Abstract
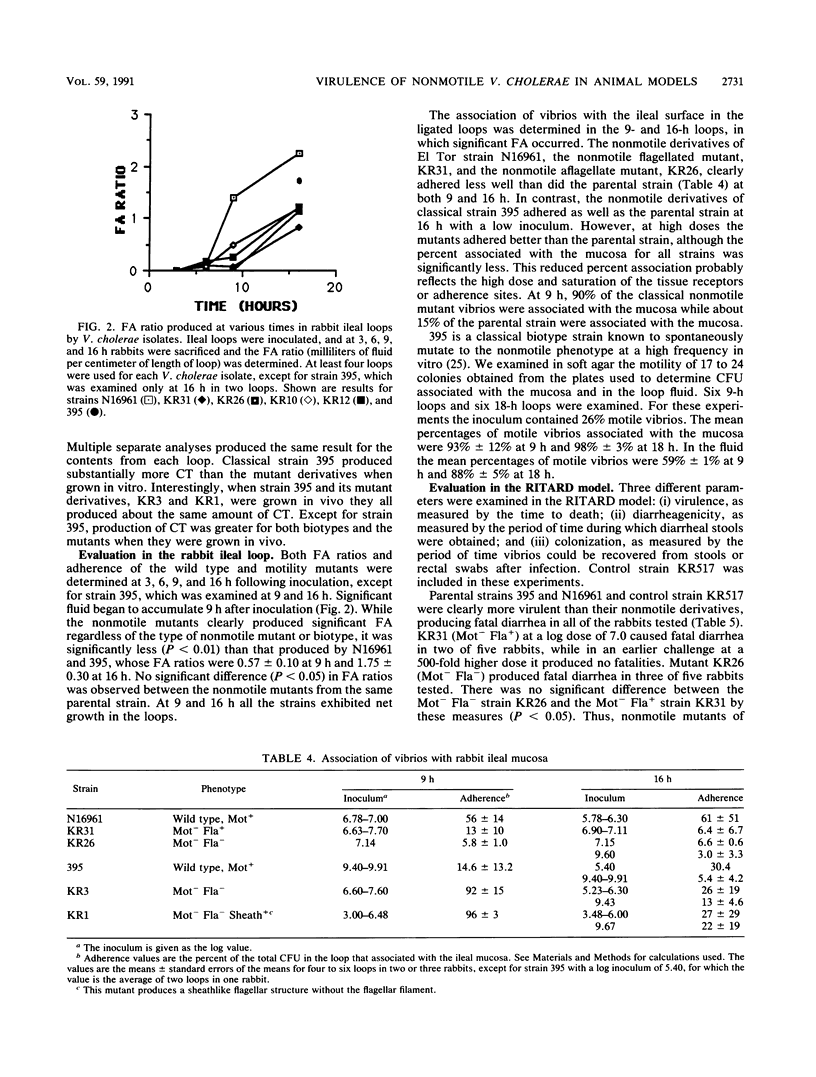
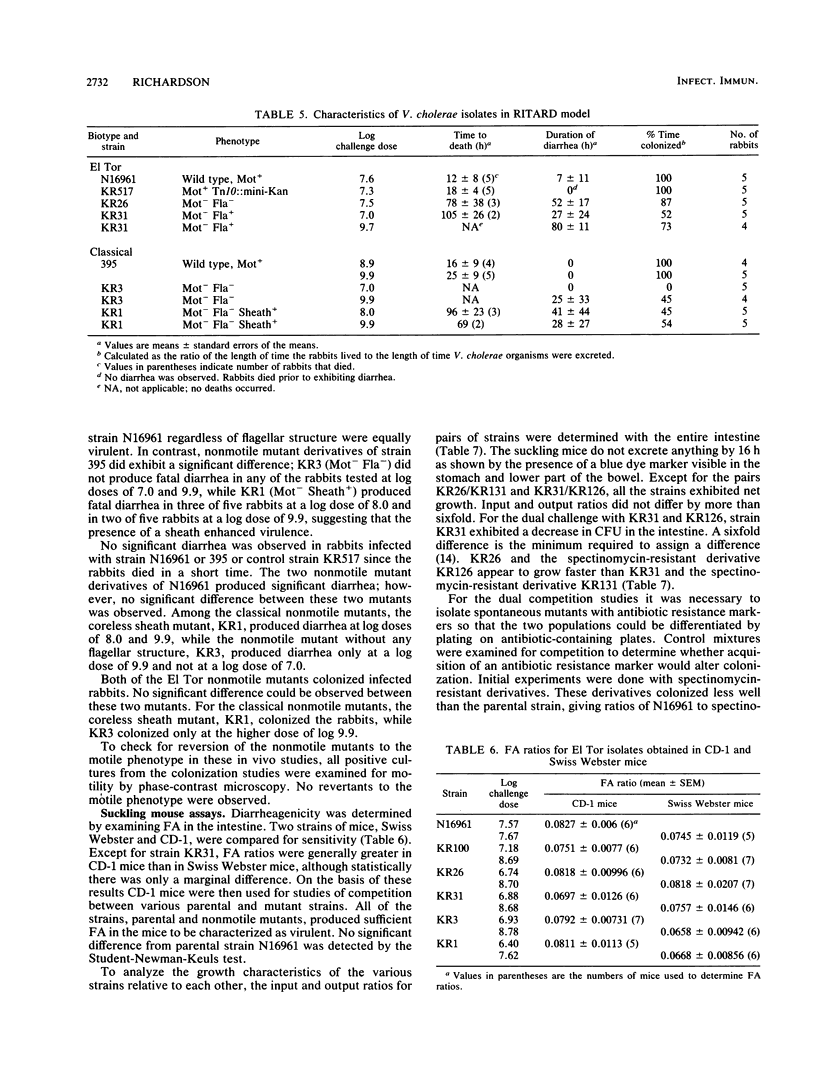
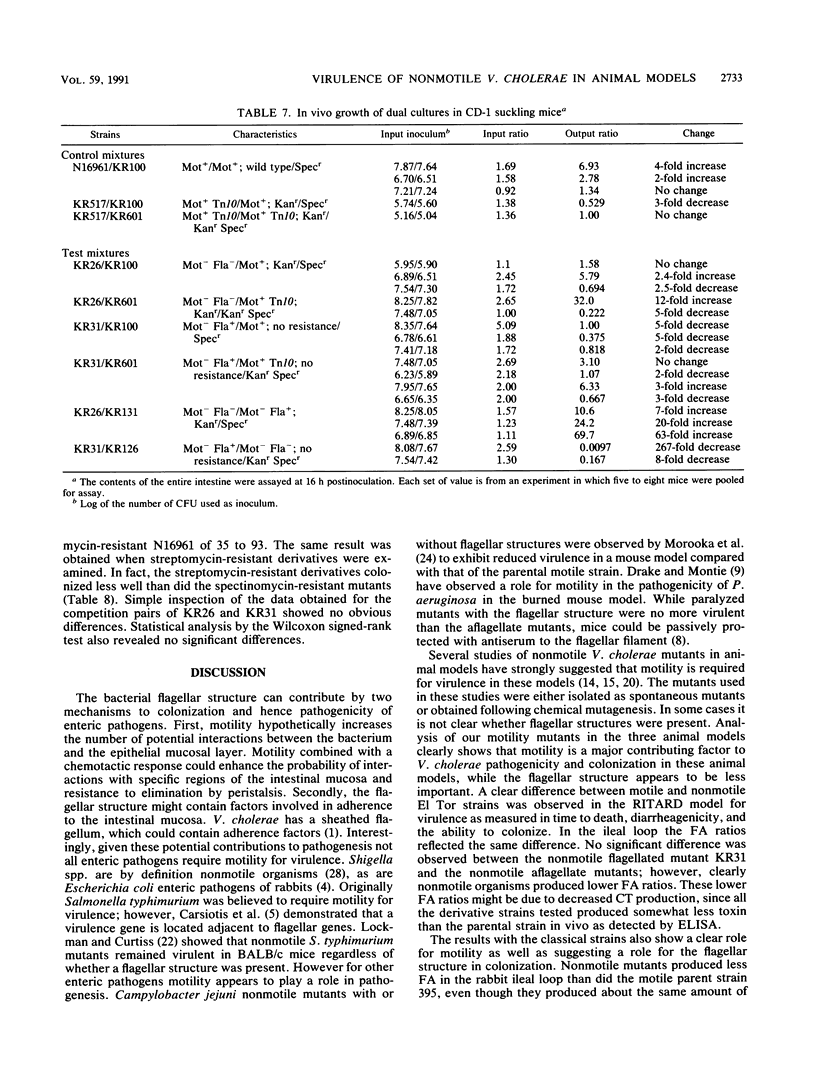
Wild-type Vibrio cholerae of both El Tor and classical biotypes (strains N16961 and 395, respectively) and nonmotile mutant derivatives with and without flagellar structures were characterized in three different animal models: (i) the rabbit ileal loop, (ii) the removable intestinal tie adult rabbit diarrhea (RITARD) model, and (iii) the suckling mouse model. Both the wild-type strains and nonmotile mutants were toxinogenic in the rabbit ileal loop and the suckling mouse models. However, all of the nonmotile mutants produced significantly less fluid accumulation than did the wild-type parental strains. The two nonmotile mutants of strain N16961 did not adhere to rabbit ileal mucosa, but both nonmotile mutants derived from strain 395 exhibited adherence. In the RITARD model, the motile El Tor strains were more virulent than both the flagellate and aflagellate nonmotile mutants (all infected rabbits died within 18 h), while the nonmotile mutants, when fatalities occurred, required 78 to 105 h to produce a fatal outcome. Likewise, the motile classical parent 395 produced a fatal outcome within ca. 25 h, while nonmotile mutants required 69 to 96 h. The nonmotile flagellate strain KR31 was not significantly more virulent than the nonmotile aflagellate strain KR26. Of the two classical nonmotile mutants, KR1, which produces a coreless sheathlike structure, was clearly more virulent (5 of 10 rabbits died within 96 h), while KR3 (nonmotile, aflagellate) did not produce fatalities in any of the 10 rabbits tested. Similarly, no significant difference in diarrheagenicity or colonizing ability was detected between the two nonmotile mutants derived from the El Tor strain, but the classical nonmotile mutant with the coreless sheath caused significantly greater diarrhea and colonized for a longer time than did the isogenic nonmotile aflagellate strain, KR3. No significant differences between the nonmotile mutants were detected in competition studies done with suckling mice. Analysis of the wild-type and mutant strains in these three animal models clearly demonstrated a role for motility in V. cholerae pathogenicity, while analysis of only the nonmotile mutants derived from the classical parent suggested a role for flagellar structures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Baselski V., Briggs R., Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977 Mar;15(3):704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Carsiotis M., Stocker B. A., Weinstein D. L., O'Brien A. D. A Salmonella typhimurium virulence gene linked to flg. Infect Immun. 1989 Nov;57(11):3276–3280. doi: 10.1128/iai.57.11.3276-3280.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements M. L., Levine M. M., Young C. R., Black R. E., Lim Y. L., Robins-Browne R. M., Craig J. P. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982 Apr;145(4):465–473. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Drake D., Montie T. C. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol. 1988 Jan;134(1):43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- Drake D., Montie T. C. Protection against Pseudomonas aeruginosa infection by passive transfer of anti-flagellar serum. Can J Microbiol. 1987 Sep;33(9):755–763. doi: 10.1139/m87-130. [DOI] [PubMed] [Google Scholar]
- Eubanks E. R., Guentzel M. N., Berry L. J. Evaluation of surface components of Vibrio cholerae as protective immunogens. Infect Immun. 1977 Feb;15(2):533–538. doi: 10.1128/iai.15.2.533-538.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT E. A., GORDON J. AN ELECTRON MICROSCOPE STUDY OF VIBRIO FLAGELLA. J Gen Microbiol. 1963 Aug;32:235–239. doi: 10.1099/00221287-32-2-235. [DOI] [PubMed] [Google Scholar]
- Finn T. M., Reiser J., Germanier R., Cryz S. J., Jr Cell-associated hemagglutinin-deficient mutant of Vibrio cholerae. Infect Immun. 1987 Apr;55(4):942–946. doi: 10.1128/iai.55.4.942-946.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Jones G. W. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976 Jul;14(1):246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981 Oct;34(1):222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Field L. H., Eubanks E. R., Berry L. J. Use of fluorescent antibody in studies of immunity to cholera in infant mice. Infect Immun. 1977 Feb;15(2):539–548. doi: 10.1128/iai.15.2.539-548.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H., Vial P. A., Kaper J. B., Mekalanos J. J., Levine M. M. Morphological studies on fimbriae expressed by Vibrio cholerae 01. Microb Pathog. 1988 Apr;4(4):257–265. doi: 10.1016/0882-4010(88)90086-1. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Hall R. H., Losonsky G., Mekalanos J. J., Taylor R. K., Levine M. M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988 Oct 1;168(4):1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Clements M. L., Cisneros L., Saah A., Nalin D. R., Gill D. M., Craig J. P., Young C. R., Ristaino P. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J Infect Dis. 1982 Mar;145(3):296–299. doi: 10.1093/infdis/145.3.296. [DOI] [PubMed] [Google Scholar]
- Lockman H. A., Curtiss R., 3rd Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990 Jan;58(1):137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Mostow P., Richardson K. High-frequency spontaneous mutation of classical Vibrio cholerae to a nonmotile phenotype. Infect Immun. 1990 Nov;58(11):3633–3639. doi: 10.1128/iai.58.11.3633-3639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick I. G., Ford C. W., Shackleford G. M., Berry L. J. Improved protection against cholera in adult rabbits with a combined flagellar-toxoid vaccine. Infect Immun. 1980 Nov;30(2):375–380. doi: 10.1128/iai.30.2.375-380.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K., Nixon L., Mostow P., Kaper J. B., Michalski J. Transposon-induced non-motile mutants of Vibrio cholerae. J Gen Microbiol. 1990 Apr;136(4):717–725. doi: 10.1099/00221287-136-4-717. [DOI] [PubMed] [Google Scholar]
- Sack D. A., Huda S., Neogi P. K., Daniel R. R., Spira W. M. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J Clin Microbiol. 1980 Jan;11(1):35–40. doi: 10.1128/jcm.11.1.35-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira W. M., Sack R. B., Froehlich J. L. Simple adult rabbit model for Vibrio cholerae and enterotoxigenic Escherichia coli diarrhea. Infect Immun. 1981 May;32(2):739–747. doi: 10.1128/iai.32.2.739-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987 May;84(9):2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema J. S., Guinée P. A., Ibrahim A. A., Pâques M., Ruitenberg E. J. In vivo adherence and colonization of Vibrio cholerae strains that differ in hemagglutinating activity and motility. Infect Immun. 1987 Sep;55(9):2093–2102. doi: 10.1128/iai.55.9.2093-2102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kamano T., Uchimura M., Iwanaga M., Yokota T. Vibrio cholerae O1 adherence to villi and lymphoid follicle epithelium: in vitro model using formalin-treated human small intestine and correlation between adherence and cell-associated hemagglutinin levels. Infect Immun. 1988 Dec;56(12):3241–3250. doi: 10.1128/iai.56.12.3241-3250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Electron microscopic study of Vibrio cholerae O1 adherence to the mucus coat and villus surface in the human small intestine. Infect Immun. 1988 Oct;56(10):2753–2759. doi: 10.1128/iai.56.10.2753-2759.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Flagella-induced immunity against experimental cholera in adult rabbits. Infect Immun. 1979 Jul;25(1):220–228. doi: 10.1128/iai.25.1.220-228.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Willis D. L., Berry L. J. Role of motility in experimental cholera in adult rabbits. Infect Immun. 1978 Nov;22(2):387–392. doi: 10.1128/iai.22.2.387-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]