Abstract
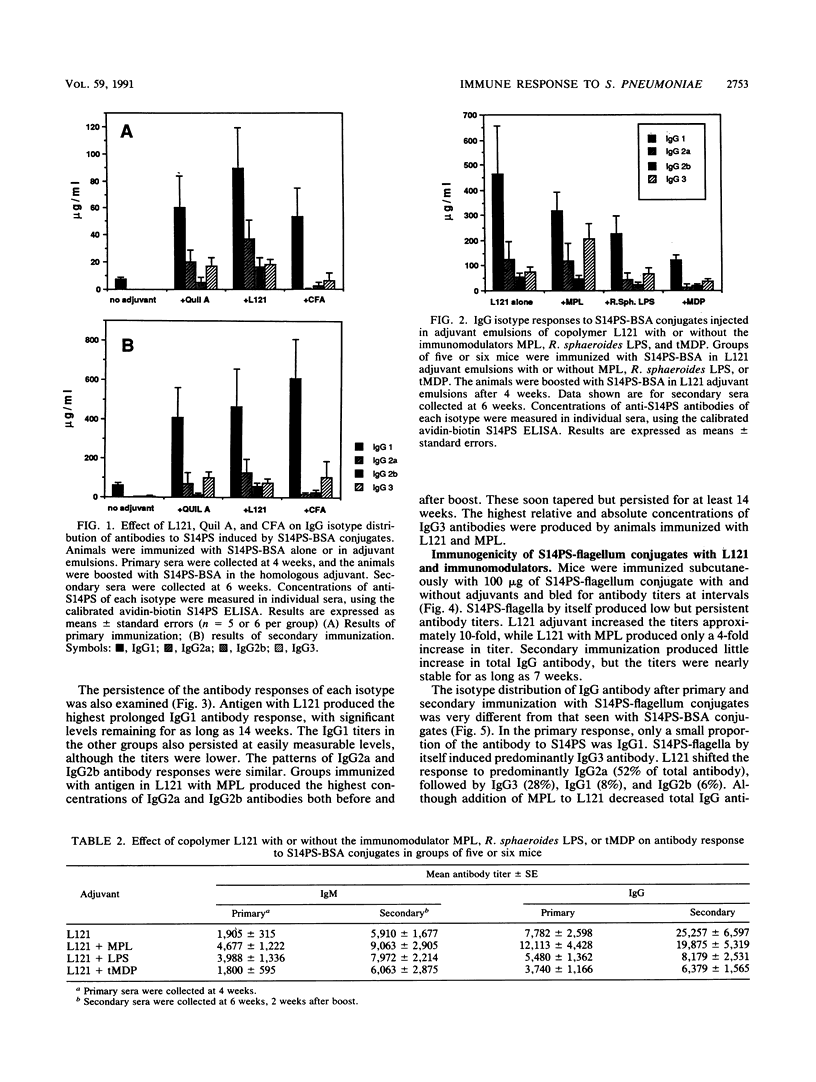
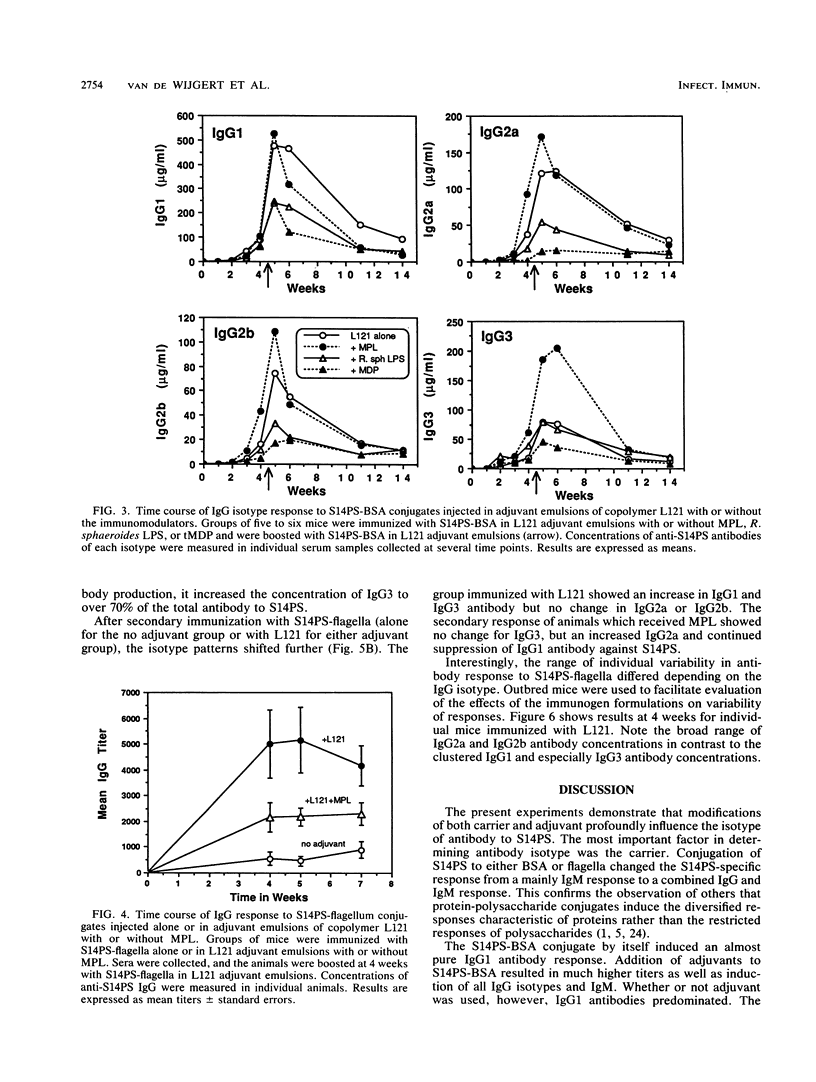
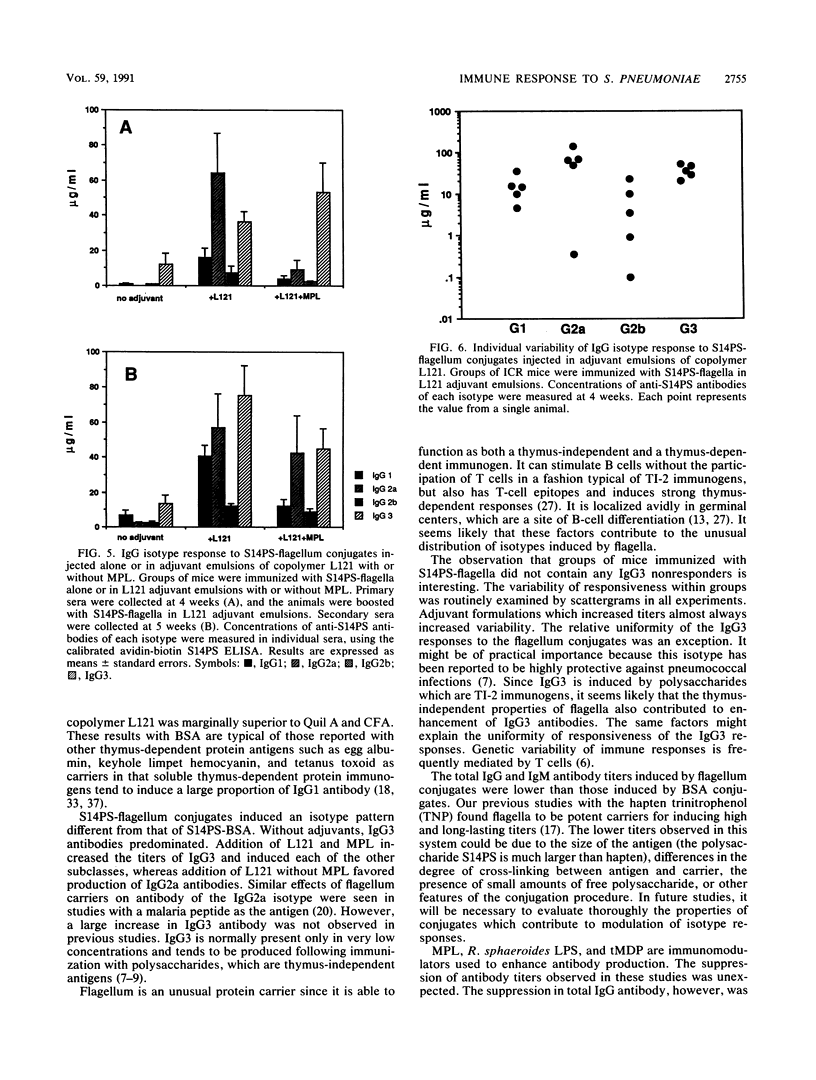
This project investigated the effects of novel carriers and adjuvants on the isotype of murine immunoglobulin G (IgG) antibody to pneumococcal capsular polysaccharide type 14 (S14PS). S14PS conjugated to bovine serum albumin induced a weak antibody response which was 100% IgG1 following injection without adjuvant. The same polysaccharide conjugated to flagella of Salmonella typhi induced an antibody response which was 88% IgG3. S14PS-bovine serum albumin was injected with block copolymer L121 or Quil A in squalane-in-water emulsions. The copolymer L121 was at least as effective as Quil A or complete Freund adjuvant in inducing IgG antibodies. IgG1 was the dominant subclass for all. Addition of monophosphoryl lipid A, but not the threonyl derivative of muramyl dipeptide or nontoxic Rhodopseudomonas sphaeroides lipopolysaccharide, to copolymer L121 increased production of the IgG2a, IgG2b, and IgG3 subclasses. S14PS-flagella with copolymer L121 induced higher titers with a markedly altered isotype distribution: 13% IgG1, 52% IgG2a, 6% IgG2b, and 29% IgG3. Monophosphoryl lipid A added to L121 reduced IgG1 antibody to 5%, but increased IgG2a antibody to 14%, IgG2b antibody to 3%, and IgG3 antibody to 78%. These studies demonstrate that both the carrier and the adjuvant can influence the titer and isotype distribution of antipolysaccharide antibody responses.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Pichichero M., Edwards K., Porch C. R., Insel R. Priming and induction of Haemophilus influenzae type b capsular antibodies in early infancy by Dpo20, an oligosaccharide-protein conjugate vaccine. J Pediatr. 1987 Nov;111(5):644–650. doi: 10.1016/s0022-3476(87)80237-8. [DOI] [PubMed] [Google Scholar]
- Aplin J. D., Wriston J. C., Jr Preparation, properties, and applications of carbohydrate conjugates of proteins and lipids. CRC Crit Rev Biochem. 1981;10(4):259–306. doi: 10.3109/10409238109113601. [DOI] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Prescott B., Cantrell J. L., Rudbach J. A. Inactivation of suppressor T-cell activity by nontoxic monophosphoryl lipid A. Infect Immun. 1988 May;56(5):1076–1083. doi: 10.1128/iai.56.5.1076-1083.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Hiernaux J. R., Fauntleroy M. B., Stashak P. W., Prescott B., Cantrell J. L., Rudbach J. A. Ability of monophosphoryl lipid A to augment the antibody response of young mice. Infect Immun. 1988 Dec;56(12):3064–3066. doi: 10.1128/iai.56.12.3064-3066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett D. J., Lee C. G., Ammann A. J., Ayoub E. M. IgG and IgM pneumococcal polysaccharide antibody responses in infants. Pediatr Res. 1984 Nov;18(11):1067–1071. doi: 10.1203/00006450-198411000-00001. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Claflin J. L., Schroer K., Forman C. Mouse Igg3 antibodies are highly protective against infection with Streptococcus pneumoniae. Nature. 1981 Nov 5;294(5836):88–90. doi: 10.1038/294088a0. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Horowitz J. C., Volanakis J. E., Benjamin W. H., Jr, McDaniel L. S., Eldridge J., Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989 May;57(5):1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. The effects of subclass on the ability of anti-phosphocholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. J Mol Cell Immunol. 1984;1(5):305–309. [PubMed] [Google Scholar]
- Coon J., Hunter R. Properties of conjugated protein immunogens which selectively stimulate delayed-type hypersensitivity. J Immunol. 1975 May;114(5):1518–1522. [PubMed] [Google Scholar]
- Coutelier J. P., van der Logt J. T., Heessen F. W., Vink A., van Snick J. Virally induced modulation of murine IgG antibody subclasses. J Exp Med. 1988 Dec 1;168(6):2373–2378. doi: 10.1084/jem.168.6.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann J. V., Lipsky B. A. Pneumococcal vaccine in the United States. A critical analysis. JAMA. 1981 Sep 25;246(13):1428–1432. [PubMed] [Google Scholar]
- Hunter R. L., Bennett B. The adjuvant activity of nonionic block polymer surfactants. II. Antibody formation and inflammation related to the structure of triblock and octablock copolymers. J Immunol. 1984 Dec;133(6):3167–3175. [PubMed] [Google Scholar]
- Hunter R. L., Bennett B. The adjuvant activity of nonionic block polymer surfactants. III. Characterization of selected biologically active surfaces. Scand J Immunol. 1986 Mar;23(3):287–300. doi: 10.1111/j.1365-3083.1986.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Hunter R., Olsen M., Buynitzky S. Adjuvant activity of non-ionic block copolymers. IV. Effect of molecular weight and formulation on titre and isotype of antibody. Vaccine. 1991 Apr;9(4):250–256. doi: 10.1016/0264-410x(91)90108-i. [DOI] [PubMed] [Google Scholar]
- Hunter R., Strickland F., Kézdy F. The adjuvant activity of nonionic block polymer surfactants. I. The role of hydrophile-lipophile balance. J Immunol. 1981 Sep;127(3):1244–1250. [PubMed] [Google Scholar]
- Kamerling J. P., Gerwig G. J., Vliegenthart J. F., Clamp J. R. Characterization by gas-liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilyl methyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem J. 1975 Dec;151(3):491–495. doi: 10.1042/bj1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney J. S., Hughes B. W., Masada M. P., Allison A. C. Influence of adjuvants on the quantity, affinity, isotype and epitope specificity of murine antibodies. J Immunol Methods. 1989 Jul 26;121(2):157–166. doi: 10.1016/0022-1759(89)90156-7. [DOI] [PubMed] [Google Scholar]
- Le Garrec Y. Immunomodifiers of bacterial origin. Comp Immunol Microbiol Infect Dis. 1986;9(2-3):137–141. doi: 10.1016/0147-9571(86)90005-6. [DOI] [PubMed] [Google Scholar]
- McCormick D. B., Roth J. A. Specificity, stereochemistry, and mechanism of the color reaction between p-dimethylaminocinnamalhyde and biotin analogs. Anal Biochem. 1970 Mar;34:226–236. doi: 10.1016/0003-2697(70)90102-8. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Mattila P., Rautonen N., Seppälä I., Eskola J., Käyhty H. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J Immunol. 1987 Sep 15;139(6):1999–2004. [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Pike B. L., Nossal G. J. A reappraisal of "T-independent" antigens. I. Effect of lymphokines on the response of single adult hapten-specific B lymphocytes. J Immunol. 1984 Apr;132(4):1687–1695. [PubMed] [Google Scholar]
- Salimath P. V., Weckesser J., Strittmatter W., Mayer H. Structural studies on the non-toxic lipid A from Rhodopseudomonas sphaeroides ATCC 17023. Eur J Biochem. 1983 Oct 17;136(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07726.x. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Sutton A., Vann W. F., Karpas A. B., Stein K. E., Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985 Oct 10;82(2):215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- Tai J. Y., Vella P. P., McLean A. A., Woodhour A. F., McAleer W. J., Sha A., Dennis-Sykes C., Hilleman M. R. Haemophilus influenzae type b polysaccharide-protein conjugate vaccine. Proc Soc Exp Biol Med. 1987 Feb;184(2):154–161. doi: 10.3181/00379727-184-42460. [DOI] [PubMed] [Google Scholar]
- Takayama K., Olsen M., Datta P., Hunter R. L. Adjuvant activity of non-ionic block copolymers. V. Modulation of antibody isotype by lipopolysaccharides, lipid A and precursors. Vaccine. 1991 Apr;9(4):257–265. doi: 10.1016/0264-410x(91)90109-j. [DOI] [PubMed] [Google Scholar]
- Takehara H. A., Perini A., da Silva M. H., Mota I. Trypanosoma cruzi: role of different antibody classes in protection against infection in the mouse. Exp Parasitol. 1981 Aug;52(1):137–146. doi: 10.1016/0014-4894(81)90069-2. [DOI] [PubMed] [Google Scholar]
- Tomai M. A., Johnson A. G. T cell and interferon-gamma involvement in the adjuvant action of a detoxified endotoxin. J Biol Response Mod. 1989 Dec;8(6):625–643. [PubMed] [Google Scholar]
- Ukei S., Iida J., Shiba T., Kusumoto S., Azuma I. Adjuvant and antitumour activities of synthetic lipid A analogues. Vaccine. 1986 Mar;4(1):21–24. doi: 10.1016/0264-410x(86)90093-9. [DOI] [PubMed] [Google Scholar]
- Verheul A. F., Versteeg A. A., De Reuver M. J., Jansze M., Snippe H. Modulation of the immune response to pneumococcal type 14 capsular polysaccharide-protein conjugates by the adjuvant Quil A depends on the properties of the conjugates. Infect Immun. 1989 Apr;57(4):1078–1083. doi: 10.1128/iai.57.4.1078-1083.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul A. F., Versteeg A. A., Westerdaal N. A., Van Dam G. J., Jansze M., Snippe H. Measurement of the humoral immune response against Streptococcus pneumoniae type 14-derived antigens by an ELISA and ELISPOT assay based on biotin-avidin technology. J Immunol Methods. 1990 Jan 24;126(1):79–87. doi: 10.1016/0022-1759(90)90014-m. [DOI] [PubMed] [Google Scholar]
- Zigterman G. J., Schotanus K., Ernste E. B., Van Dam G. J., Jansze M., Snippe H., Willers J. M. Nonionic block polymer surfactants modulate the humoral immune response against Streptococcus pneumoniae-derived hexasaccharide-protein conjugates. Infect Immun. 1989 Sep;57(9):2712–2718. doi: 10.1128/iai.57.9.2712-2718.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


