Abstract
Recent research in our laboratory has centered on studies of polyacrylate and polyacrylamide nanoparticle emulsions for use in antibiotic delivery. Our goal is to develop these nanoparticle emulsions for treatment of life-threatening bacterial infections such as those caused by methicillin-resistant Staphylococcus aureus (MRSA). For this intended application, it is necessary to ensure that the biological activity of the emulsion is due only to the drug attached to the polymeric chain, rather than to any extraneous components. To investigate this, we evaluated cytotoxicity and microbiological activity of the nanoparticle emulsions before and after purification by centrifugation, dialysis, and gel filtration. Depending on the amount of surfactant used, all or most of the microbial and cellular toxicity can be removed by a simple purification procedure.
Keywords: polyacrylate nanoparticles, emulsions, cytotoxicity, sodium dodecyl sulfate
Background
The tremendous impact that antibiotics have had on human health is well-appreciated.[1,2] However, many microorganisms have rapidly acquired resistance for most of the currently available antimicrobials[3,4], displaying an amazing versatility to overcome the cidal or static effects of antibiotics on microbial growth and proliferation. The continuing rise in the numbers and prevalence of drug-resistant microbes that cause infections, most notably methicillin-resistant Staphylococcus aureus (MRSA), is further exasperated by the sheer difficulty and expense of developing new antimicrobially-active molecules.[5,6] Moreover, many of the most promising drug candidates suffer from poor water solubility and systemic stability that limits their clinical development. Our laboratory has been working on ways to improve the performance of such antibiotic compounds, and for enhancing the activity of older classes of antibiotics, using polyacrylate nanoparticles.[7] These nanoparticles can be easily prepared by emulsion polymerization by pre-solubilizing an acrylated derivative of the antibiotic in a warm mixture of butyl acrylate-styrene, adding sodium dodecyl sulfate (SDS) in water, and inducing polymerization with a water-soluble radical initiator such as potassium persulfate.
In our previous studies, we characterized several types of antibiotic-conjugated polyacrylate emulsions prepared in this manner by dynamic light scattering (DLS), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and atomic force microscopy (AFM), and showed that they contained nanoparticles measuring 30–60 nm in diameter. Microbiological testing of these emulsions revealed promising growth inhibition against MRSA that related to both the antibiotic structure as well as to the type of covalent linkage to the nanoparticle matrix.[7–10] At the time, we were not able to definitively establish whether the increase in bioactivity (relative to the free drug itself) came solely from the covalently-attached antibiotic nanoparticles, from other unidentified materials that may have also been present in the media, or perhaps from a combination of both. To decipher this is clearly an important objective to more fully assess bioactivity of the nanoparticle-bound drugs, and their potential therapeutic applications. During emulsion polymerization, impurities such as unreacted monomers, surfactant, initiator, salts and large polymeric aggregates[11] are likely to be present in varying amounts, and may contribute to the physical and biological properties of the emulsion. Therefore, we set out to develop methods to remove such potential interferences in order to ensure that the physical characteristics and biological activity of the emulsion is due to the nanoparticles. Furthermore, purification procedures were required before we could initiate more detailed structural characterization of the emulsified nanoparticles or investigate their application as treatments in animal infection models.[12]
In this report, we describe purification methods for this purpose, and the effect the purification procedure has on the overall microbiological and cytotoxic properties of the resulting nanoparticle emulsion. In particular, we pay close attention to the role that the surfactant, SDS, may have on the antibacterial and cellular toxicity of the nanoparticle emulsions.[13] Although SDS as a surfactant has excellent dispersion properties in the homogenization process,[14] it has been widely reported in the literature that certain concentrations of SDS (and similar surfactants) can display antimicrobial activity[15] due to detergent effects on cellular differentiation,[16,17] and barrier lipids,[18–21] and even induce inflammatory response[14] in studies done in keratinocytes and fibroblast cells. Thus, questions about SDS at the concentration levels used in this nanoparticle formulation need to be addressed and that is a primary focus in this study.
Methods
General procedures
The following starting materials were used for this study: styrene, stabilized p.a. (Acros Organics), n-butyl acrylate, stabilized 99% (Acros Organics); potassium persulfate (KPS), electrophoresis grade (Fisher Bioreagents); sodium dodecyl sulfate (SDS), 99% (Acros); anthracene-9-carboxylic acid, 96%, (Acros Organics); 2-hydroxyethyl acrylate, 96% (Aldrich); diisopropyl azodicarboxylate, 94% (Acros Organics); triphenylphosphine, 99% (Aldrich); tetrahydrofuran, 99.9% (Sigma-Aldrich). No further purification of these materials was necessary.
Preparation of the anthracene monomer 1
In a 50 ml round bottom flask, 150 μl (1.3 mmol) of 2-hydroxyethyl acrylate dissolved in 5 ml of THF was cooled to 0°C, then 186 μl of diisopropyl azodicarboxylate was added, followed by addition of 250 mg (1.12 mmol) of anthracene-9-carboxylic acid, and 295 mg (1.12 mmol) of triphenylphosphine. The solution was kept at 0°C for 5 hours, and the reaction progress was monitored by thin layer chromatograph (TLC). Once the reaction was completed, the solvent was evaporated and the resulting compound was purified by flash column chromatography using hexanes:ethyl acetate (90:10) yielding a yellowish solid in 64% with a melting point of 66°C. 1H NMR (400 MHz, CDCl3) δ 8.53 (1H, s), 8.07-7.99 (4H, m), 7.52-7.47, (4H, m), 6.47 (1H, d, J = 17.2 Hz), 6.18, (1H, dd, J = 10.8, 10.4 Hz), 5.88, (1H, d, J = 10.8 Hz), 4.86, (2H, t, J = 4.4 Hz), 4.59, (2H, t, J = 2.4 Hz). 13C NMR (100 MHz, CDCl3) δ 131.8, 131.2, 129.9, 128.9, 128.2, 127.3, 125.7, 125.2, 77.5, 77.2, 76.9, 63.4, 62.5.
Preparation of the polyacrylate nanoparticles
Poly(butyl acrylate-styrene) nanoparticles were prepared by emulsion polymerization as described in previous publications.[7–10] A 7:3 w:w mixture of butyl acrylate and styrene (total volume 1084 uL) was heated at 80°C for 10 min, followed by pre-emulsification in deionized water (4.0 mL) with simultaneous addition of the desired amount of surfactant (10–100 mg, 1–10 weight %) with rapid stirring. After 30 minutes the radical initiator, potassium persulfate (10 mg, 1 weight %), was added to the homogeneous emulsion to induce polymerization. The mixture was then stirred for 6 hours at 80°C. For the anthracene-containing samples, the anthracene acrylate monomer (10–50 mg, 1–5 weight %) was added to the warm 7:3 butyl acrylate-styrene mixture prior to emulsification in water.
Physicochemical characterization of the emulsions
Particle size analysis of the emulsions was determined by DLS using an UPA 150 Honeywell MicroTrac instrument, after diluting the emulsions with deionized water to about 100 μg/ml. Analysis was performed in triplicate (180 seconds per run per sample). Determination of the particle size by (mv) volume-average, (mn) number-average and (ma) area-average was calculated directly by the instrument with the respective standard deviation value. Zeta potential measurements were done in triplicate by micro electrophoresis on a Brookhaven ZetaPALS instrument. For these measurements, the emulsion was first diluted to 1.5% of its initial solid content (20%). For each sample, 2 × 10 runs were performed.
Dialysis of the emulsions
Spectra/Por® dialysis tubing of different pore sizes, including 6–8K, 12–14K, 50K and 100K molecular weight cut-offs (MWCO), were used (Sigma-Aldrich). Different dialysis times were examined, from 6 hour to 48 hours, to determine the optimal duration of dialysis.
Centrifugation of the emulsions
Centrifugation was carried out using an Eppendorf centrifuge 5415D at 13K rpm (16K × g) for 30 minutes in 2.0 ml Eppendorf Safe-Lock centrifugation tubes. Additionally, centrifugation was evaluated at higher centrifugal forces using Nanosep centrifugal devices from PALL Life Sciences: 10K, 30K, 100K, 300K, and 1000K.
Measuring the solid content of the emulsions
Solid content of each nanoparticle emulsion was determined by freeze-drying a weighed, 2 ml volume of emulsion on a Virtix Sentra Freezemobile 12XL instrument for 24 hours, and the dried residue was then weighed on a Sartorius CP124S balance. The weight % was calculated by dividing the dried weight by the initial weight of the emulsion, and multiplying by 100.
Measuring anthracene concentrations in the emulsions
The concentration of anthracene in the emulsions was determined after each purification procedure on a UV-2401PC, Shimadzu UV-VIS recording spectrophotometer, after diluting the original sample by 100-fold with deionized water to reach the linear range of the calibration curve.
Assay of in vitro microbiological activity of the nanoparticle emulsions against S. aureus and MRSA
The minimum inhibitory concentration (MIC) of each emulsion against S. aureus and MRSA was determined by serial dilution, according to NCCLS protocols.[22] The test medium was prepared by adding the test emulsion to the appropriate volume of Mueller Hinton broth. The total volume in each tube was 1.0 ml, such that each sequential tube contained half the concentration of emulsion. Bacterial cultures were grown overnight at 37°C on Trypticase Soy Agar (TSA) plates. The bacterial suspension was then prepared by inoculating the Mueller Hinton broth with several bacterial colonies then subsequently adjusting the bacterial load to 0.5 McFarland Standard (~1.5 × 108 CFU/ml). Bacterial cultures were incubated at 37°C for approximately 2 hours. The absorbance of each culture was determined at 625 nm, and the cultures were adjusted by increasing the incubation time or diluting with broth until the absorbance was equal to 0.08–0.10. The cultures were then diluted 1:100 in Mueller Hinton broth to reach approximately 1.5 × 106 CFU/ml. The dilution tubes were inoculated with an equal volume of the inoculum (1.0 ml), resulting in a final bacterial concentration of 5 × 10 CFU/ml. The tubes were incubated at 37°C for 16–20 hours. After incubation, the absorbance was read at 625 nm to determine the MIC. The MIC was determined as the lowest concentration of emulsion (zero absorbance) that completely inhibited bacterial growth in the tubes.
Assay of in vitro cytotoxicity of the nanoparticle emulsions
Cytotoxicity of the nanoparticle emulsions was evaluated using either human dermal fibroblasts or keratinocytes, which were grown in Dubelco’s Modified Eagle Medium (DMEM) at 37°C with a 5% CO2 atmosphere for several days until cells were confluent. The cells were harvested and re-suspended in DMEM containing 10% Fetal Bovine Serum (FBS) and 0.1% gentamycin. The cells were counted using a hemocytometer, the total number of cells was determined and the cells were seeded into 96-well plates at 50,000 cells per well. Each well contained 150 μl DMEM with 10% FBS and 0.1% gentamycin. Cells were allowed to grow for 4–6 hours prior to treatment with the nanoparticle emulsions. The emulsions being assayed were added directly to the media in each well at the following dilutions (volume of emulsion to volume of media): 1:150, 1:125, 1:100, 1:75, 1:50 and 1:25. Testing of each emulsion at each concentration was performed in triplicate. On each 96-well plate, three wells were left untreated for use in calculating the 100% absorbance value. The plates were then incubated for 48 hours and observed under the microscope at various time points. A 5 mg/ml solution of 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) in phosphate buffered saline (PBS) was prepared and 15 μl (10% of the total culture volume) was added to each well except those designated as instrument blanks. The plates were incubated for 4 hours to allow sufficient time for the conversion of the MTT dye (yellow liquid) to the water-insoluble formazan derivative, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (purple solid) by the mitochondrial dehydrogenases in the living cells. After incubation, purple crystals were observed and the media was removed from each well by aspiration. The crystals were then dissolved by adding 100 μl dimethyl sulfoxide (DMSO) to each well. DMSO was also added to the wells designated as blanks. Viable cell count was determined spectrophotometrically using a microplate reader by measuring the absorbance at two discrete wavelengths (595 and 630 nm). For each emulsion at each concentration, the absorbance values were averaged and the percent cell viability was determined as percentage of the average absorbance obtained from the untreated cells.
Results
The concentration of surfactant used for preparing the polyacrylate emulsion plays an important role, not only in stabilizing the final emulsion, but also in promoting formation of micelles where the molecule of interest, an antibiotic for example, may migrate into during the emulsification process. Because of the intended application of this type of emulsion as a drug delivery system, it is necessary to evaluate possible biological activity the surfactant and other components may have within the emulsion itself. Polyacrylates are commonly used in biomedical applications but toxicity associated with nanoparticle forms has not been systematically determined. Likewise, evaluation of microcidal activity and cytotoxicity of surfactants, while thoroughly investigated, needs closer scrutiny in terms of their utilization in nanoparticle-based drug delivery. Indeed, it has been observed before that the toxicity of surfactants, including SDS in particular, depends on whether they are unassociated (in bulk media) or bound to the surface of nanoparticles.[14]
A variety of methods to purify nanoparticles have been reported in the literature, including centrifugation,[23] ultracentrifugation,[24] gel filtration,[12] ultrafiltration,[25] dialysis,[26] diafiltration centrifugal device and tangential flow filtration.[27] The various applications and sample compositions make it difficult to predetermine an effective, standardized and practical purification method for the polyacrylate system. In fact, given the nature of the different potential impurities (small molecule contaminants versus large amorphous aggregates), we considered the possibility of employing a combination of purification methods.
We first attempted to purify the nanoparticle emulsions by simple extraction with organic solvents. The hope was to remove any residual contaminants such as unreacted monomers or organic-soluble materials in the media and to quantify the effects of the extraction on nanoparticle size, structure, and bioactivity. Common solvents such as methylene chloride and ethyl acetate led to significant loss of solid content from the aqueous emulsion, with what appeared to be coagulation of the polymerized or oligomeric material as a pasty white film, while cyclohexane seemed to work in that it did not change the appearance of the emulsion even after 72 hours of continuous extraction. NMR analysis of the extract showed small amounts of butyl acrylate and styrene, and for drug containing samples, minute amounts (<1%) of the unreacted drug acrylate. This confirmed our previous NMR studies which suggested that nearly all of the drug acrylate used for the polymerization was covalently incorporated into the prepared polyacrylate matrix. AFM imaging of the showed that the average particle sizes and spherical morphology of the nanoparticles were essentially the same for the cyclohexane-extracted and untreated samples. The removal of the small amount of drug acrylate from the drug-containing polyacrylate emulsions resulted in only a minimal decrease in anti-Staphylococcal activity. From this initial investigation, we learned that cyclohexane can be used to extract residual organics (unreacted monomers) from the freshly-prepared nanoparticle emulsions without damaging or altering the size, morphology, or microbiological effects of the nanoparticles.
In follow-up to this, we conducted cytotoxicity studies of the original and extracted emulsions against human dermal fibroblasts. We observed that emulsions formulated with 3–5% SDS produced a thin precipitate on the surface of the fibroblast cells which inhibited their ability to elongate properly onto the glass surface. We correlated this to the presence of some excess (unassociated) SDS in the emulsion, since the same results were obtained when fibroblasts were treated with a saline solution doped with 1–3% (by weight) of SDS, but not with a freshly-prepared emulsion of poly(butyl acrylate-styrene) formulated with only 1% SDS. This agrees with a prior report that SDS in aqueous media is cytotoxic, but SDS associated with a matrix such as a polymeric nanoparticle is not.[14] This led us to consider the possibility that any unassociated (toxic) SDS present in the emulsion could perhaps be removed after the formation of the nanoparticle by polymerization, in order to decrease cytotoxic effects without changing the morphological or microbiological features of the emulsified nanoparticles. Thus, our focus turned to ways to remove SDS and non-nanoparticle contaminants (oligomers, non-stabilized polymers, along with residual unreacted monomers) from the emulsion.
After several trials of testing different purification conditions and techniques, we found to that the poly(butyl acrylate-styrene) nanoparticle emulsions could be effectively purified by a combination of mild centrifugation and dialysis. The centrifugation time and strength (g’s) could be adjusted until consistent results were obtained. The purification protocol began with a centrifugation of the initial (unpurified) nanoparticle emulsion at 16K gravities for 30 minutes to remove large polymeric aggregates formed during the polymerization process. Sample volumes of 2 ml or less were most conveniently centrifuged using a standard-model bench top microcentrifuge (Figure 1). We found that longer centrifugation times or stronger centrifugal forces was unnecessary and did not improve separation. The supernatant that was obtained by decantation was then subjected to dialysis to remove excess surfactant, initiator and small molecule contaminants from the emulsion (Figure 2). Several different pore sizes were examined, ranging from molecular weight cut-offs (MWCO) of 6–8K, 12–14K, 50K, and 100K. Dialysis times were also varied from 2–24 hours, with samples removed for analysis every 2 hours. Finally, the material recovered from the dialysis bag was subjected to a second mild centrifugation at 16K × g for 30 minutes to remove residual cloudy material that sometimes appears after dialysis. The resulting emulsions are much more transparent and suitable for biological screening. We found that it is also possible to include a final gel filtration step to further refine the product. However, analysis of the gel-filtered samples by dynamic light scattering (DLS) showed a unique population of particles (~13 nm) when this final gel filtration step was included in the purification scheme. Interestingly, we did not find this unique population in the absence of gel filtration.
Figure 1.
2-ml volumes of the emulsions were centrifuged for 30 minutes at 16K × g to remove large aggregates formed during the polymerization process. The compacted pellet obtained after removal of the supernatant is shown.
Figure 2.
Dialysis of the emulsion was conducted with different dialysis membranes (MWCO’s of 6–8K, 12–14K, 50K and 100K) and dialysis times (4–24 hours) in bulk water.
In order to quantify the amount of co-monomer (eg., acrylated drug) remaining in the nanoparticle emulsion after each step of purification, we employed a UV-active, fluorescent acrylate monomer 1 (as a drug equivalent), which was prepared from anthracene-9-carboxylic acid as shown in Figure 3. The selection of this particular UV- and fluorescent-active chromophore was based on its similar overall size and lipophilic nature to that of the drug co-monomers we typically employ for preparing antibiotic-conjugated nanoparticles.[7–10] Employing this labeled monomer, UV- and fluorescently-active nanoparticles could be produced (using 1 weight % of labeled acrylate 1) so we could easily monitor the “drug” concentrations in the emulsion throughout the purification process. Thus, anthracene-labeled samples were subjected to the same purification techniques as the non-labeled system to conduct these studies. We began these investigations by preparing drug-free polyacrylate emulsions of 7:3 w:w butyl acrylate-styrene by varying the SDS concentration from 1 weight % to 10 weight %. We immediately noticed that the physical appearance of the emulsion becomes progressively less cloudy as the SDS concentration is incrementally increased from 1 weight % to 10 weight %, indicating decreasing aggregate formation in the media. This runs parallel with the results of DLS studies, which show that the size of the nanoparticles steadily decreases as SDS concentrations are increased from 1 weight % (~57 nm) to 3 weight % (~42 nm) to 10% (~33 nm). After each stage of purification (initial centrifugation, dialysis, and final centrifugation), dynamic light scattering was performed on the samples. The data shows that the average diameters and the surface charges of the nanoparticles in these emulsions do not change throughout the purification stages. This indicates that the emulsions are unaltered with respect to the nature of the nanoparticles present, regardless of the state of purity of the aqueous medium.
Figure 3.
Synthesis of acrylated anthracene monomer 1.
Next, we set out to determine how much of the solid particulate in the emulsion was being removed upon purification. For this study, a standard poly(butyl acrylate-styrene) emulsion was prepared with 3 weight % of SDS and centrifugated at 16K × g for 30 minutes, and then divided into equivolume (2 ml) portions. Each portion was put into one of four different pore size dialysis membrane tubing (6–8K, 12–14K, 50K, 100K MWCO). Each bag was then placed in a beaker and dialyzed into 400 ml of deionized water with rapid stirring; the water was changed every two hours. At the end of the dialysis period (2–24 hours), the contents of each bag was emptied and lyophilized to determine the final dry mass of the remaining material. We found that the initial centrifugation removes less than 10% of the overall solid content (presumed to be primarily large particulates), depending on how cloudy and non-homogeneous the original emulsion was (10% SDS < 5% SDS < 3% SDS < 1% SDS), while subsequent dialysis more significantly reduces the amount of solid material. In fact, almost half of the solid content in the supernatant can be removed during just 8–10 hours of dialysis, depending on the membrane pore size. It appears that the 6–8K MWCO, the 12–14K MWCO, and the 50K MWCO membranes all have similar properties, each leading to the loss of about 50% of the solid content within a 10-hour period of dialysis. On the other hand, use of the 100 MWCO dialysis membrane causes a much more rapid and complete loss of solid material (about 80%) just within the first two hours.
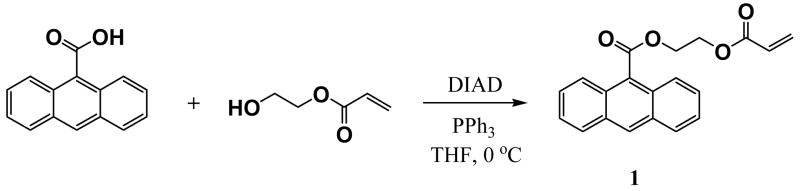
This study was then repeated with a similar emulsion containing the anthracene label to quantify how much of the co-monomer is being retained in the solid material during purification. We measured the concentration of the anthracene label (as a model for a small lipophilic drug molecule) spectroscopically at different stages of purification. Calibration curves were first prepared using different amounts of anthracene monomer 1 in order to determine the linear range corresponding to the final concentration of anthracene being introduced into the nanoparticle matrix Figure 4 illustrates the UV and fluorescence curves for nanoparticle emulsions prepared by adding a calculated amount of anthracene monomer 1 to a pre-made diluted sample of the 7:3 w:w butyl acrylate-styrene nanoparticle emulsion. The emulsion was first diluted 100 fold to reduce loss due to light scattering.
Figure 4.
Spectrophotometric calibration curves prepared using 7:3 butyl acrylate:styrene nanoparticle emulsions treated with different concentrations of anthracene monomer 1 which corresponding to that in emulsion (after 100-fold dilution) Absorbance and fluorescence was measured at 258 nm, and values are the averages of triplicate measurements. Note the downward progression of fluorescence intensity with increasing anthracene monomer concentration.
While the UV absorption curve shows a linear relationship between intensity absorbance versus anthracene concentration, fluorescence shows a parabolic response most likely due to quantum quenching at higher concentrations. Therefore, UV absorbance was chosen as the parameter for monitoring anthracene concentration in the emulsion. To determine the optimal concentration of anthracene monomer to use in these model experiments, we first had to evaluate how much could be introduced covalently into the matrix as a function of SDS concentration. We observed that when 1% SDS is used in naonoparticle formation, the initial (unpurified) emulsion was very milky, and UV absorption of the sample (even when diluted 100-fold) suffered from severe light scattering. However, when the concentration of SDS in the emulsion was 3 weight percent or greater, UV absorption values were essentially identical to those in Figure 3 (after 100-fold dilution). Next, we ran experiments to evaluate the limits in the anthracene concentration that could be covalently introduced into the nanoparticle matrix. For the emulsion containing 3 weight percent of SDS, it was possible to completely incorporate up to 1 weight percent of the anthracene monomer 1 into the polymeric framework; attempts to use more than 3 weight percent led to unstable, non-homogeneous mixtures. For the emulsion made with 10 weight percent of SDS, up to 3 weight percent of the anthracene monomer 1 could be introduced into a stable emulsion.
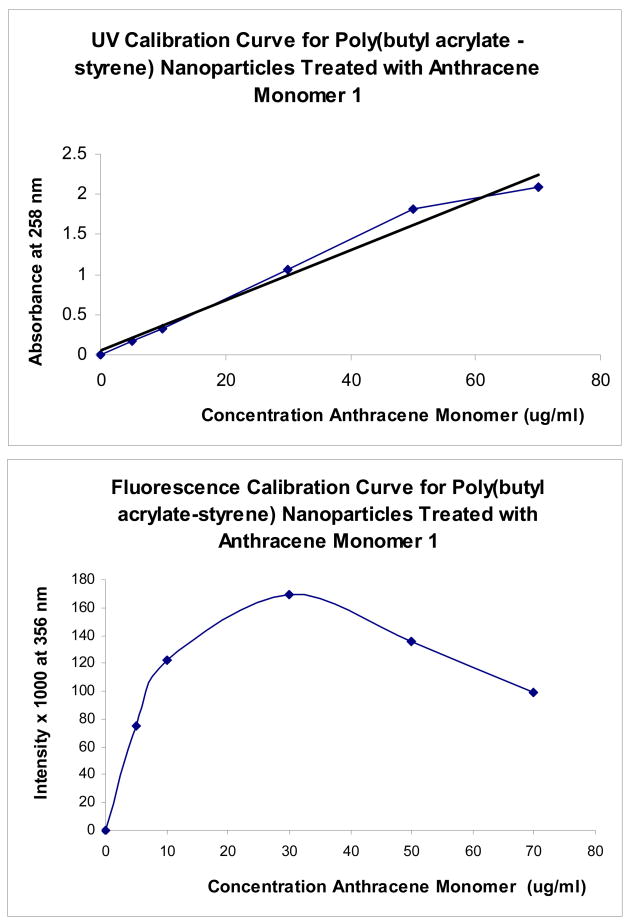
In our previous experiments with the non-labeled nanoparticle emulsions, we found that most of the solid material loss from the emulsion occurs during the first 10 hours of dialysis, but not centrifugation. Using the anthracene-labeled samples, we next repeated the purification steps to see if the rate of loss of the anthracene-labeled components in the emulsion during purification might be comparable to the rate of loss of the solid content. The issue was whether the anthracene monomer (as a drug model) is equally distributed in the various solid materials in the emulsion, or selectively into the nanoparticle matrix. For this evaluation, we used the UV-labeled emulsion containing 3 weight percent of SDS and 1 weight percent of anthracene monomer, and calculated the amount of remaining concentration from UV absorption measurements (after 100-fold dilution of the sample). In Figure 5, we plot the rate of loss of the anthracene-labeled material in the emulsion during centrifugation (denoted as A to B on x-axis) and at different time points during dialysis (2–4 hours) as a function of dialysis tubing MWCO.
Figure 5.
Variation of concentration of anthracene remaining in the emulsion prepared with 3 weight % of SDS and 1 weight % of anthracene monomer, expressed in percentage as a function of the MWCO vs. the time of dialysis. (A) is the unpurified emulsion, (B) is after centrifugation at 16K × g for 30 minutes, 2h-10h is the time of dialysis, and (D) is the final centrifugation. Absorbance was measured at 258 nm (in triplicate).
Once again, we see that the initial centrifugation removes less than 10% of the original anthracene-labeled material. This material is primarily from the large non-stabilized aggregates that cause cloudiness in the emulsion. As expected, dialysis removes considerably more of the solid content, and along with it, a corresponding amount of the anthracene-labeled material. It appears that the rate of loss of anthracene label corresponds to the rate of loss of solid content observed in the previous experiment, indicating that the anthracene label seems to be nearly equally distributed throughout all polymerized material in the media. Moreover, as before, the extent of this loss depends on the MWCO of the dialysis tubing and the duration of dialysis.
Evaluation of microbiological activities of nanoparticle emulsions
Even though the nanoparticle emulsions examined in this study do not contain an antibiotic per se, the presence of a surfactant and potentially other bacteriocidal components caused us to look into possible antimicrobial effects. Initially, in vitro testing of the polyacrylate emulsions did in fact determine that the unpurified emulsions do have some antimicrobial activity of their own. This was studied by determining the broth minimum inhibitory concentration (MIC) value of the emulsified nanoparticle against S. aureus (ATCC 25923) and MRSA (ATCC 43300). In particular, the emulsion containing 10 weight percent of SDS affords an MIC of ~16 ug/ml against both S. aureus and MRSA, indicating that even without an antibiotic the unpurified emulsion possesses antimicrobial activity at this unusually high surfactant concentration (10 weight percent). This is not unexpected given the well-known microcidal effects of SDS as a detergent.[19,28] As anticipated, bacteriocidal activity drops rapidly as the surfactant concentration is diminished, with the emulsions that contain 5, 3, and 1 weight percent of SDS having MICs of 32, 64, and 128 ug/ml, respectively, against S. aureus and MRSA..
We then tested the same emulsions after subjecting them to the purification protocol of centrifugation for 30 minutes at 16K × g, dialysis for 8 hours in a 50-MWCO dialysis tubing, and centrifugation for 30 minutes at 16K × g. None of the purified emulsions containing 1, 3, 5, and 10 weight % of SDS showed antimicrobial activity below 128 ug/ml, confirming that the original biocidal activity of the unpurified samples is due to components such as free (unassociated) surfactant in the bulk media that can be removed by purification.
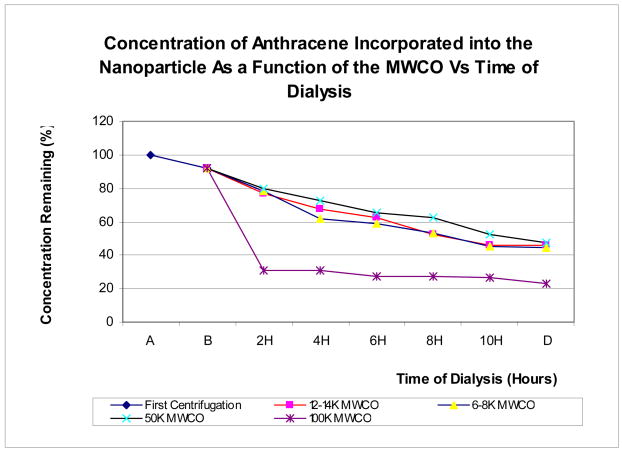
Cytotoxicity studies of emulsions prepared with different concentrations of SDS
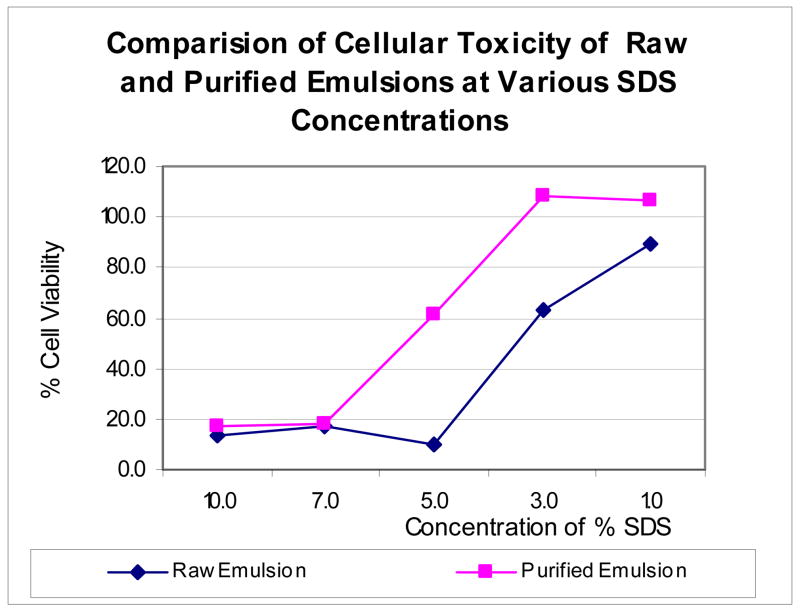
Next, we wanted to learn more about the potential effects of the nanoparticle emulsions on human tissue, which ultimately is a decisive factor in whether these nanoparticles can be used for human drug therapy. Thus, in vitro cytotoxicity studies were conducted with the unpurified and purified emulsions against keratinocytes as our toxicity screen. Keratinocytes are more sensitive to the effects of surfactants such as SDS than fibroblasts, and thus seemed to be a better indicator of nanoparticle toxicity for this study. This was done by subjecting cultured keratinocytes to different concentrations of the emulsions (unpurified versus purified) for 48 hours and determining cell viability by MTT assay. The results are shown in Figure 6. Clearly, cytotoxicity of the nanoparticles increased in relation to the amount of SDS used for polymerization. Cell viability measurements indicate that unpurified nanoparticle emulsions prepared with 5, 7, or 10 weight percent of SDS killed more than 80% of the cells after 48 hours (bottom line), while the 3% and 1 % SDS emulsions were much less toxic. Even at 3%, SDS-containing emulsions reduced cell viability by almost 50%. After purification of the emulsions as outlined above, however, only those made with 1–3 weight % of SDS were completely non-toxic in this assay, while those made with 7 to 10 weight percent of SDS remained cytotoxic. At around 5%, about half the cells were unaffected after 48 hours. This data suggests that even though the purified emulsions were all effectively microbiologically inactive against S. aureus and MRSA, those having more than 3 weight percent of SDS remained cytotoxic to human keratinocytes even after purification. This provides important insight in terms of the design and intended use of SDS-stabilized polyacrylate nanoparticles as a drug delivery motif.
Figure 6.
Cytotoxicity profile of the emulsions prepared with different concentrations of SDS, tested in keratinocytes, 1:100 dilution, 50,000 cells/well, 150 μl DMEM, 10% FBS and 0.1% gentamycin, 48 hours of incubation, 37 °C, 5% CO2, measured at 595 and 630 nm.
Discussion
The initial purpose of this study was to understand the role of the nanoparticles on antibacterial activity. To do this required a means for removing potential interferences in the bioassays. In fact, removing the antibiotic molecule from the nanoparticle preparation did not remove all the microbiological effects, which we have now traced to the presence of impurities (mostly SDS surfactant). With proper purification, the emulsions can be cleaned up satisfactorily to permit a more detailed examination of the microbiological properties and in vivo efficacies of antibiotic-conjugated nanoparticle emulsions, without these spurious interferences. This is now being conducted in our laboratory. The antibacterial activity and cytotoxicity of the emulsions was evaluated at different concentrations of SDS. When the concentration of the surfactant during the nanoparticle formation is greater than 3 weight percent, the cytotoxicity increases significantly. The established purification protocol of mild centrifugation-overnight dialysis-mild centrifugation was effective in reducing unwanted microbiological activity and cellular toxicity in emulsions having SDS concentrations at or below 3 weight %. Observation that higher concentrations of surfactant used in forming the nanoparticles leads to smaller particle size may come into play as well, since particle size in itself may very dictate the toxicity and drug delivery capabilities of nanomaterials particularly for systemic applications. Again, the amount of free surfactant versus nanoparticle-bound surfactant on bioactivity is likely to be important, and this in turn relates to nanoparticle dimensions and composition. Clearly more studies on this are needed. In vivo experiments are now being conducted in our laboratory to assess this.
We also have learned from this study that as solid (polymeric) material is removed from the emulsion during purification, that the rate of loss of anthracene (and thus antibiotic, if used) is comparable, indicating that the anthracene (drug) is incorporated almost equally into the nanoparticle polymer as in the various oligomeric and polymeric chains present in the initial emulsion (which are removed by purification). This allows us to more accurately estimate the concentration of covalently-bound anthracene (drug) in the final nanoparticle emulsion after purification for biological experiments. Thus, even though about half of the solid (polymeric) content is lost by purification, the final (nearly transparent) sample does not cause coagulation and is highly enriched in the nanoparticle component. These purification procedures are being used in our laboratory currently to prepare drug-conjugated nanoparticles for in vivo studies in various animal infection models.
Acknowledgments
Financial support from the National Institutes of Health (R01 AI01535) and National Science Foundation (NSF 0419903, NSF 0620572), US Department of Homeland Security (fellowship to KG), University of South Florida and the Florida Center of Excellence in Biomolecular Identification and Targeted Therapeutics (for a Graduate Multidisciplinary Scholarship to JG), and the University of South Florida Office of Technology Development for a Florida High Tech Corridor matching grant is deeply appreciated.
Sources of support: National Institutes of Health (R01 AI01535) and National Science Foundation (NSF 0419903, NSF 0620572), US Department of Homeland Security (fellowship to KG), University of South Florida and the Florida Center of Excellence in Biomolecular Identification and Targeted Therapeutics (for a Graduate Multidisciplinary Scholarship to JG), and the University of South Florida Office of Technology Development for a Florida High Tech Corridor matching grant.
Footnotes
Conflict of Interest Statement: Edward Turos is co-inventor on a US patent application by the University of South Florida for the polyacrylate nanoparticle antibiotics, the subject of this publication. Dr. Turos is also co-founder, chief scientific advisor, and shareholder of Nanopharma Technologies, Inc., a University of South Florida spin-out company. Nanopharma Technologies, Inc., has licensed the nanoparticles technology from University of South Florida for potential commercial development.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julio C. Garay-Jimenez, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
Ashley Young, Nanopharma Technologies, Inc., 3650 Spectrum Boulevard, Suite 160, Tampa, FL 33612 USA.
Danielle Gergeres, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
Kerriann Greenhalgh, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA.
Edward Turos, Center for Molecular Diversity in Drug Design, Discovery, and Delivery, Department of Chemistry CHE 205, 4202 East Fowler Avenue, University of South Florida, Tampa, FL 33620 USA, Turos, Professor of Chemistry.
References
- 1.Spellberg B, Powers J, Brass E, Miller LG, Edwards JE. Trends in antimicrobial drug development: implication for the future. Clin Infect Dis. 2004;178:1279–86. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 2.Leeb M. Antibiotics: A shot in the arm. Nature. 2004;431:892–3. doi: 10.1038/431892a. [DOI] [PubMed] [Google Scholar]
- 3.Weber JT, Courvalin P. An emptying quiver: antimicrobial drugs and resistance. Emerg Infect Dis. 2005;11:791–3. doi: 10.3201/eid1106.050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbarth S, Samore M. Antimicrobial resistance determinants and future control. Emerg Infect Dis. 2001;11:794–801. doi: 10.3201/eid1106.050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMassa JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development cost. J Health Econ. 2003;22:151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert DN, Edwards JE. Is there hope for the prevention of future antimicrobial shortages? Clin Infect Dis. 2002;35:215–6. doi: 10.1086/341958. [DOI] [PubMed] [Google Scholar]
- 7.Turos E, Shim JY, Wang Y, Greenhalgh K, Reddy GS, Dickey S, Lim DV. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17:53–6. doi: 10.1016/j.bmcl.2006.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abeylath S, Turos E. Glycosylated polyacrylate nanoparticles by emulsion polymerization. Carb Polym. 2007;70:32–7. doi: 10.1016/j.carbpol.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turos E, Reddy GSK, Greenhalgh K, Ramaraju P, Abeylath SC, Jang S, Dickey S, Lim DV. Penicillin-bound polyacrylate nanoparticles: Restoring the activity of β-lactam antibiotics against MRSA. Bioorg Med Chem Lett. 2007;17:3468–72. doi: 10.1016/j.bmcl.2007.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turos E, Shim JY. WO 2005020933 PCT Int Appl. 2005
- 11.Alleman E, Doelker E, Gurny R. Drug loaded poly(lactic acid) nanoparticles produced by a reversible saltin-out process: purification on an injectable dosage. Eur J Pharm Biopharm. 1993;39:13–8. [Google Scholar]
- 12.Beck P, Scherer D, Kreuter J. Separation of drug-loaded nanoparticles from free drug by gel filtration. J Microencapsul. 1990;7:491–6. doi: 10.3109/02652049009040471. [DOI] [PubMed] [Google Scholar]
- 13.Verhulst C, Coiffard C, Coiffard L, Rivalland P, De Roeck-Holzhauer Y. In vitro correlation between two colorimetric assays and the pyruvic acid consumption by fibroblasts cultured to determine the sodium laurysulfate Citotoxicity. J Pharmacol Toxicol Methods. 1998;39:143–6. doi: 10.1016/s1056-8719(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 14.Mueller R, Ruehl D, Runge S, Schulze-Forster K, Mehnert W. Cytotoxicity of solid lipid nanoparticles as a function of the lipid matrix and the surfactant. Pharma Res. 1997;14:458–62. doi: 10.1023/a:1012043315093. [DOI] [PubMed] [Google Scholar]
- 15.Babich H, Babich J. Sodium lauryl sulfate and triclosan: in vitro cytotoxicity studies in gingival cells. Toxicol Lett. 1997;91:189–96. doi: 10.1016/s0378-4274(97)00022-2. [DOI] [PubMed] [Google Scholar]
- 16.Craig S, Newby C, Barr R, Greaves M, Mallet A. Cytokine release and cytotoxicity in human keratinocytes and fibroblast induced by phenols and sodium dodecyl sulfate. J Invest Dermatol. 2000;115:292–8. doi: 10.1046/j.1523-1747.2000.00056.x. [DOI] [PubMed] [Google Scholar]
- 17.Ruissen F, Carroll J, Vander Valk P, Schalkwijk J. Differential effects of detergents on keratinocyte gene expression. J Invest Dermatol. 1998;110:358–63. doi: 10.1046/j.1523-1747.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 18.Törmä H, Geijer S, Gester T, Alpholm K, Berne B, Lindberg M. Variations in the mRNA expression of inflammatory mediators, markers of differentiation and lipid-metabolizing enzymes caused by sodium lauryl sulphate in cultured human keratinocytes. Toxicol in vitro. 2006;20:472–9. doi: 10.1016/j.tiv.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Krebs F, Miller S, Catalone B, Welsh P, Malamud D, Howett M, Wigdahl B. Sodium dodecyl sulfate and C#1G as microbicidal alternatives to nonoxynol 9: comparative sensitivity of primary human vaginal keratinocytes. Antimicrob Agents Chemother. 2000;44:1954–60. doi: 10.1128/aac.44.7.1954-1960.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varani J, Astrom A, Griffiths C, Voorhees J. Induction of proliferation of growth-inhibited keratinocytes and fibroblasts in monolayer culture by sodium lauryl sulfate: comparison with all-trans retinoic acid. J Invest Dermatol. 1991;97:917–21. doi: 10.1111/1523-1747.ep12491682. [DOI] [PubMed] [Google Scholar]
- 21.Wei T, Geijer S, Lindberg M, Berne B, Törmä H. Detergents with different chemical properties induce variable degree of cytotoxicity and mRNA expression of lipid-metabolizing enzymes and differentiation markers in cultured keratinocytes. Toxicol in vitro. 2006;20:1387–94. doi: 10.1016/j.tiv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.NCCLS (National Committee for Clinical Laboratory Standards) Methods for Dilution of Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. NCCLS Document M7–A4, 1997; Vol. 17, No. 2.
- 23.Murakami H, Kobayashi H, Takeuchi H, Kawashima Y. Further application of a modified spontaneous emulsification solvent diffusion method to various types of PLGA and PLA polymers for preparation of nanoparticles. Powder Technol. 2000;107:137–143. [Google Scholar]
- 24.Kreuse HJ, Schwartz A, Rohdewald P. Interfacial polymerization, a useful method for the preparation of poly(methyl cyanoacrylate) nanoparticles. Drug Dev Ind Pharm. 1986;12:527–52. [Google Scholar]
- 25.Zahka L, Mir L. Ultrafiltration of latex emulsions. Chem Eng Prog. 1977;73:53–5. [Google Scholar]
- 26.Kwon HY, Lee JY, Choi SW, Jang J, Kim JH. Preparation of PLGA nanoparticles containing estrogen by emulsification-diffusion method. Colloids Surf A Physicochem Eng Asp. 2001;182:123–30. [Google Scholar]
- 27.Dalwadi G, Heather A, Benson E, Chen Y. Comparison of diafiltration and tangential flow filtration for purification of nanoparticle suspensions. Pharm Res. 2005;22:2152–62. doi: 10.1007/s11095-005-7781-z. [DOI] [PubMed] [Google Scholar]
- 28.Liwarska-Bizukojc E, Miksch K, Malachowska-Jutsz A. Kalka, Acute toxicity and genotoxicity of five selected anionic and nonionic surfactants. J Chemosphere. 2005;58:1249–53. doi: 10.1016/j.chemosphere.2004.10.031. [DOI] [PubMed] [Google Scholar]