increase the K+ concentration in the intrastrial space, while probably also decreasing [K+]i in marginal cells (Nin et al, 2008). This rise in intrastrial [K+] will decrease the K+-diffusion potential across the apical membrane of intermediate cells—the main source of the EP (Salt et al, 1987; Nin et al, 2008). A decreased [K+]i of marginal cells might further contribute to a decrease of the EP by changing the apical K+-diffusion potential. In addition, the parallel operation of the electroneutral Nkcc1 cotransporter and the electrogenic ClC-K/barttin predicts a depolarizing Cl− efflux across the basolateral membrane of marginal cells, thereby largely determining the very low voltage (estimated to be −10 mV inside) across this membrane. As the Na,K-ATPase is electrogenic, the disruption of ClC-K/barttin is expected to shift the basolateral membrane voltage of marginal cells to more inside-negative values if some Na,K-ATPase activity persists (as suggested by normal endolymphatic [K+]), an effect that likely contributes to the decreased EP of barttin KO mice. Hence the model (Figure 6) can easily account for the drastic decrease in EP.
Function of barttin in strial K+ secretion
The model also predicts a drastic decrease in K+ and fluid secretion, as the lack of Cl− recycling would inhibit both basolateral K+-uptake processes into marginal cells, that is, Nkcc1 and the Na,K-ATPase (Quraishi and Raphael, 2006). Nkcc1 KO mice are profoundly deaf and display severe vestibular symptoms (Delpire et al, 1999). These are associated with a collapse of Reissner's membrane and the vestibular lumen, in stark contrast to the present Bsndlox/lox Sox10::Cre mice. One should also note that the hearing loss of Nkcc1−/− mice was more pronounced than that of mice lacking barttin (Figure 2A), although the difference in genetic background (C57Bl6 for Nkcc1−/− mice) might be a contributing factor. The normal endolymphatic volume and [K+] of Bsndlox/lox Sox10::Cre mice therefore suggest that, in the absence of barttin, some Cl− conductance remains in marginal cells, which can support residual Nkcc1 activity and K+ secretion. As our immunohistochemistry indicates that a contribution of ClC-K1 (partially functional without barttin (Uchida et al, 1993; Waldegger and Jentsch, 2000)) is negligible, there might be other Cl− channels in marginal cells like swelling- or Ca++-activated ones.
We propose that strial K+ secretion of Bsndlox/lox Sox10::Cre mice is reduced to an extent that just suffices to establish normal [K+] and volume of the endolymph. This notion is strongly supported by the collapse of Reissner's membrane in Bsndlox/lox FoxG1::Cre mice. Their renal salt and fluid loss—which is less than that in the complete KO mice, but severe enough to prevent these mice from reaching adulthood—may tip the balance and decrease K+ secretion below the threshold necessary for generating enough hydrostatic pressure in the scala media. The further impairment of K+ secretion in Bsndlox/lox FoxG1::Cre mice might be owed directly to changed concentrations of extracellular electrolytes, or more likely is the consequence of alterations in hormones like ADH and aldosterone that respond to renal salt and fluid loss.
Considering the model of strial transport depicted in Figure 6, one may wonder why [K+] and EP did not decrease in parallel in Bsndlox/lox Sox10::Cre mice. However, this situation is not without precedent. For instance, the EP of Kir4.1 KO mice was totally abolished, whereas Reissner's membrane had only partially collapsed and endolymph [K+] was roughly halved (Marcus et al, 2002). Furthermore, endolympathic [K+] reaches its final value earlier than the EP during development (Yamasaki et al, 2000). These differences might be owed to ion conductances and transporters in the stria that are not depicted in Figure 6, or to differences between K+-efflux pathways from the endolymph and the electrical resistance of the tissue enclosing it (Wangemann, 2006).
The disruption of barttin also led to morphological changes in the stria vascularis. This degeneration progressed with time, but neither resulted in a complete loss of any of the strial cell types, nor in a disruption of strial tight junction barriers. Nonetheless, it is most likely that the loss of interdigitations, which normally increase the transport surface of intermediate and marginal cells, will contribute to the impairment of strial function.
Mechanism leading to hearing loss in Bartter syndrome IV
As the hearing loss of Bsndlox/lox Sox10::Cre mice is already fully present at 3 weeks of age, a time when OHCs have not yet degenerated, it must be a direct consequence of the endolymphatic changes. The decreased EP (+15 instead of +100 mV) drastically reduces, but does not abolish, the driving force for K+ entry through mechanosensitive cation channels of both inner and OHCs. With an intracellular potential of 60 mV more negative than perilymph (Kharkovets et al, 2006) and assuming that intracellular and endolymphatic [K+] are equal, the driving force for apical K+ entry into OHCs should be reduced from −160 mV to −75 mV. The absence of DPOAEs suggests that this ∼55% reduction of driving force sufficed to abolish sound-induced OHC contractions. This may be surprising, but similar observations were made in mice lacking tight junctions between basal cells as a consequence of claudin-11 disruption (Gow et al, 2004): in the presence of normal endolymphatic [K+] and an EP of 25 mV (that is, a less severe loss than in our mice), their DPOAEs were severely reduced. Bsndlox/lox Sox10::Cre OHCs also expressed less KCNQ4 K+ channels, suggesting that the driving force for K+ entry is decreased somewhat further by a slight depolarization of OHCs (Kcnq4−/− OHCs were depolarized by ∼15 mV (Kharkovets et al, 2006)). Lowering KCNQ4-mediated K+ conductance also increases the OHC membrane time constant and thereby reduces the AC component of prestin-mediated sound amplification (Kharkovets et al, 2006). The decrease in OHC KCNQ4 expression might be a sign of incipient OHC degeneration, as it was also observed in mice null for the BK K+ channel (Rüttiger et al, 2004).
A total loss of OHC function decreases hearing sensitivity by 30–50 dB (Ryan and Dallos, 1975). We therefore conclude that a large component of the barttin-related ∼60 dB hearing loss is owed to a loss of OHC function. The fact that the hearing loss of Bsndlox/lox Sox10::Cre mice did not get worse in spite of the progressive degeneration of OHCs corroborates our DPOAE analysis, which indicated a loss of amplifier function already at 3 weeks. Another likely and important component is a decrease in the mechanoelectrical transduction current of IHCs caused by the drop in EP. The relative decrease of their transduction current may be slightly less than in OHCs because their resting potential is ∼10 mV more negative than in OHCs (Kharkovets et al, 2006).
Barttin and the vestibular organ
Although Bsndlox/lox Sox10::Cre mice lacked obvious vestibular symptoms, more sensitive tests revealed abnormal VORs. This seems surprising, as vestibular endolymph has high [K+], but lacks a positive potential—and only the potential, but not [K+], was changed in the scala media of KO mice. We suggest that the drastic drop of the EP of Bsndlox/lox Sox10::Cre mice changes the potential of the vestibular endolymph with which it is contiguous and thus electrically connected. Vestibular EP might be slightly negative in the KO mice, a hypothesis that is difficult to prove, as measuring that compartment is exceedingly difficult. The vestibular disturbances of Bsndlox/lox Sox10::Cre mice were subtle and humans can compensate vestibular disturbances much better than mice. It is therefore not surprising that there are no reports on vestibular symptoms in patients with Bartter IV.
Bsnd KO mice as model for deafness in human Bartter syndrome IV
There are few published data on the extent of hearing loss in patients with Bartter IV. For two patients, it was reported to be in excess of 115 dB (Miyamura et al, 2003; Zaffanello et al, 2006), considerably more than that in the present mice. A larger hearing loss in humans with Bartter IV than in Bsndlox/lox Sox10::Cre mice might be related to their kidney disease, as suggested by the collapse of Reissner's membrane in salt-losing Bsndlox/lox FoxG1::Cre mice. The comparison between both mouse models suggests that an efficient and early treatment of renal symptoms in patients with Bartter IV may be beneficial to keep some, although severely reduced, sensitivity to sound. Even an optimal symptomatic treatment will not, however, prevent the profound ∼60 dB hearing loss that is caused by the breakdown of the EP and the resulting decrease of hair cell transduction currents and loss of sound amplification by OHCs.
Materials and methods
Mice
Bsndlox/lox mice in which exon 2 was flanked by loxP sites were generated by homologous recombination of an appropriate targeting vector into MPI II embryonic stem cells, which were injected into blastocysts using standard procedures. Details will be described elsewhere together with a description of the renal phenotype. Sox10::Cre mice (Matsuoka et al, 2005) were obtained from W.D. Richardson, and FoxG1::Cre mice (Hébert and McConnell, 2000) were obtained from S.K. McConnell. Nkcc1−/− mice (Pace et al, 2000) were obtained from Beverly H. Koller and backcrossed to a C57/Bl6 genetic background. The barttin mouse models were in a mixed (C57Bl6–129SVJ) genetic background and were compared with littermate controls. Animal experiments were performed according to the ethical guidelines of the institutions involved.
Immunohistochemical and histological analysis
Mice were anaesthetized with ketamin/rompun and perfused through the heart with 4% paraformaldehyde (PFA) in PBS. After removal of temporal bones, inner ears were fixed at 4°C for 2 h in 4% PFA in PBS. From P7 onwards, samples were decalcified with 10% EDTA (in PBS, pH 7.4) at 4 °C for 24 h (P7-P14), 36 h (P15-P19), 48 h (P20 to adult), fixed in 4% PFA for 1 h, and decalcified again in 10% EDTA for 12 h (P15-P19), 24–48 h (P20 to adult). For immunohistochemical staining, inner ears were embedded in Neg-50 (Richard-Allan Scientific) after cryoprotection by immersion in 30% sucrose for 12 h at 4 °C. Sections (8 μm) were blocked with 3% normal goat serum (NGS), 2% BSA and 0.5 % NP-40 in PBS for 1 h. Antibodies were diluted in 1.5% NGS, 1% BSA and 0.25% NP-40 in PBS. The following primary antibodies were used: Barttin (Estévez et al, 2001), KCNQ1 (Dedek and Waldegger, 2001), prestin (Weber et al, 2002), calretinin (1:2000; Swant), KCNQ4 (Kharkovets et al, 2000), Na,K-ATPase (1:300; α1-subunit; Upstate Biotechnology), H,K-ATPase (1:200; α-subunit; Calbiochem), Kir4.1 (Ando and Takeuchi, 1999), GLUT-1 (1:200; Dianova). We raised a new antibody in guinea pigs against the amino-terminal ClC-K peptide MEELVGLREGSSKKPC-amide, which was coupled to KLH using MBS. Resulting sera were purified against the peptide. Fluorescence-labelled secondary antibodies were obtained from Molecular Probes. TO-PRO®-3 (Molecular Probes) was used for staining nuclei. For haematoxylin–eosin (HE) staining, inner ears were paraffin-embedded and sectioned to 6 μm. Tissues from a minimum of three WT/KO mouse pairs at each time point were investigated for all data shown.
Tracer permeability assay
After dissecting inner ears from temporal bones of 3 WT and 3 KO mice at the age of 3, 6 and 9 months, both the round and oval windows were opened in PBS containing 1 mM CaCl2. The scala tympani and scala vestibuli of cochleae were perfused through the round and oval windows with 500 μl EZ-LinkTM Sulfo-NHS-LC-Biotin (10 mg/ml; Pierce) in PBS for 5 min (Kitajiri et al, 2004). During injection, the Reissner's membrane was damaged allowing the tracer to enter the scala media. After washing through the windows with PBS containing 1 mM CaCl2 for 5 min at 4°C, inner ears were fixed with 4% PFA in PBS for 12 h and processed for cryosectioning as described above. Biotin tracer was detected by incubating frozen sections with FITC–streptavidin conjugate (Zymed) for 30 min.
Semithin and ultrathin sections
After perfusion of mice with a mixture of 2.5% glutaraldehyde and 2% PFA in PBS, inner ears were dissected from temporal bones and post-fixed overnight at 4 °C. Decalcification was done in 10% EDTA (in PBS, pH 7.4) at 4 °C. Cochleae were cut in two, post-fixed in 1% osmium tetroxide for 1 h, dehydrated in a graded series of ethanol solutions and embedded in Poly/Bed®812 (Polysciences Inc.). The blocks were cut in semithin (0.7 μm) and ultrathin (50 nm) sections. Semithin sections were stained with toluidine blue. Ultrathin sections stained with uranyl acetate and lead citrate were examined with a Zeiss 902 electron microscope.
Auditory brain stem response
Auditory evoked brain stem responses to clicks were recorded in anaesthetized animals as described (Kharkovets et al, 2006) and as detailed in Supplementary data. There was no indication for differences in the measured physiological parameters between mice expressing barttin from both alleles, that is, between Bsnd+/+, Bsnd+/+ Sox10::Cre, Bsnd+/lox and Bsndlox/lox mice. Therefore, results from these mice were pooled and labelled ‘WT' in Figure 2. For instance, in Figure 2A and for 6 weeks of age, there were three Bsnd+/+ Sox10::Cre mice, one Bsnd+/lox and three Bsndlox/lox mice.
EP and potassium concentration
Measurements of EP and K+ concentration were performed in anaesthetized mice. Bullae were opened ventrolaterally and the bone over the first turn of the cochlea was thinned and opened apically to the stapedial artery. The stria vascularis was penetrated with a double-barreled microelectrode and the potential and [K+] were measured in the scala media. For details, see Supplementary data.
Distortion product otoacoustic emission
Distortion product otoacoustic emissions were measured on anaesthetized mice using two primary tones essentially as described (Kharkovets et al, 2006) and as detailed in Supplementary data.
Vestibular tests
The vestibular system was studied by accelerating 12- to 20-week-old restrained mice on a turntable and measuring the movements of their eyes, essentially as described by Stahl et al (2000) and as detailed in Supplementary data.
Supplementary Material
Supplementary Information
Acknowledgments
We thank the following colleagues for generously making their mice available: WD Richardson and N Tekki-Kessaris for Sox10::Cre mice, SK McConnell for FoxG1::Cre mice and BH Koller for Nkcc1 KO mice. We thank S Takeuchi for the Kir4.1 antibody, M Knipper for the prestin antibody, D Lorenz for electron microscopy, N Krönke, R Pareja Alcaraz and I Freyert for technical assistance, F Hildebrandt for exchanging information in an early stage of the project and T Moser for critical reading of the manuscript. This work was supported, in part, by the European Union through the Eurohear and Euregene FP6 programs and the Deutsche Forschungsgemeinschaft to TJJ, and Eurohear to Tobias Moser, supporting the DPOAE measurements of NS.
References
- Ando M, Takeuchi S (1999) Immunological identification of an inward rectifier K+ channel (Kir4.1) in the intermediate cell (melanocyte) of the cochlear stria vascularis of gerbils and rats. Cell Tissue Res 298: 179–183 [DOI] [PubMed] [Google Scholar]
- Birkenhäger R, Otto E, Schürmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NVAM, Antignac C, Sudbrack R, Kispert A, Hildebrandt F (2001) Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29: 310–314 [DOI] [PubMed] [Google Scholar]
- Boettger T, Hübner C, Maier H, Rust MB, Beck FX, Jentsch TJ (2002) Deafness and renal tubular acidosis in mice lacking the K-Cl cotransporter Kcc4. Nature 416: 874–878 [DOI] [PubMed] [Google Scholar]
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Völkl H, Hübner CA, Jentsch TJ (2003) Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J 22: 5422–5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Hardisty-Hughes RE, Mburu P (2008) Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet 9: 277–290 [DOI] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C (2002) Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol 12: 1106–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Waldegger S (2001) Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflügers Arch 442: 896–902 [DOI] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22: 192–195 [DOI] [PubMed] [Google Scholar]
- Estévez R, Boettger T, Stein V, Birkenhäger R, Otto M, Hildebrandt F, Jentsch TJ (2001) Barttin is a Cl−-channel β-subunit crucial for renal Cl−-reabsorption and inner ear K+-secretion. Nature 414: 558–561 [DOI] [PubMed] [Google Scholar]
- Forge A, Wright T (2002) The molecular architecture of the inner ear. Br Med Bull 63: 5–24 [DOI] [PubMed] [Google Scholar]
- Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B (2004) Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci 24: 7051–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert JM, McConnell SK (2000) Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol 222: 296–306 [DOI] [PubMed] [Google Scholar]
- Hibino H, Kurachi Y (2006) Molecular and physiological bases of the K+ circulation in the mammalian inner ear. Physiology (Bethesda) 21: 336–345 [DOI] [PubMed] [Google Scholar]
- Ikeda K, Morizono T (1989) Electrochemical profiles for monovalent ions in the stria vascularis: cellular model of ion transport mechanisms. Hear Res 39: 279–286 [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ (2006) Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J 25: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ (2000) KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA 97: 4333–4338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ (1994) Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci USA 91: 6943–6947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC (2000) Gap junction systems in the mammalian cochlea. Brain Res Rev 32: 163–166 [DOI] [PubMed] [Google Scholar]
- Kitajiri S, Miyamoto T, Mineharu A, Sonoda N, Furuse K, Hata M, Sasaki H, Mori Y, Kubota T, Ito J, Furuse M, Tsukita S (2004) Compartmentalization established by claudin-11-based tight junctions in stria vascularis is required for hearing through generation of endocochlear potential. J Cell Sci 117: 5087–5096 [DOI] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ (1999) KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell 96: 437–446 [DOI] [PubMed] [Google Scholar]
- Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) ClC-7 requires Ostm1 as a β-subunit to support bone resorption and lysosomal function. Nature 440: 220–223 [DOI] [PubMed] [Google Scholar]
- Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, Brandenburg SA, Litzi TJ, Bunton TE, Limb C, Francis H, Gorelikow M, Gu H, Washington K, Argani P, Goldenring JR, Coffey RJ, Feinberg AP (2000) Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest 106: 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Wu T, Wangemann P, Kofuji P (2002) KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. Am J Physiol Cell Physiol 282: C403–C407 [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G (2005) Neural crest origins of the neck and shoulder. Nature 436: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirt JP, Schulte BA (1994) Distribution of immunoreactive α- and β-subunit isoforms of Na,K-ATPase in the gerbil inner ear. J Histochem Cytochem 42: 843–853 [DOI] [PubMed] [Google Scholar]
- Miyamura N, Matsumoto K, Taguchi T, Tokunaga H, Nishikawa T, Nishida K, Toyonaga T, Sakakida M, Araki E (2003) Atypical Bartter syndrome with sensorineural deafness with G47R mutation of the β-subunit for ClC-Ka and ClC-Kb chloride channels, Barttin. J Clin Endocrinol Metab 88: 781–786 [DOI] [PubMed] [Google Scholar]
- Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P (1997) A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange–Nielsen cardioauditory syndrome. Nat Genet 15: 186–189 [DOI] [PubMed] [Google Scholar]
- Nin F, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y (2008) The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc Natl Acad Sci USA 105: 1751–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace AJ, Lee E, Athirakul K, Coffman TM, O'Brien DA, Koller BH (2000) Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl− cotransporter. J Clin Invest 105: 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quraishi IH, Raphael RM (2006) A computational model of vectorial potassium transport by cochlear marginal cells and vestibular dark cells. Am J Physiol Cell Physiol 292: C591–C602 [DOI] [PubMed] [Google Scholar]
- Rüttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Müller M, Köpschall I, Pfister M, Münkner S, Rohbock K, Pfaff I, Rüsch A, Ruth P, Knipper M (2004) Deletion of the Ca2+-activated potassium (BK) α-subunit but not the BK β1-subunit leads to progressive hearing loss. Proc Natl Acad Sci USA 101: 12922–12927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Dallos P (1975) Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature 253: 44–46 [DOI] [PubMed] [Google Scholar]
- Sage CL, Marcus DC (2001) Immunolocalization of ClC-K chloride channel in strial marginal cells and vestibular dark cells. Hear Res 160: 1–9 [DOI] [PubMed] [Google Scholar]
- Salt AN, Melichar I, Thalmann R (1987) Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97: 984–991 [PubMed] [Google Scholar]
- Schlingmann KP, Konrad M, Jeck N, Waldegger P, Reinalter SC, Holder M, Seyberth HW, Waldegger S (2004) Salt wasting and deafness resulting from mutations in two chloride channels. N Engl J Med 350: 1314–1319 [DOI] [PubMed] [Google Scholar]
- Scholl U, Hebeisen S, Janssen AG, Müller-Newen G, Alekov A, Fahlke C (2006) Barttin modulates trafficking and function of ClC-K channels. Proc Natl Acad Sci USA 103: 11411–11416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, Rubie C, Hordt M, Towbin JA, Borggrefe M, Assmann G, Qu X, Somberg JC, Breithardt G, Oberti C, Funke H (1997) KCNE1 mutations cause Jervell and Lange–Nielsen syndrome. Nat Genet 17: 267–268 [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K (1995) A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Hibino H, Doi K, Suzuki T, Hisa Y, Kurachi Y (2006) Gastric type H+,K+-ATPase in the cochlear lateral wall is critically involved in formation of the endocochlear potential. Am J Physiol Cell Physiol 291: C1038–C1048 [DOI] [PubMed] [Google Scholar]
- Stahl JS, van Alphen AM, De Zeeuw CI (2000) A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Methods 99: 101–110 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Ando M, Kakigi A (2000) Mechanism generating endocochlear potential: role played by intermediate cells in stria vascularis. Biophys J 79: 2572–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23: 99–103 [DOI] [PubMed] [Google Scholar]
- Uchida S, Sasaki S, Furukawa T, Hiraoka M, Imai T, Hirata Y, Marumo F (1993) Molecular cloning of a chloride channel that is regulated by dehydration and expressed predominantly in kidney medulla [published erratum appears in J Biol Chem 1994 Jul 22;269(29):19192]. J Biol Chem 268: 3821–3824 [PubMed] [Google Scholar]
- Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17: 1251–1264 [DOI] [PubMed] [Google Scholar]
- Waldegger S, Jeck N, Barth P, Peters M, Vitzthum H, Wolf K, Kurtz A, Konrad M, Seyberth HW (2002) Barttin increases surface expression and changes current properties of ClC-K channels. Pflügers Arch 444: 411–418 [DOI] [PubMed] [Google Scholar]
- Waldegger S, Jentsch TJ (2000) Functional and structural analysis of ClC-K chloride channels involved in renal disease. J Biol Chem 275: 24527–24533 [DOI] [PubMed] [Google Scholar]
- Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zimmermann U, Winter H, Mack A, Kopschall I, Rohbock K, Zenner HP, Knipper M (2002) Thyroid hormone is a critical determinant for the regulation of the cochlear motor protein prestin. Proc Natl Acad Sci USA 99: 2901–2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Komune S, Shimozono M, Matsuda K, Haruta A (2000) Development of monovalent ions in the endolymph in mouse cochlea. ORL J Otorhinolaryngol Relat Spec 62: 241–246 [DOI] [PubMed] [Google Scholar]
- Zaffanello M, Taranta A, Palma A, Bettinelli A, Marseglia GL, Emma F (2006) Type IV Bartter syndrome: report of two new cases. Pediatr Nephrol 21: 766–770 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information