Abstract
Human DNA polymerase iota (polι) is a unique member of the Y-family of specialised polymerases that displays a 5′deoxyribose phosphate (dRP) lyase activity. Although polι is well conserved in higher eukaryotes, its role in mammalian cells remains unclear. To investigate the biological importance of polι in human cells, we generated fibroblasts stably downregulating polι (MRC5-polιKD) and examined their response to several types of DNA-damaging agents. We show that cell lines downregulating polι exhibit hypersensitivity to DNA damage induced by hydrogen peroxide (H2O2) or menadione but not to ethylmethane sulphonate (EMS), UVC or UVA. Interestingly, extracts from cells downregulating polι show reduced base excision repair (BER) activity. In addition, polι binds to chromatin after treatment of cells with H2O2 and interacts with the BER factor XRCC1. Finally, green fluorescent protein-tagged polι accumulates at the sites of oxidative DNA damage in living cells. This recruitment is partially mediated by its dRP lyase domain and ubiquitin-binding domains. These data reveal a novel role of human polι in protecting cells from oxidative damage.
Keywords: BER, DNA polymerase, oxidative DNA damage, Y-family polymerase
Introduction
The genome is continuously exposed to damaging agents, both endogenous, resulting from hydrolysis and oxidation during normal cell metabolism, and exogenous such as UV light, ionising radiation and a wide variety of chemical carcinogens. Among several mechanisms employed to protect genetic integrity from the detrimental effects of genotoxic compounds, base excision repair (BER) is a crucial process that eliminates many types of base damage (Lindahl, 1982). On the basis of current models, mammalian BER is divided into at least two subpathways that are designated ‘single nucleotide BER' (SN BER) and ‘long-patch BER' (LP BER). In both subpathways, repair is initiated by damage-specific DNA glycosylases that specifically recognise and remove the damaged base by cleavage of the bond between the base and the deoxyribose sugar of the DNA strands. Strand incision can be carried out by the same glycosylases (bifunctional glycosylases) or by an AP endonuclease. The resulting gaps are filled either with one nucleotide (SN BER) or with 2–10 nucleotides (LP BER) followed by a ligation step. For the SN BER, the DNA polymerase β (polβ) contributes to both gap-filling and removal of the 5′deoxyribose phosphate (dRP) (Srivastava et al, 1998; Almeida and Sobol, 2007). Mouse embryonic fibroblasts (MEFs) deficient in polβ have demonstrated the requirement of polβ mainly in the repair of alkylated lesions. In contrast, a defect in polβ has very little impact on cell proliferation after exposure of cells to oxidative DNA damage suggesting that other polymerases might have an important function in this BER pathway (Sobol et al, 1996; Fortini et al, 2000; Horton et al, 2002, 2005; Polosina et al, 2004; Yoshimura et al, 2006).
polι is a member of the recently discovered Y-family of DNA polymerases (Ohmori et al, 2001), which have been best characterised in vitro for their lesion-bypassing properties (reviewed in Goodman, 2002). Human polι is a highly distributive low-fidelity enzyme lacking an intrinsic exonuclease proofreading activity. Strikingly, polι incorporates dGMP opposite T 3–10 times more frequently than the correct nucleotide dAMP (Johnson et al, 2000; Tissier et al, 2000; Zhang et al, 2000). In addition to its polymerisation activity, polι also displays a dRP lyase activity (Bebenek et al, 2001). In reactions reconstituted with uracil-DNA glycosylase, AP endonuclease and a DNA ligase, polι can use both its dRP lyase and polymerase activities to repair G·U and A·U pairs on the DNA. Moreover, polι is able to complement in vitro the single-nucleotide BER deficiency of a DNA polβ-null cell extract (Bebenek et al, 2001; Prasad et al, 2003). Taken together, these features suggested a putative role for polι in BER in human cells.
In an effort to further clarify the role of polι in BER in vivo, we generated human fibroblasts stably downregulating polι (hereafter abbreviated as MRC5-polιKD) and found that these cells exhibit hypersensitivity to oxidative stress. This sensitivity is correlated with an accumulation of MRC5-polιKD cells in G1 phase. Interestingly, extracts from cells downregulating polι show reduced BER activity. Furthermore, we observed that polι is recruited to the chromatin after H2O2 treatment and is associated with XRCC1, a scaffold protein involved in single-strand DNA break (SSB) repair. In addition, polι is recruited to sites of oxidised chromatin in living cells. Altogether, these data suggest that polι is implicated in particular forms of BER in human cells.
Results
Generation of human fibroblasts downregulating pol protein
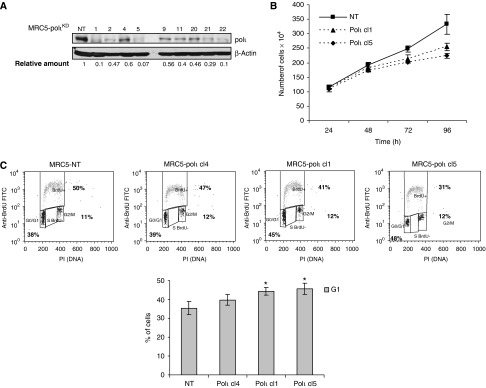
As no human cell line lacking polι has been identified, we generated human fibroblasts stably downregulating polι protein. The MRC5-SV cell line was transfected with the vector pSUPER.puro harbouring an shRNA directed against POLI mRNA. In parallel, a scrambled sequence was also employed as a control. Isolation of stable clones after puromycin selection and protein quantification by western blotting revealed that the level of polι was greatly decreased (up to 90%) in different MRC5-polιKD clones when compared with clones harbouring a nonspecific sequence (MRC5-NT) (Figure 1A). In this study, we used two clones displaying a drastic reduction of polι level (clone nos. 1 and 5) and one clone with an intermediate level of polι protein (clone no. 4). The growth properties of MRC5-polιKD cells were monitored by measuring growth curves. We observed a small delay in the growth of cells downregulating polι when compared with MRC5-NT cells (Figure 1B). Likewise, cell cycle analysis showed that MRC5-polιKD remained longer in G1 phase than did the control cells (Figure 1C, top panel). This accumulation in G1 was correlated with the extent of downregulation of polι as clone nos. 1 and 5 displayed a greater percentage of cells in G1 than clone no. 4 (Figure 1C, bottom panel). Hence, these results indicated that human cells expressing only 5–10% of polι protein are able to grow almost normally, except a slight accumulation of cells in G1 phase.
Figure 1.
Characterisation of cell lines stably downregulating polι (MRC5-polιKD). (A) Western blotting analysis of MRC5-polιKD clones (numbered above). Relative amount was calculated as a ratio of polι/NT after normalisation with β-actin amount. (B) Growth kinetics over 4 days of two MRC5-polιKD clones (nos. 1 and 5) and control cells (MRC5-NT). (C) Cell cycle analysis of MRC5-polιKD and MRC5-NT cell lines. The top panel shows a representative cell cycle distribution of the indicated cell line as measured by BrdU incorporation in flow cytometric analysis. The percentage of cells in all phases (G1/S/G2–M) is indicated. In the bottom panel, the histograms report the percentages of cells in G1 phase. The reported values are the means±s.d. of nine independent experiments (*P<0.05).
Downregulation of polι provokes an increase in sensitivity to killing by H2O2, but not to an alkylating agent or UVC
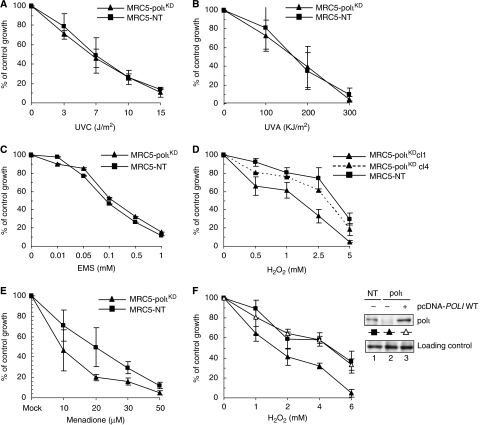
To study the biological function of human polι, we exposed MRC5-polιKD cells, to different DNA-damaging agents. MRC5-polιKD cells showed no significant increase in sensitivity either to UVC and UVA irradiations or to ethylmethane sulphonate (EMS) treatment (Figure 2A–C). In great contrast, MRC5-polιKD cells were found to be hypersensitive to hydrogen peroxide (H2O2), which causes mainly oxidation of DNA bases and SSBs (Figure 2D), and to menadione (Figure 2E), a redox-cycling quinone that generates intracellular ROS (Watanabe et al, 2004). Moreover, these cells were moderately sensitive to low doses of potassium bromate (data not shown), an agent that allows a more specific introduction of 8-oxo-G in the genome (Ballmaier and Epe, 1995). To rule out the possibility that this sensitivity could be a side effect of the stable shRNA procedure, we transiently complemented the MRC5-polιKD cells with POLI cDNA bearing silent mutations to avoid the recognition of the resulting mRNA by shRNA polι (Figure 2F). We observed that the sensitivity of complemented MRC5-polιKD cells to H2O2 was rescued to the level of the control cell line. This indicates that the sensitivity of the knockdown cell line to H2O2 treatment is due to the low level of polι protein.
Figure 2.
Sensitivities of MRC5-polιKD cells to killing by various DNA-damaging agents. For each survival experiment, cells were exposed to increasing doses of the indicated genotoxic agent and counted 72 h later. Percentage of control growth was plotted for each data point, representing the mean of three independent experiments. (A) Cells were exposed to UVC, (B) UVA and (C) EMS for 1 h at 37°C in serum-free medium. (D) Cells were exposed to H2O2 for 10 min at 4°C. (E) Cells were exposed to menadione for 1 h at 37°C. (F) On the left panel, MRC5-polιKD cells were transfected with either an empty vector (closed triangle) or a vector expressing polι (open triangle). After 24 h, cells were exposed to H2O2 as described above. On the right panel, western blots of extracts of MRC5-polιKD and MRC5-NT cells containing an empty vector or polι expression vector, which were separated by SDS–PAGE and blotted using anti-polι antibody, are shown.
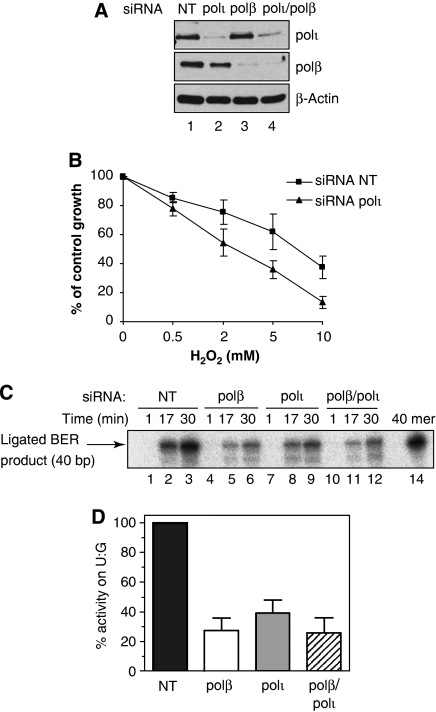
Because polβ−/− MEFs are only slightly sensitive to H2O2 exposure (Sobol et al, 1996; Fortini et al, 2000; Horton et al, 2002, 2005; Polosina et al, 2004; Yoshimura et al, 2006), we investigated whether human cells expressing low levels of both polι and polβ show a stronger sensitivity to oxidative DNA damage. We therefore reduced the endogenous levels of polβ up to 80% using siRNA duplexes in MRC5-polιKD and MRC5-NT cells (Figure 3A) and we then monitored cell survival after H2O2 treatment. We did not observe any difference in the sensitivity of MRC5-polιKD/siRNA polβ when compared with MRC5-polιKD/siRNA NT (Figure 3B). Furthermore, the MRC5-NT cells transiently downregulating polβ were not sensitive to H2O2. However, we could observe a moderate sensitivity of MRC5-NT/polβKD cells to the thymidine analogue 5-hydroxymethyl-2′-deoxyuridine (hmdUrd), demonstrating that the reduction in the levels of polβ we obtained by siRNA had a biological effect (Figure 3C). Our latest observation is in agreement with the reported study of Horton et al (2005) who used polβ−/− MEFs. Altogether, these data suggest that some types of oxidation-induced DNA damages are repaired in a polβ-independent and polι-dependent manner in human cells.
Figure 3.
Human cells downregulating polβ are not sensitive to oxidative damaging agents. (A) MRC5-polιKD and MRC5-NT cells were transfected with polβ-specific siRNAs (lanes 2 and 4) or scrambled siRNAs (lanes 1 and 3). After 72 h, cells were harvested and extracts were separated by SDS–PAGE, and incubated with anti-polι and anti-polβ antibodies. β-Actin detection was used as a loading control. (B) MRC5-polιKD and MRC5-NT cells were transfected with a pool of polβ-specific siRNAs 24 h before treatment with increasing doses of H2O2 for 10 min at 4°C. Cells were counted 72 h later. Percentage of control growth was plotted for each data point, representing the mean of three independent experiments. (C) MRC5 cells were transfected with polβ-specific siRNA. After 48 h, they were exposed to hmdUrd for 24 h and surviving cells were counted 72 h later and plotted as described above.
BER activity is reduced in extracts from cells downregulating pol
To examine whether polι can participate in SN-patch BER pathway, we reconstituted the repair of G·U oligonucleotide duplex in the presence of radioactively labelled dCTP and whole-cell extracts of MRC5 depleted for specialised DNA polymerases (Biade et al, 1998). To compare the BER activity in cell extracts having the same genetic background, we prepared extracts from MRC5 fibroblasts transiently transfected with siRNA specific to polβ, polι or both polβ/polι and not from the stable clone MRC5-polιKD. We used non-targeted siRNA (NT) as a negative control. Downregulation of the DNA polymerases was verified by western blot (Figure 4A). Likewise, the H2O2 sensitivity of MRC5 transiently transfected with siRNA against polι was confirmed (Figure 4B). As shown in Figure 4C, after 30 min incubation of G·U oligonucleotide duplex, a full-length 40 mer product was generated with high efficiency in control NT-MRC5 cell extract (lane 3). This indicates that uracil-DNA glycosylase excises U base, AP endonuclease cleaves resulting abasic site and generates single-strand nick with 5′ deoxyribose phosphate residue. A DNA repair polymerase then removes dRP residue and incorporates 32P-labelled dCMP nucleotide. Finally DNA ligase completes the restoration of the full-length 40 mer G·C duplex. In agreement with previous observations (Yoshimura et al, 2006), the polβ-depleted cell extract showed dramatic (up to 70%) decrease in the formation of fully repaired 40 mer duplex (Figure 4C, lanes 5 and 6, and Figure 4D) as compared with that of wild-type cell extract (Figure 4C, lanes 2 and 3, and Figure 4D). Interestingly, the polι-deficient cell extract also showed a strong reduction in the generation of a 40-mer product (Figure 4C, lanes 8 and 9, and Figure 4D) similar to that of the polβ-deficient extract (Figure 4C, lanes 5 and 6), suggesting that the DNA polymerase polι is involved in the BER pathway. However, depletion of both polβ and polι in MRC5 cell extracts did not decrease further formation of a 40-mer product (Figure 4D), suggesting (i) that the residual amount of polι and/or polβ is responsible for the remaining BER activity or (ii) the presence of third BER-specific DNA repair polymerase.
Figure 4.
Cells downregulating polι show an impaired uracil-initiated base excision DNA repair synthesis in human cell-free extracts. (A) MRC5 cells were transfected with scrambled siRNAs (lane 1) or polι-specific siRNAs (lanes 2 and 4) or with polβ-specific siRNAs (lanes 3 and 4). After 72 h, cells were harvested and extracts were separated by SDS–PAGE. Immunoblots were incubated with anti-polι or anti-polβ antibodies. β-Actin detection was used as a loading control. (B) MRC5 cells were transfected with a pool of polι-specific siRNAs 24 h before treatment with increasing doses of H2O2 for 10 min at 4°C. Cells were counted 72 h later. Percentage of control growth was plotted for each data point, representing the mean of three independent experiments. (C) Denaturing gel of G·U repair products after incubation with siRNA-transfected MRC5 whole-cell extracts: 50 nM of G·U was incubated with 10 μg extract at 37°C, and aliquots were withdrawn at different time intervals and analysed on a 20% denaturing PAGE. Formation of 40 mer repair product was quantified by phosphorimaging. (D) The average initial rate of BER DNA repair synthesis in whole-cell extracts. For comparison, the DNA repair synthesis in extract from cells transfected with scrambled siRNA (NT) was assumed as 100%. Error bars show the s.d. of the mean of three independent experiments.
pol-deficient cells display a delay in G1 phase following exposure to H2O2
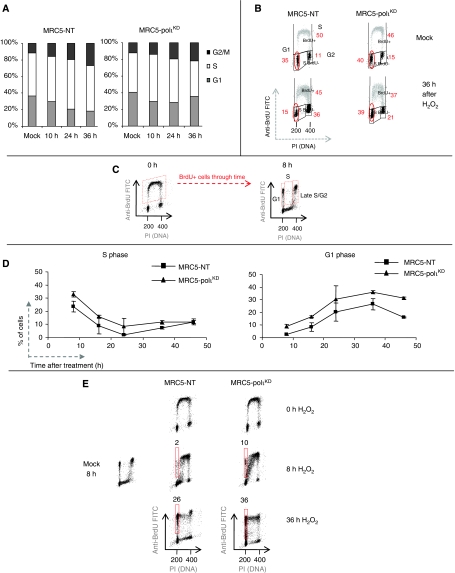
To better characterise the impact of polι downregulation on the response to oxidative DNA damage, we analysed the cell cycle profile of MRC5-polιKD and MRC5-NT cells following H2O2 treatment. We, therefore, employed two different approaches to measure the progression of cells. In the first set of experiments, MRC5-polιKD and MRC5-NT cells were exposed to H2O2, and were then pulse-labelled with BrdU just before harvesting at the indicated time points. In MRC5-NT cells, 36 h after treatment the number of cells in G1 phase decreased from 35 to 15%, whereas S-phase content remained unchanged (Figure 5A and B). In polιKD cells, the number of cells in G1 was moderately affected post-treatment. However, 36 h later it re-increased, whereas S-phase cell number decreased (Figure 5A). At this time point, both cell lines displayed a slight accumulation in G2/M phase. This shows that polιKD cells accumulate in G1 phase at later times after H2O2 and have difficulties in entering into a new S phase.
Figure 5.
MRC5-polιKD cells are delayed in G1 phase after H2O2 treatment. (A) MRC5-polιKD and MRC5-NT cells were exposed to 2.5 mM H2O2 for 10 min at 4°C and pulse-labelled with BrdU for 20 min before harvesting at indicated time points. The percentage of cells in all phases (G1/S/G2–M) is indicated in the histogram (mean of three independent experiments). (B) Cells were treated as described above and representative cell cycle distribution of the indicated cells as measured by BrdU incorporation (vertical axis, log scale) and DNA content (horizontal axis, linear scale) in flow cytometric analysis is shown. The top panels show the cell cycle distributions in mock-treated cells and bottom panels show cell cycle distribution 36 h after H2O2 treatment. The percentage of cells in all phases (G1/S/G2–M) is indicated. (C, D) Cells were pulse-labelled with 10 μm BrdU for 10 min prior to H2O2 treatment (10 min at 4°C). (C) Progression of BrdU-positive cells through time in the cell cycle was assessed by flow cytometry analysis. Representative distribution of mock-treated MRC5-NT is shown at T=0 and 8 h. BrdU-positive cells were gated as G1/early S, S and lateS/G2 cells. (D) MRC5-polιKD and MRC5-NT cells were treated as described above then the curves report the percentages of BrdU+ cells in S phase (left) or in G1 phase (right) at the indicated time points. The reported values are the mean of at least two independent experiments. (E) Representative cell cycle distribution of the indicated cells as measured by BrdU incorporation in flow cytometric analysis is shown. In red squares, the amount of BrdU+ cells in G1 phase is highlighted. A full-colour version of this figure is available at The EMBO Journal Online.
We asked whether this delay was a consequence of the fraction of cells exposed to H2O2 during DNA replication. To specifically monitor the progression of cells treated during the S phase, we pulse-labelled asynchronous cells with BrdU just before exposure or not to H2O2 (Figure 5C, left panel, the big box corresponds to cells treated in S phase). The cells were then grown in fresh medium for 8–48 h before analysis by flow cytometry. As they progressed through the cell cycle, BrdU-positive cells were gated in G1/early S-, S-, late S/G2-phase compartments according to their total DNA content (Figure 5C, right panel). We observed that both cell lines needed 24 h to progress through S phase after treatment (Figure 5D, left panel), whereas S phase lasted approximately 10–12 h in mock-treated cells (data not shown). This shows that H2O2 causes a S-phase delay in both cell lines. Interestingly, when we examined the progression of BrdU-positive cells through the G1 phase, we found that the percentage of MRC5-polιKD cells was higher than control cells, especially for the late time points (Figure 5D, right panel and Figure 5E). Altogether, these results suggested that the absence of polι affects the progression of cells through G1/S phase, following exposure to an oxidising agent. It raises the possibility that polι might operate in a ‘backup' mechanism to repair oxidising DNA lesions that were either bypassed or not repaired at all during the S and G2/M phases, and were, as such, found present in the G1 phase of the next cell cycle.
pol is recruited to sites of DNA damage in human cells
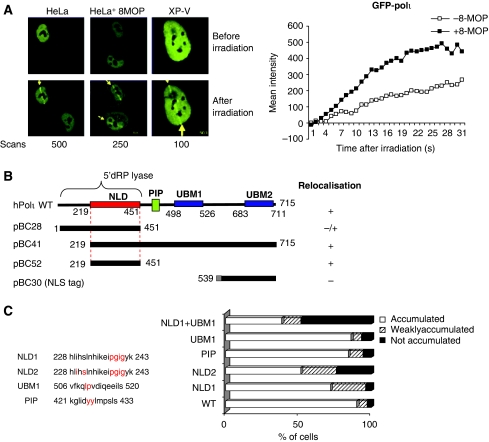
So far, our results suggested a role of polι in the repair of oxidative DNA damage. We next investigated the localisation pattern of green fluorescent protein (GFP)-tagged polι in living cells after inducing DNA damage in a restricted area of the nucleus by laser (Lan et al, 2005). We therefore overexpressed a full-length GFP–polι in HeLa cells and irradiated the cell nucleus with a 405 nm laser light. As shown in Figure 6A, left panel, polι accumulation was observed immediately after irradiation with 500 scans, which produces SSBs, base damage and double-strand breaks (Lan et al, 2005). As GFP–polι does not accumulate at the sites irradiated with lower laser dose, which mainly produces SSBs, possible substrates for GFP–polι may be either base damage or/and DNA double-strand breaks. However, the accumulation of polι was further enhanced by the presence of a photosensitiser (8-methoxypsoralen (8-MOP)) (Figure 6A, right panel) that increases accumulation of DNA glycosylases after irradiation (Orimo et al, 2006; Prasad et al, 2007), suggesting that polι is recruited to sites of base damage in living cells.
Figure 6.
Accumulation of polι at the sites of base damage induced by UV laser irradiation. (A) HeLa or XP-V cells transiently expressing GFP–polι were irradiated with 405 nm laser light (with or without preincubation with the photosensitiser 8-MOP). GFP signals before (top) and after (bottom) irradiation are shown (left panel). Numbers of scans are indicated below. Time course for the accumulation of GFP–polι without (open squares) or with (open squares) 8-MOP, after irradiation with a 405 nm laser (right panel). (B) Schematic representation of polι domains: UBM, ubiquitin-binding motif; NLD, nuclear localisation domain; PIP, interaction domain with PCNA; 5′dRP lyase domain. polι deletion constructs were made as described earlier (Kannouche et al, 2003). The fragments were cloned downstream of the GFP tag and transfected into HeLa cells. The cells were irradiated with a 405 nm laser light and the accumulation of mutants at the sites of DNA damage was analysed as indicated: +: accumulation in all scanned cells; +/−: accumulation in some cells; −: no accumulation. (C) Point mutations were introduced in GFP–polι by site-directed mutagenesis. The residues that were changed to alanine are indicated in red. The first and the last amino acids of each sequence are numbered. In the right panel, HeLa cells were transfected with different GFP–polι mutants and incubated for 24 h. Cells were then irradiated with a 405 nm laser light and the percentage of GFP–polι-expressing cells in which the protein was localised at the sites of oxidative damage was determined.
After UVC irradiation, the accumulation of polι into replication foci is largely dependent on polη (Kannouche et al, 2003). We aimed to ask whether polι could relocalise at the sites of laser-induced DNA damage in the absence of polη. XP-variant cells (XP2SASV) were transfected with peGFP–polι and were then irradiated. We observed an accumulation of GFP–polι at the irradiated sites (Figure 6A, right panel), suggesting that the recruitment of polι at the sites of oxidative DNA damage does not require the presence of polη.
The recruitment of pol to sites of DNA damage in human cells requires the NLD and UBM domains
To identify the domains of polι required for this relocalisation, we examined the localisation pattern of different GFP–polι mutants containing gross deletions and/or point mutation after irradiation. The primary sequence of polι (715 amino acids) contains no clear NLS. However, it has been reported that the sequences necessary for nuclear localisation are in the region of amino acids 219–451 (named NLD for nuclear localisation domain) (Kannouche et al, 2003). In this region (aa 228–252), Bebenek et al (2001) identified a helix-hairpin-helix (HhH) motif similar to that found in the NH2-terminal domain of polβ, which catalyses excision of a 5′dRP group from DNA. Recently, two ubiquitin-binding motifs (UBM1 and UBM2) were identified in the C terminus of polι at positions 498–526 and 679–707 in the human sequence. These UBM domains bind ubiquitin in a direct and non-covalent manner (Bienko et al, 2005; Plosky et al, 2006) (Figure 6B).
As shown in Figure 6B, removal of the N-terminal 218 amino acids (pBC41) did not affect the relocalisation of the protein at irradiated sites. pBC28, which contains a deletion of the C terminus from amino acid 451–715, was also able to relocalise but less efficiently, suggesting that the UBM domains might have a function in the recruitment of polι. Surprisingly, the construct containing amino acid 219–451 (pBC52), which corresponds to the NLD was sufficient for relocalisation. In contrast, the last 80 aa of polι are not sufficient to allow a relocalisation of the fusion protein at irradiated sites. These results indicate that the sequences necessary for relocalisation of polι at the sites of oxidative DNA damage are probably within the NLD region, even though the UBMs might also have a function in these dynamics.
We then investigated more precisely the importance of these domains in the relocalisation of polι by making point mutations (Figure 6C). The HhH of polι, which is containing the NLD, is also conserved among X-family proteins such as polβ and polλ (Moon et al, 2007). In contrast, this conservation is not detected among Y-family DNA polymerases (Supplementary Figure 1A). The 3D schematic structure shows some overlapping of the residues of the HhH of polβ and polι, mainly with the hairpin and the second helix (Supplementary Figure 1B). However, this overlap is less obvious than the one reported for polβ and polλ (Moon et al, 2007). We introduced point mutations in the helix 1 (H1) as well as in the hairpin of the polι sequence (Figure 6C). In addition, we constructed mutants harbouring a point mutation in the UBM1 domain. The protein with a mutation in the PCNA-interacting protein (PIP) box motif has been described earlier (Vidal et al, 2004). All these mutants were made as fusions with GFP protein to allow analysis of their localisation after irradiation by the laser. As expected, wild-type polι was able to accumulate at the sites of laser-induced DNA damage in 91% of irradiated cells (Figure 6C, right panel). Mutations in the UBM1 or PIP domain did not affect the relocalisation of the protein. In striking contrast, the NLD1–UBM1 mutant relocalised in only 38% of irradiated cells, whereas the single mutants NLD1 and NLD2 accumulated at the sites of DNA damage in 69 and 63% of cells, respectively. We therefore conclude that the HhH motif of polι, together with the UBM, contribute to an efficient recruitment to sites of laser-induced DNA damage.
To validate these observations, we examined the importance of these polι domains in the sensibility of cells to H2O2. In addition, the lysine residue K72 of polβ functions as a Schiff base during the β-elimination reaction. We speculated that the Lys237 of polι is its orthologue (see the arrow in Supplementary Figure 1). We checked whether the Lys237 was required to complement the MRC5-polιKD phenotype. We then transiently complemented the MRC5-polιKD cells with POLI cDNA-bearing silent mutations and different point mutations in NLD or UBM domains (Supplementary Figure 2). We observed that the sensitivity of complemented MRC5-polιKD cells to H2O2 was rescued with most of the polι mutants. Interestingly, we found an incomplete complementation of MRC5-polιKD phenotype when we expressed the NLD1–UBM1 mutant confirming that the HhH motif, together with the UBM of polι, is required for its function in the response to oxidative stress.
pol binds to chromatin after oxidative damage
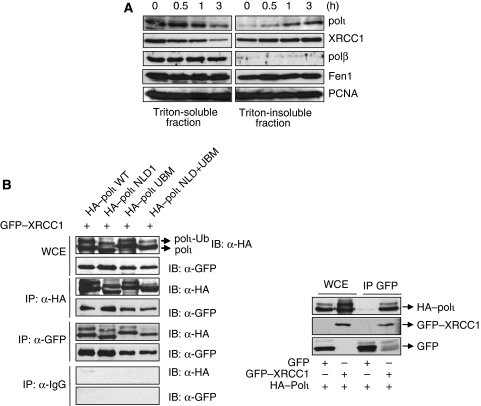
We reported earlier that polι accumulates together with polη and PCNA in replication factories following UVC irradiation. Yet whereas polη foci were completely resistant to extraction by triton, a non-ionic detergent, polι was totally solubilised, suggesting that polι is less tightly bound to the chromatin than polη after UVC exposure (Kannouche et al, 2003; Kannouche and Lehmann, 2004). To determine the subnuclear localisation of polι and of different BER factors after oxidative damage, MRC5 fibroblasts were treated with H2O2, harvested and lysed in an appropriate buffer containing triton (Kannouche et al, 2004). After centrifugation, the supernatant was kept (triton-soluble fraction), whereas the pellet was solubilised in a buffer containing DNase enzyme (chromatin-bound fraction). The fractions were analysed for polι by western blotting. We found that the amount of polι in the chromatin-bound fraction was increased substantially following H2O2 treatment (Figure 7A). Similar results were obtained after irradiation of the cells with UVA (200 KJ/m2) (Supplementary Figure 3A). Additionally, polι foci induced by UVA irradiation were resistant to triton extraction (Supplementary Figure 3B). We also found that the scaffold factor XRCC1 became triton-resistant under conditions of oxidative stress as previously observed in HeLa cells (Heale et al, 2006). Unexpectedly, polβ was found in the triton-soluble fraction after both H2O2 (Figure 7A) and methylmethane sulphonate (MMS) treatment (data not shown), suggesting that its interaction with chromatin during BER must be very transient. The flap endonuclease 1 (FEN1), which is involved in LP BER as well as in DNA replication, was partially found in the triton-insoluble fraction. These data indicate that polι and XRCC1 are recruited to the chromatin after H2O2 treatment and we assume that this recruitment reflects a biological role of polι following oxidative damage in human cells.
Figure 7.
polι becomes chromatin-bound after H2O2 and interacts with XRCC1. (A) MRC5 cells were exposed to 5 mM H2O2 for 10 min at 4°C and incubated for the indicated times prior to harvesting and subjected to fractionation into triton-soluble and triton-insoluble fractions. Extracts were analysed by western blotting with the indicated antibodies. (B) HEK293 cells were co-transfected with GFP–XRCC1 and different HA–polι mutants as indicated. For immunoprecipitation, anti-HA, anti-GFP or pre-immune IgG antibodies were incubated with cell lysates. On the left, immunoprecipitates were analysed by immunoblotting with α-HA or α-GFP antibodies. WCE: whole-cell extracts containing 1/20 of the lysates used for immunoprecipitation. On the right, immunoprecipitation of GFP alone was used as a negative control for interaction with HA–polι.
pol interacts with XRCC1 in human cells
After oxidative DNA damage, the BER factor XRCC1 has a major function by interacting with multiple repair partners, including PARP-1, polβ, PCNA, ligase III, APE1 and PNK (reviewed in Caldecott, 2003a). As our cellular fractionation experiments showed that both polι and XRCC1 became triton resistant following H2O2 treatment, we sought to see whether they could also interact physically in vivo. To check this hypothesis, both full-length and mutated polι proteins were tagged with N-terminal HA epitopes and were co-expressed in cells with GFP–XRCC1. Cell lysates were immunoprecipitated with anti-HA antibody. Interestingly, GFP–XRCC1 was observed in the immunoprecipitate containing the full length of polι or the mutants, reflecting a tight association of the two proteins in vivo (Figure 7B). This interaction was also confirmed by a reverse immunoprecipitation of GFP–XRCC1 with α-GFP antibodies and immunoblotting with anti-HA antibodies. These results strongly support a role for polι in some aspect of BER in vivo. However, as all polι mutants were able to interact with XRCC1, we concluded that the HhH and UBM1 motifs are not required for this interaction.
Discussion
Nine years after its discovery, the function of the Y-family DNA polymerase iota has remained an enigma. Mice lacking polι have a normal phenotype (McDonald et al, 2003). We have now uncovered a clear role of polι in protecting cells from oxidative damage.
We have demonstrated that human cells downregulating polι exhibit hypersensitivity to oxidative base damage induced by H2O2. We also found that polι becomes triton resistant after H2O2 treatment and interacts with the BER factor XRCC1. Furthermore, GFP-tagged polι accumulates at the sites of oxidative DNA damage in living cells. Our mutagenesis experiments suggest that two different domains of polι are required for this relocalisation: its dRP lyase region and its UBM1. Altogether, our results strongly suggest that polι can protect cells against oxidative damage.
Human pol is involved in the cellular response to oxidising agents
This is the first report that shows a menadione and H2O2 sensitivities in human fibroblasts deficient for a DNA polymerase. This sensitivity is a direct result of the reduction of functional polι as transfection of MRC5-polιKD cells with full-length POLI cDNA reversed this phenotype. So far, sensitivities to DNA-damaging agents have been observed in chicken DT40 cells and/or MEFs inactivated or mutated in POLB, POLL or POLQ genes (Horton et al, 2002; Braithwaite et al, 2005; Yoshimura et al, 2006; Tano et al, 2007).
polβ is the classical BER protein for removal of the 5′dRP moiety resulting from APE1 cleavage (reviewed in Almeida and Sobol, 2007). It has been extensively reported that MEF deficient for polβ is hypersensitive to mono-functional DNA-alkylating agents such as MMS. In contrast, polβ−/− cells are not or are only slightly sensitive to oxidising agents that produce oxidative DNA damage, suggesting that polβ is not of primary importance in BER of oxidised DNA, or its activity can be complemented by another DNA polymerase. polι has been shown to be capable of removing the 5′dRP lesion subsequent to APE1 strand cleavage, but a deficiency in polι does not render cells sensitive to alkylation (Trivedi et al, 2005 and this paper). In contrast, cells with a reduced level of polι are sensitive to H2O2 and this phenotype is not synergistically increased by concomitant decrease of polβ level, suggesting that human DNA polymerase iota is important for protection of cells against cell killing due to oxidative stress. Altogether, these data indicate that, although both DNA polymerases exhibit dRP lyase activity, the two polymerases do not share the same lesion substrate. Presumably, polι participates in a specialised form of BER as speculated earlier by Bebenek et al (2001). This assumption is strengthened by the fact that extracts from cells downregulating polι show a reduced BER activity.
Accumulation of pol-deficient cells in G1 phase after H2O2 treatment
We showed that reduced levels of the polι protein have an impact on cell cycle progression before and after DNA damage. Indeed, we detected a slight accumulation of MRC5-polιKD cells in G1 phase in untreated cells, probably due to spontaneous DNA damage (Figure 1C). This effect is increased after exposure to H2O2 (Figure 5). This G1 arrest was not observed immediately after treatment but rather occurred at late time points (24–36 h post-treatment). When we analysed the cell cycle progression of cells treated with H2O2 in S phase (revealed with BrdU pulse labelling prior to treatment), we obtained the same FACS profile with an accumulation of MRC5-polιKD cells in G1 phase 24–36 h post-treatment as compared with the control cells. These data revealed that when MRC5-polιKD cells are exposed to H2O2 during S phase, replication rates are reduced in the first S phase to the same extent as in control cells (Figure 5D). However, progression into the second cell cycle causes a G1 arrest in polι-deficient cells, whereas the control cells can progress through the second S phase. The simplest interpretation is that when MRC5-polιKD cells arrive in the G1 phase, the limited quantity of polι leads to a reduced capacity in repairing or tolerating the remaining oxidised lesions present on the DNA inducing G1 arrest.
Nevertheless, it is noteworthy that in normal cells, polι is located in replication foci during S phase (Kannouche et al, 2003). This polymerase is able to incorporate a guanine opposite uracil and its derivatives providing a possible S-phase-dependent mechanism that can decrease the mutagenic potential of lesions formed by the deamination of cytosines (Vaisman and Woodgate, 2001). Therefore, even if the MRC5-polιKD cells are allowed to undergo the first round of replication, we hypothesise that the absence of polι in S phase after H2O2 exposure affects the repair and/or bypass of some oxidised DNA lesions. Thus, MRC5-polιKD cells will contain more mispairs and/or unrepaired lesions as compared with the control cells when cells will progress through the G1 phase inducing a G1 arrest.
pol interacts with XRCC1 and is recruited to sites of oxidative DNA damage
We observed that polι was bound to chromatin following H2O2 treatment. This recruitment was also detected after 200 kJ/m2 of UVA irradiation. In contrast, polι remained triton soluble after UVC irradiation (Kannouche and Lehmann, 2004), demonstrating a specific involvement of polι in the response to oxidative stress. Further in vivo evidence for a role of polι in some aspect of BER is provided by its interaction with XRCC1, which has been shown to have central function in the repair of breaks in DNA (Caldecott, 2003b; Lan et al, 2004; Almeida and Sobol, 2007; Dianov and Parsons, 2007). This indicates that XRCC1 and polι exist in a common complex in vivo.
We also found that polι was recruited to sites of oxidative DNA damage induced with high laser dose. Although the laser treatment used in this study produces oxidative base damage, SSBs and double-strand DNA breaks (DSBs), we assume that polι is localised to sites of oxidative base damage as we observed that the accumulation of this polymerase was enhanced in the presence of 8-MOP, a photosensitiser that enhances the accumulation of human OGG1 and NTH1 after laser irradiation (Orimo et al, 2006; Prasad et al, 2007). Moreover, we excluded the possibility that polι was implicated in double-strand break repair as cells downregulating polι were not sensitive to neocarzinostatin (data not shown), a drug that induces high ratio of DSBs (Ohtsuki and Ishida, 1975; Favaudon, 1982).
We showed that the NLD regions of polι and its UBM1 domain were required for an efficient localisation of the protein to sites of oxidative DNA damage. Likewise, these domains are required to rescue the H2O2 sensibility of MRC5-polιKD cells. In contrast, they appeared to be independent of the XRCC1–polι interaction, as all proteins mutated in NLD and/or UBM domains are still able to bind to XRCC1. It is possible that these domains of polι might target it to complexes assembled at the sites of damage. The UBM1 of polι binds ubiquitin in a direct and non-covalent manner (Bienko et al, 2005; Plosky et al, 2006). Therefore, polι could bind to any number of proteins that are specifically ubiquitinated. It has been shown that polι is able to bind monoubiquitinated PCNA through its PIP and UBM1 domains (Vidal et al, 2004; Bienko et al, 2005; Plosky et al, 2006). In our experiments, it seems unlikely that the localisation of polι at the sites of oxidative damage was dependent on PCNA as (1) polι mutated in the PIP domain is still localised to irradiated sites and (2) PCNA accumulates at SSBs, whereas polι relocalises to oxidative base damage (Lan et al, 2004; Hashiguchi et al, 2007). Interestingly, we also observed that the recruitment of polι at the sites of oxidative base damage was independent of polη, in contrast to its recruitment into replication factories during S phase (Kannouche et al, 2003). Thus, after UVC polι interacts with polη and PCNA into replication factories, whereas after oxidative DNA damage, polι is probably assembled into a new complex involving ubiquitinated proteins and XRCC1.
Are our data consistent with other features of pol?
Several lines of evidence are consistent with our finding that polι is involved in the response to oxidative DNA damage:
At the biochemical level, polι displays a 5′dRP activity. In reactions reconstituted in vitro with uracil-DNA glycosylase, AP endonuclease and DNA ligase, polι can use its dRP lyase and polymerase activities to repair G·U and A·U pairs in DNA. Moreover, polι is partially able to complement in vitro single-nucleotide BER deficiency of a DNA polβ null-cell extract (Bebenek et al, 2001; Prasad et al, 2003), suggesting that polι and polβ have at least some degree of mechanistic overlap.
At the gene expression level, in response to hypoxia/reoxygenation, hsPOLI transcript level accumulates in a hypoxia-inducible factor 1 (HIF1)-dependent way. This induction is not observed for the other Y-family polymerases (Ito et al, 2006). As reoxygenation produces reactive oxygen species (ROS) that generate DNA damage (Hammond et al, 2003a, 2003b), this upregulation of POLI strongly suggests that polι protein is required to repair and/or bypass the ROS-induced DNA damage. In addition, it has been shown that POLI is overexpressed in a wide range of tumour types (Albertella et al, 2005). This observation could be explained by the fact that within a three-dimensional microenvironment, tumour cells can be exposed to fluctuating levels of cycling hypoxia (acute hypoxia followed by reoxygenation) (Bristow and Hill, 2008).
At the genetic level, Ohkumo et al (2006) have reported that UVB induces mesenchymal tumours in mice deficient for polι. The authors concluded that UV-induced DNA lesions are primarily responsible for mesenchymal skin tumour formation in POLI-deficient mice. However, chronic UVB exposure of mice also produces ROS (Pelle et al, 2003; Wulff et al, 2008). Regarding our data, we can speculate that UVB-induced mesenchymal tumours in POLI-deficient mice are caused, to a certain extent, by ROS, which induce oxidative DNA lesions that are not properly repaired in dermis, in the absence of the polι protein.
Taken together, our data reveal a novel role of human DNA polι in the maintenance of the genome integrity following exposure of cells to oxidative stress. We show for the first time a phenotype of human cells downregulating polι, and we have, thus, expanded our understanding to elucidate the biological role of this DNA polymerase. The fact that polι exists as a complex together with XRCC1 in cells implicates of a TLS polymerase in mechanisms of repair of oxidative lesions. Recent data have reported that other Y-family polymerases are involved in non-TLS processes such as polη in homologous recombination and polκ in NER (Kawamoto et al, 2005; McIlwraith et al, 2005; Ogi et al, 2006). The next step will be to understand how polι is targeted to use its intrinsic polymerase and dRP lyase activities only at the appropriate time and place in human cells.
Materials and methods
Transfection and plasmid constructions
Downregulation of polι was carried out with the vector pSUPER.puro (Oligoengine) to express a short hairpin RNA (shRNA) directed against the POLI mRNA. The 21-nucleotide sequence against polι was synthesised by Oligoengine and is located towards the C terminus of the protein (5′-GAAGGAAGCCTCATACAGTTT-3′ and 3′-ACTGTATGAGGCTTCCTTCTT-5′). As a control, we used a scrambled sequence (5′-CCCCGCGCGCTTTGTAGGATT-3′ and 3′-AAAAGCGCGCTTTGTAGGATT-5′).
The protocol used to obtain the shRNA-polι plasmid was in accordance with the manufacturer's protocol. For stable transfectants, MRC5 cells were transfected with pSUPER plasmids containing the appropriate shRNA. At 48 h after transfection, cells were incubated in a selection medium containing 0.45 μg/ml puromycin (Sigma-Aldrich). Selection was continued for 2 weeks and stable transfectants were isolated. We designated the cell lines downregulating polι MRC5-polιKD followed by the clone number. In parallel, we made cell line expressing scrambled sequence as a negative control and it was named MRC5-NT. The GFP–XRCC1 and GFP–polι plasmid have been described earlier (Kannouche et al, 2003; Campalans et al, 2005, respectively). To generate the HA–polι, the full-length cDNA for human polι was inserted into the vector phCMV2 (Gene Therapy Systems) by cloning into restriction sites XhoI–BamHI. The sequence of polι was confirmed by DNA sequencing. Cells were transfected with the plasmids using Exgen500 (Fermentas) according to the manufacturer's protocol. For polβ siRNA transfection, we used the Dharmacon SMARTpool of four polβ-specific siRNAs. Likewise, for transient downregulation of polι, we used the Dharmacon SMARTpool of four polι-specific siRNAs. Cells were transfected with 30 nM of siRNA pool using Interferin reagent (Polyplus) according to the manufacturer's instructions, and were incubated for the indicated time points.
In vitro site-directed mutagenesis
Point mutations were introduced in different positions of GFP–polι using QuickChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. The sequences of the primers used are described in Table I. Mutant NLD1 was made using the primers NLD 240–243, mutant NLD2 was made using the primers NLD 240–243 and NLD 230–232, mutant UBM1 was made using the primers UBM 510–511 and mutant PIP was constructed as described earlier (Vidal et al, 2004). The panel of polι mutants were cloned into phCMV2 vector as described above. A silencing mutation in the wt POLI cDNA was introduced using primers 336–340.
Table 1.
Primers used in site-directed mutagenesis
| Primer name | Sequence |
|---|---|
| NLD 240_243 | 5′-TATTCATAGTTTGAATCACATAAAGGAAATAGCTGCTGC TGCCTATAAAACTGCCAAATGTCTTGAAGCACTG-3′ |
| NLD 230_232 | 5′-GTCTTATTACCTGAAAGTTGTCAACATCTTGCTCATGCT TTGAATCACATAAAGGAAATAGCTGCTGC-3′ |
| UBM 510_511 | 5′-GAAGGTGTTGACCAAGAAGTCTTCAAGGCGGCCCAAGTA GATATTCAAGAAGAAATCCTT-3′ |
| Silencing 336_340 | 5′-CTTTTAAACAGAGTATGCCAAGATGGTCGGAAACCACAC ACAGTGAGATTAATAATCCGTCGGTA-3′ |
Proliferation assay
MRC5-polιKD or MRC5-NT cells were plated at 2 × 105 per well of a six-well plate and incubated for 24 h. Cells were then exposed to increasing doses of H2O2 (0–7 mM; Sigma-Aldrich) in cold PBS at 4°C for 10 min; or EMS (0.01–1 mM; Sigma-Aldrich) in a medium without FCS at 37°C for 1 h or were treated with 0–20 μM of 5-hydroxymethyl-2′-deoxyuridine (hmdUrd; Sigma-Aldrich) for 24 h or exposed to increasing doses of menadione for 1 h in complete MEM medium at 37°C (0–50 μM; Sigma-Aldrich). UVA (320–400 nm) radiation was achieved by a metal halide lamp equipped with anticaloric filter KG1. The fluency of the UVA lamp was 12.3 J/s/m2 and the cells were irradiated in MEM medium without phenol red. For the UVC (254 nm) lamp, the fluency was 0.35 J/s/m2 and the cells were irradiated without any medium. After each treatment, cells were rinsed twice with PBS, incubated for 72 h in complete MEM medium and counted by Trypan blue staining using a Neubauer haemocytometer or an automatic counter.
Flow cytometric analysis
To study the cell cycle distribution, cells were treated for 10 min with 2.5 mM H2O2 in cold PBS, at 4°C and, at indicated times, 10 μM of BrdU (Sigma-Aldrich) was added to the dishes for 20 min prior harvesting. Alternatively, S-phase cells were pulse-labelled with 10 μM BrdU in complete medium for 10 min at 37°C. Cells were then treated as described above and re-incubated in fresh medium for the indicated time. Cells were then trypsinised, washed in PBS and fixed in 80% ice-cold ethanol overnight at −20°C. For BrdU staining, samples were incubated in 15 mM pepsin for 20 min at 37°C. The cells were pelleted and incubated with 2 N HCl and incubated for 20 min at room temperature. The pellet was washed in buffer Bu (0.1% FCS, 0.1% Tween 20, 0.1 M Hepes in PBS), and was incubated with anti-BrdU antibody diluted at 1:50 in buffer Bu (clone Bu20a; Dako) for 45 min. After centrifugation, cells were resuspended and incubated with anti-mouse-FITC antibody diluted at 1:50 in buffer Bu (Vector). For total DNA staining, cells were centrifuged and resuspended in PBS containing 25 μg/ml propidium iodide (Sigma-Aldrich) and 50 μg/μl RNAse (Sigma-Aldrich). The samples were analysed by flow cytometry on a FACScalibur (Becton Dickinson, BD Sciences) using the CellQuest Pro software (BD Sciences).
Laser light irradiation and microscopy
Fluorescence images were obtained and processed using a FV-500 confocal scanning laser microscopy system (Olympus). The microscope is coupled with microirradiation facilities to emit 405 nm laser light as described earlier (Lan et al, 2005). Precisely, we used the 405 nm scan laser system for irradiating the cells in the epifluorescence path of the microscope system. The power of the scan laser can be controlled by altering the scanning times (250 or 500 scans). The scan laser light of 405 nm wavelength delivers within 5 ms around 1600 nW of energy. Thus, cells were incubated on a 37°C hot plate in glass-bottomed dishes placed in chambers to prevent evaporation and then irradiated. After irradiation, images were captured at 10- to 20-s intervals for up to 5–10 min. The energy of the fluorescent light at the irradiated site was measured. The mean intensity of the track in the irradiated cell was obtained after subtracting the background intensity.
Further details of materials and methods are presented as Supplementary data.
Supplementary Material
Supplementary Figures
Supplementary Information
Acknowledgments
We thank Dr R Woodgate (NIH Bethesda, MD, USA) for providing purified antibody for polι, Dr P Radicella for the GFP–XRCC1 plasmid and Dr V Favaudon for providing the neocarzinostatin drug. We also thank AR Lehmann for the GFP–polι plasmids and for his critical comments on the paper. We are grateful to Caroline Pouvelle for assistance with cell culture and Yann Lecluse for FACS analysis. This study was supported by Association pour la Recherche sur le Cancer (ARC, no. 3573), by Fondation pour la Recherche Medicale (INE20040300464) and by Institut National du Cancer no. PL28 to PK, by Genome Network Project Japan to AY, and by CNRS GDRE182, ARC No. 4032, 3655 and EDF RB2007-02 to Murat Saparbaev. TBP was supported by an ARC fellowship and AZ was supported by Contract MRTN-Ct-2003-503618.
References
- Albertella MR, Lau A, O'Connor MJ (2005) The overexpression of specialized DNA polymerases in cancer. DNA Repair (Amst) 4: 583–593 [DOI] [PubMed] [Google Scholar]
- Almeida KH, Sobol RW (2007) A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst) 6: 695–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier D, Epe B (1995) Oxidative DNA damage induced by potassium bromate under cell-free conditions and in mammalian cells. Carcinogenesis 16: 335–342 [DOI] [PubMed] [Google Scholar]
- Bebenek K, Tissier A, Frank EG, McDonald JP, Prasad R, Wilson SH, Woodgate R, Kunkel TA (2001) 5′-Deoxyribose phosphate lyase activity of human DNA polymerase iota in vitro. Science 291: 2156–2159 [DOI] [PubMed] [Google Scholar]
- Biade S, Sobol RW, Wilson SH, Matsumoto Y (1998) Impairment of proliferating cell nuclear antigen-dependent apurinic/apyrimidinic site repair on linear DNA. J Biol Chem 273: 898–902 [DOI] [PubMed] [Google Scholar]
- Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310: 1821–1824 [DOI] [PubMed] [Google Scholar]
- Braithwaite EK, Kedar PS, Lan L, Polosina YY, Asagoshi K, Poltoratsky VP, Horton JK, Miller H, Teebor GW, Yasui A, Wilson SH (2005) DNA polymerase lambda protects mouse fibroblasts against oxidative DNA damage and is recruited to sites of DNA damage/repair. J Biol Chem 280: 31641–31647 [DOI] [PubMed] [Google Scholar]
- Bristow RG, Hill RP (2008) Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer 8: 180–192 [DOI] [PubMed] [Google Scholar]
- Caldecott KW (2003a) Protein–protein interactions during mammalian DNA single-strand break repair. Biochem Soc Trans 31: 247–251 [DOI] [PubMed] [Google Scholar]
- Caldecott KW (2003b) XRCC1 and DNA strand break repair. DNA Repair (Amst) 2: 955–969 [DOI] [PubMed] [Google Scholar]
- Campalans A, Marsin S, Nakabeppu Y, O'Connor TR, Boiteux S, Radicella JP (2005) XRCC1 interactions with multiple DNA glycosylases: a model for its recruitment to base excision repair. DNA Repair (Amst) 4: 826–835 [DOI] [PubMed] [Google Scholar]
- Dianov GL, Parsons JL (2007) Co-ordination of DNA single strand break repair. DNA Repair (Amst) 6: 454–460 [DOI] [PubMed] [Google Scholar]
- Favaudon V (1982) On the mechanism of reductive activation in the mode of action of some anticancer drugs. Biochimie 64: 457–475 [DOI] [PubMed] [Google Scholar]
- Fortini P, Pascucci B, Belisario F, Dogliotti E (2000) DNA polymerase beta is required for efficient DNA strand break repair induced by methyl methanesulfonate but not by hydrogen peroxide. Nucleic Acids Res 28: 3040–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MF (2002) Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem 71: 17–50 [DOI] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ (2003a) ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem 278: 12207–12213 [DOI] [PubMed] [Google Scholar]
- Hammond EM, Green SL, Giaccia AJ (2003b) Comparison of hypoxia-induced replication arrest with hydroxyurea and aphidicolin-induced arrest. Mutat Res 532: 205–213 [DOI] [PubMed] [Google Scholar]
- Hashiguchi K, Matsumoto Y, Yasui A (2007) Recruitment of DNA repair synthesis machinery to sites of DNA damage/repair in living human cells. Nucleic Acids Res 35: 2913–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale JT, Ball AR Jr, Schmiesing JA, Kim JS, Kong X, Zhou S, Hudson DF, Earnshaw WC, Yokomori K (2006) Condensin I interacts with the PARP-1–XRCC1 complex and functions in DNA single-strand break repair. Mol Cell 21: 837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JK, Baker A, Berg BJ, Sobol RW, Wilson SH (2002) Involvement of DNA polymerase beta in protection against the cytotoxicity of oxidative DNA damage. DNA Repair (Amst) 1: 317–333 [DOI] [PubMed] [Google Scholar]
- Horton JK, Stefanick DF, Naron JM, Kedar PS, Wilson SH (2005) Poly(ADP-ribose) polymerase activity prevents signaling pathways for cell cycle arrest after DNA methylating agent exposure. J Biol Chem 280: 15773–15785 [DOI] [PubMed] [Google Scholar]
- Ito A, Koshikawa N, Mochizuki S, Omura K, Takenaga K (2006) Hypoxia-inducible factor-1 mediates the expression of DNA polymerase iota in human tumor cells. Biochem Biophys Res Commun 351: 306–311 [DOI] [PubMed] [Google Scholar]
- Johnson RE, Washington MT, Haracska L, Prakash S, Prakash L (2000) Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature 406: 1015–1019 [DOI] [PubMed] [Google Scholar]
- Kannouche P, Fernandez de Henestrosa AR, Coull B, Vidal AE, Gray C, Zicha D, Woodgate R, Lehmann AR (2003) Localization of DNA polymerases eta and iota to the replication machinery is tightly co-ordinated in human cells. EMBO J 22: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannouche PL, Lehmann AR (2004) Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3: 1011–1013 [PubMed] [Google Scholar]
- Kannouche PL, Wing J, Lehmann AR (2004) Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14: 491–500 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, Masutani C, Hanaoka F, Nozaki K, Hashimoto N, Takeda S (2005) Dual roles for DNA polymerase in homologous DNA recombination and translesion DNA synthesis. Mol Cell 20: 793–799 [DOI] [PubMed] [Google Scholar]
- Lan L, Nakajima S, Komatsu K, Nussenzweig A, Shimamoto A, Oshima J, Yasui A (2005) Accumulation of Werner protein at DNA double-strand breaks in human cells. J Cell Sci 118: 4153–4162 [DOI] [PubMed] [Google Scholar]
- Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson SH, Yasui A (2004) In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc Natl Acad Sci USA 101: 13738–13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T (1982) DNA repair enzymes. Annu Rev Biochem 51: 61–87 [DOI] [PubMed] [Google Scholar]
- McDonald JP, Frank EG, Plosky BS, Rogozin IB, Masutani C, Hanaoka F, Woodgate R, Gearhart PJ (2003) 129-derived strains of mice are deficient in DNA polymerase ι and have normal immunoglobulin hypermutation. J Exp Med 198: 635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC (2005) Human DNA polymerase eta promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell 20: 783–792 [DOI] [PubMed] [Google Scholar]
- Moon AF, Garcia-Diaz M, Batra VK, Beard WA, Bebenek K, Kunkel TA, Wilson SH, Pedersen LC (2007) The X family portrait: structural insights into biological functions of X family polymerases. DNA Repair (Amst) 6: 1709–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogi T, Lehmann AR (2006) The Y-family DNA polymerase (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol 8: 640–642 [DOI] [PubMed] [Google Scholar]
- Ohkumo T, Kondo Y, Yokoi M, Tsukamoto T, Yamada A, Sugimoto T, Kanao R, Higashi Y, Kondoh H, Tatematsu M, Masutani C, Hanaoka F (2006) UV-B radiation induces epithelial tumors in mice lacking DNA polymerase eta and mesenchymal tumors in mice deficient for DNA polymerase iota. Mol Cell Biol 26: 7696–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker GC, Wang Z, Woodgate R (2001) The Y-family of DNA polymerases. Mol Cell 8: 7–8 [DOI] [PubMed] [Google Scholar]
- Ohtsuki K, Ishida N (1975) Neocarzinostatin-induced breakdown of deoxyribonucleic acid in HeLa-S3 cells. J Antibiot (Tokyo) 28: 143–148 [DOI] [PubMed] [Google Scholar]
- Orimo H, Tokura Y, Hino R, Kasai H (2006) Formation of 8-hydroxy-2′-deoxyguanosine in the DNA of cultured human keratinocytes by clinically used doses of narrowband and broadband ultraviolet B and psoralen plus ultraviolet A. Cancer Sci 97: 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelle E, Huang X, Mammone T, Marenus K, Maes D, Frenkel K (2003) Ultraviolet-B-induced oxidative DNA base damage in primary normal human epidermal keratinocytes and inhibition by a hydroxyl radical scavenger. J Invest Dermatol 121: 177–183 [DOI] [PubMed] [Google Scholar]
- Plosky BS, Vidal AE, Fernandez de Henestrosa AR, McLenigan MP, McDonald JP, Mead S, Woodgate R (2006) Controlling the subcellular localization of DNA polymerases iota and eta via interactions with ubiquitin. EMBO J 25: 2847–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosina YY, Rosenquist TA, Grollman AP, Miller H (2004) ‘Knock down' of DNA polymerase beta by RNA interference: recapitulation of null phenotype. DNA Repair (Amst) 3: 1469–1474 [DOI] [PubMed] [Google Scholar]
- Prasad R, Bebenek K, Hou E, Shock DD, Beard WA, Woodgate R, Kunkel TA, Wilson SH (2003) Localization of the deoxyribose phosphate lyase active site in human DNA polymerase iota by controlled proteolysis. J Biol Chem 278: 29649–29654 [DOI] [PubMed] [Google Scholar]
- Prasad R, Liu Y, Deterding LJ, Poltoratsky VP, Kedar PS, Horton JK, Kanno S, Asagoshi K, Hou EW, Khodyreva SN, Lavrik OI, Tomer KB, Yasui A, Wilson SH (2007) HMGB1 is a cofactor in mammalian base excision repair. Mol Cell 27: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol RW, Horton JK, Kuhn R, Gu H, Singhal RK, Prasad R, Rajewsky K, Wilson SH (1996) Requirement of mammalian DNA polymerase-beta in base-excision repair. Nature 379: 183–186 [DOI] [PubMed] [Google Scholar]
- Srivastava DK, Berg BJ, Prasad R, Molina JT, Beard WA, Tomkinson AE, Wilson SH (1998) Mammalian abasic site base excision repair. Identification of the reaction sequence and rate-determining steps. J Biol Chem 273: 21203–21209 [DOI] [PubMed] [Google Scholar]
- Tano K, Nakamura J, Asagoshi K, Arakawa H, Sonoda E, Braithwaite EK, Prasad R, Buerstedde JM, Takeda S, Watanabe M, Wilson SH (2007) Interplay between DNA polymerases beta and lambda in repair of oxidation DNA damage in chicken DT40 cells. DNA Repair (Amst) 6: 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissier A, Frank EG, McDonald JP, Iwai S, Hanaoka F, Woodgate R (2000) Misinsertion and bypass of thymine–thymine dimers by human DNA polymerase iota. EMBO J 19: 5259–5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW (2005) The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res 65: 6394–6400 [DOI] [PubMed] [Google Scholar]
- Vaisman A, Woodgate R (2001) Unique misinsertion specificity of poliota may decrease the mutagenic potential of deaminated cytosines. EMBO J 20: 6520–6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal AE, Kannouche P, Podust VN, Yang W, Lehmann AR, Woodgate R (2004) Proliferating cell nuclear antigen-dependent coordination of the biological functions of human DNA polymerase iota. J Biol Chem 279: 48360–48368 [DOI] [PubMed] [Google Scholar]
- Watanabe N, Dickinson DA, Liu RM, Forman HJ (2004) Quinones and glutathione metabolism. Methods Enzymol 378: 319–340 [DOI] [PubMed] [Google Scholar]
- Wulff BC, Schick JS, Thomas-Ahner JM, Kusewitt DF, Yarosh DB, Oberyszyn TM (2008) Topical treatment with OGG1 enzyme affects UVB-induced skin carcinogenesis. Photochem Photobiol 84: 317–321 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Kohzaki M, Nakamura J, Asagoshi K, Sonoda E, Hou E, Prasad R, Wilson SH, Tano K, Yasui A, Lan L, Seki M, Wood RD, Arakawa H, Buerstedde JM, Hochegger H, Okada T, Hiraoka M, Takeda S (2006) Vertebrate POLQ and POLbeta cooperate in base excision repair of oxidative DNA damage. Mol Cell 24: 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yuan F, Wu X, Wang Z (2000) Preferential incorporation of G opposite template T by the low-fidelity human DNA polymerase iota. Mol Cell Biol 20: 7099–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Information