Abstract
Unlike other branched organs, the mammary gland undergoes most of its branching during adolescent rather than embryonic development. Its morphogenesis begins in utero, pauses between birth and puberty, and resumes in response to ovarian estrogens to form an open ductal tree that eventually fills the entire mammary fat pad of the young female adult. Importantly, this “open” architecture leaves room during pregnancy for the organ to develop milk-producing alveoli like leaves on otherwise bare branches. Thereafter, the ducts serve to deliver the milk that is produced throughout lactation. The hormonal cues that elicit these various phases of mammary development utilize local signaling cascades and reciprocal stromal–epithelial interactions to orchestrate the tissue reorganization, differentiation and specific activities that define each phase. Fortunately, the mammary gland is rather amenable to experimental inquiry and, as a result, we have a fair, although incomplete, understanding of the mechanisms that control its development. This review discusses our current sense and understanding of those mechanisms as they pertain to mammary branching, with the caveat that many more aspects are still waiting to be solved.
Keywords: mammary gland, branching morphogenesis, epithelial-stromal crosstalk
Introduction
The mammary gland—the one structure that distinguishes mammals from all other animals—accomplishes its unique task of producing and delivering adequate amounts of milk from mother to newborn in part by forming an extensive network of branched ducts (Sternlicht, 2006). This strategy of forming a branched system in order to pack a large epithelial surface area into an otherwise limited tissue volume is clearly not unique to the mammary gland. Rather, it is seen throughout biology. Broadly speaking, the development of any branched organ entails a number of discrete stages. These include the initial specification, formation, and in-growth of its so-called “anlage,” the initiation and outgrowth of its earliest rudimentary branches, the spatial organization of the organ through iterative branching and tissue remodeling events, the formation of a continuous hollow lumen, and the functional differentiation of its proximal and terminal elements (Affolter et al., 2003). Many of the key mechanisms that underlie these processes appear to be conserved among all branched organs, from the trachea and air sacs of insects to the lungs, kidneys, and salivary glands of higher organisms. However, tissue-specific mechanisms must also apply, as all of these organs clearly differ in form and function. Thus both common and unique mechanisms govern branching morphogenesis and its outcome in each particular setting. Some of these mechanisms are well or partly understood; others are not. Our aim here is to address these mechanisms as they pertain to normal mammary branching, with the understanding that some may be bypassed, corrupted, or hijacked during the development and progression of certain diseases, most notably cancer.
In mice, mammary development begins shortly after mid-gestation with the specification of two bi-lateral epidermal ridges—or “milk lines”—that run from forelimb to hindlimb on each side of the animal (Veltmaat et al., 2003; Hens and Wysolmerski, 2005). Five pairs of disk-shaped placodes then segregate along these lines at the site of each future nipple and grow into the underlying mesenchyme to form a bulb-shaped bud that represents the primary mammary rudiment or “anlage.” Once this epithelial bud penetrates the mammary mesenchyme, it enters a cluster of preadipocytes that eventually becomes the mammary fat pad. It then branches ~ 10–20 times to form a rudimentary ductal tree that occupies a minor portion of the overall fat pad at birth. The nascent gland then enters a period of relative quiescence during which it increases in size just enough to keep pace with normal body growth until puberty, when its branching morphogenesis truly takes off. In response to the onset of ovarian stimulation, multicellular club-shaped terminal end buds (TEBs) form at the ends of the ducts and penetrate further into the fat pad as the ducts elongate (Hinck and Silberstein, 2005). New “primary” ducts continue to form by bifurcation and perhaps also trifurcation of the TEBs. Meanwhile, “secondary” branches sprout laterally from the trailing primary ducts until the fat pad becomes filled by an extensive network of branched ducts (Fig. 1). Thereafter, short “tertiary” side-branches form in response to cyclic ovarian stimulation, further filling out the mature mammary tree, and alveolar structures develop at the ends of the tertiary branches. However, the mechanisms that regulate alveolar development and differentiation are distinct from those that regulate ductal development (Oakes et al., 2006).
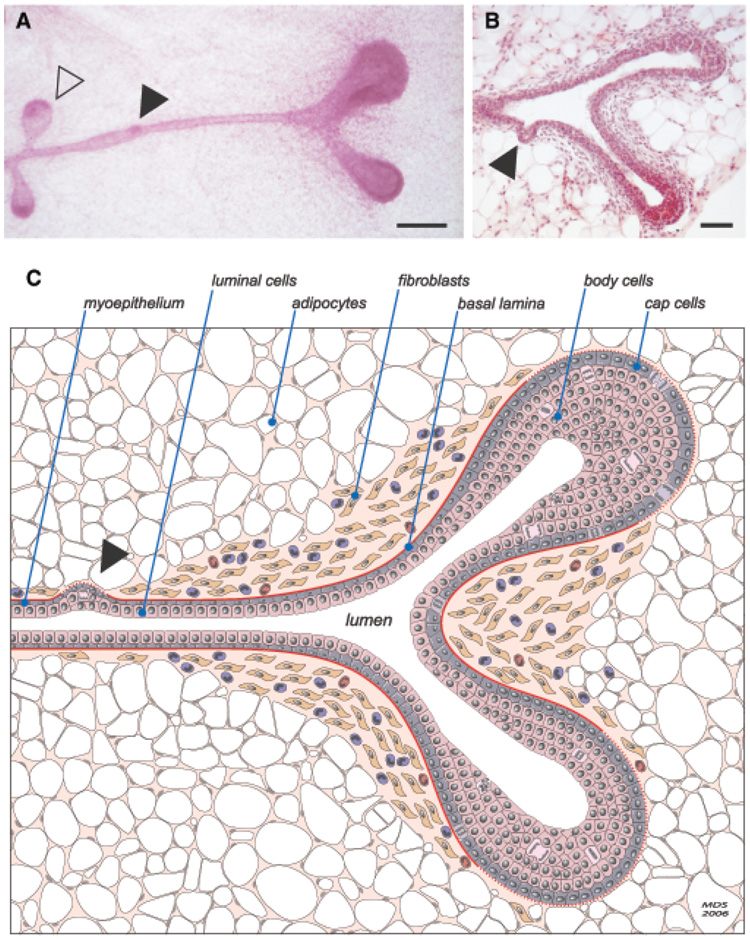
Fig. 1.
Murine terminal end bud (TEB) and duct morphology. (A) High-magnification carmine alum-stained whole mount of a TEB that has recently bifurcated to form two new primary ducts. Two new secondary side-branches are also present along the trailing duct (open arrowhead), as is an area of increased cellularity that may represent a nascent lateral bud (closed arrowhead). Increased stromal cellularity is apparent around the bifurcating TEB. Scale bar: 200 µm. (B) Hematoxylin and eosin-stained section of a bifurcating TEB with an early lateral side-branch (closed arrowhead). Scale bar: 100 µm. (Image courtesy of A.J. Ewald, UCSF) (C) Schematic diagram depicting the major features of a bifurcating TEB. Notable features include the considerable proliferative activity (mitoses) within the TEBs, the single layer of TEB cap cells and multilayered pre-luminal body cells, the characteristic presence of a fibroblast-and collagen-rich stromal collar surrounding the neck of the bifurcating TEB, and its conspicuous absence beyond the invading distal cap of each new TEB. An increased number of macrophages and eosinophils is also shown. Although there is no evidence that normal ductal cells ever cross the basal lamina, thinning of the basement membrane (dashed lines) at the leading edge of the invading ducts may reflect partial enzymatic degradation and/or incomplete de novo synthesis of the basal lamina.
Mammary development in humans is slightly different from that seen in rodents. The mammary milk-lines form earlier in human embryos (i.e., during the first trimester) and generally give rise to only one pair of mammary placodes (Howard and Gusterson, 2000). Also, rather than forming a single ductal tree, each human anlage forms several trees that coalesce at the nipple. Here there is also a conspicuous absence of surrounding hair pegs, suggesting the lateral inhibition of these structures by the nipple. In addition, fetal exposure to maternal hormones results in a low level of secretory activity and colostrum production by the mammary gland in the late-term human fetus and newborn infant, a phenomenon that does not occur in rodents. Once these endocrine influences subside, the human infant breast undergoes menopausal-like involution and the residual ductal structures enter a fairly quiescent allometric growth phase that lasts until puberty. Another major difference between humans and mice is that in the latter species, the male mammary rudiment is destroyed on or near gestational day 14 in response to androgen-dependent condensation of the surrounding mammary mesenchyme (Kratochwil and Schwartz, 1976). By contrast, in humans, breast development in males and females remains indistinguishable until puberty. Thereafter, the female human breast undergoes robust branching, terminal duct lobular unit (TDLU) formation and stromal expansion, whereas the male breast remains quiescent but capable of further development (e.g., in gynecomastia) or even malignant conversion. Perhaps most importantly, however, the ducts and TDLU of the human breast are ultimately surrounded by a dense, fibroblastic interlobular and loose intra-lobular stroma that is far more pronounced than the scant periductal fibrous, but otherwise adipose-rich mammary stroma of rodents.
Mammary branching may thus be separated into embryonic, adolescent, and adult phases, each of which is differentially regulated. Adolescent branching, for instance, requires growth hormone (GH), estrogen, and estrogen receptor α (ERα); adult tertiary side-branching, on the other hand, requires progesterone and its receptor (PR); and embryonic branching apparently proceeds without any hormonal requirement at all, as it occurs normally in the absence of either ERα, ERβ, PR, or the receptors for GH or prolactin (Bocchinfuso et al., 2000; Curtis Hewitt et al., 2000). Branching is also co-ordinated by local bi-directional crosstalk between the developing duct epithelium and nearby stromal cells (Fig. 2). For instance, in vivo transplantation studies show that embryonic mammary epithelium forms salivary gland-like structures if it is recombined with salivary gland mesenchyme and then grafted under the renal capsule of a host mouse (Sakakura et al., 1976). However, even though the mammary epithelium exhibits a salivary branching pattern, it retains its ability to produce milk proteins in response to pituitary prolactin. On the other hand, when 8.5 day embryonic Rathke’s pouch (prospective pituitary) epithelium is recombined with salivary mesenchyme and transplanted under the renal capsule of a host mouse, it apparently forms salivary-like structures that possess differentiated (α-amylase-positive) salivary acini (Kusakabe et al., 1985). Likewise, 13-day embryonic mouse skin epithelium forms functional (milk producing) mammary ducts and alveoli if it is grown in vivo within mammary mesenchyme (Cunha et al., 1995). Thus, under certain circumstances, mesenchymal cues can control both the branching pattern and the differentiation of the epithelium. In fact, even non-mammalian chick and duck epidermis, which normally forms feathers, instead becomes branched mammary glandular tissue if it is grown within rabbit mammary mesenchyme, thus not only highlighting the dominant instructive nature of the mesenchyme, but also giving new meaning to the term “chicken breast” (Propper and Gomot, 1973).
Fig. 2.
Provisional model depicting some of the key endocrine and paracrine pathways involved in mammary branching morphogenesis. Solid arrows indicate interactions that influence mammary branching, whereas hashed lines indicate putative interconnections between known signaling cascades or indirect interactions for which signaling intermediates (e.g., mitogen-activated protein kinase) have not yet been determined.
In addition, not only is the embryonic mammary mesenchyme instructive, so too is the adult mammary stroma. In one study, for instance, mammary epithelium from a mouse strain that characteristically has highly branched mammary glands were recombined with adult fat pads of a strain that has poorly branched glands, and vice versa (Naylor and Ormandy, 2002). When the re-combined glands were transplanted to immune-compromised host mice, their branching pattern reflected the genetic background of the mammary stroma in which they were grown rather than that of the donor epithelium or host mouse. Thus, stromal rather than epithelial or systemic factors are responsible for the different adult mammary side-branching patterns that distinguish different strains of mice. Moreover, xeno-transplantation studies suggest that human breast epithelial cells will only form functional ductal, lobular, and acinar structures when transplanted to chimeric “humanized” fat pads that contain human breast fibroblasts (Kuperwasser et al., 2004) or within collagen gels that contain normal human or mouse mammary fibroblasts (Parmar et al., 2002). These data support the notion that stromal factors also regulate human breast development. Indeed, although some species-specific differences undoubtedly exist, many of the basic mechanisms that control mammary development are probably conserved among all mammals. Thus, this review outlines our basic understanding of the initial endocrine stimuli and local molecular interactions that regulate mammary branching in rodents as a model for mammary morphogenesis in general. In addition, we compare and contrast the mechanisms that govern mammary branching with those that govern the development of other branched organs in order to obtain a better understanding of the fundamental mechanisms that control branching morphogenesis itself.
Pattern formation in the mammary gland and other organs
The initial appearance of murine mammary placodes is an orderly process—beginning with thoracic pair 3, followed by pairs 4 (abdominal), 5 (inguinal), 1 (cervical), and then 2 (upper thoracic) (Veltmaat et al., 2003). In addition, the two thoracic pairs tend to be more highly branched than all others in newborn mice, while the inguinal pair tends to be least developed. However, any branching patterns that form thereafter are highly variable and stochastic. In fact, even embryonic branching patterns differ from one gland to another. In contrast, the branching program of another organ system, the embryonic lung, is highly stereotyped in terms of when, where and along which axis-specific branches arise, suggesting that its primary branching routine and subroutines are genetically “hard-wired” (Metzger and Krasnow, 1999). Likewise, branching of the Drosophila tracheal system is initially highly stereotyped, while its terminal branching pattern varies as it depends not on hard-wired signaling events but on local oxygen levels in the surrounding tissue. So what dictates the ductal pattern—stochastic as it may be—of any particular mammary gland? One appealing possibility is that, in the face of local driving forces, the spacing between adjacent branches depends on inhibitor gradients that are generated by neighboring ducts to insure their relative isolation from one another. Such intrinsic negative controls would tend to produce and maintain an open ductal architecture. Therefore, the path of a particular branch may simply depend, in part, on where its prior neighbors happened to settle. In addition, less sophisticated mechanisms may also contribute. For instance, the approximately planar nature of most branching events in the mouse mammary gland may simply reflect the constraints of the stromal space within which they develop. Consequently, a very different branching pattern might emerge if they had a larger three-dimensional (3-D) volume in which to develop. In addition, the non-homogeneous nature of the mammary stroma itself may provide alternative paths of greater or lesser resistance or attractiveness along which the branches may prefer to grow.
Much of our understanding of the modes of branching that occur during mammary development—the primary bifurcation of TEBs and lateral sprouting of secondary and tertiary branches—has been deduced from “end-point” analysis of glands viewed at one instant in time rather than from direct continuous observation of the branching events themselves. Thus, the detailed cellular dynamics of TEB bifurcation and side-branching are still unclear. For instance, do the cells at the leading edge of a TEB roll inward to form an initial furrow that cleaves the TEB into two or do they bulge outward on each side of a belt of cells that has become stabilized or obstructed? In contrast to our lack of knowledge concerning the cellular behaviors that take place during mammary gland branching, time-lapse imaging of embryonic kidney cultures in which the epithelium is labeled with green-fluorescent protein (GFP) has provided novel insights (Watanabe and Costantini, 2004). In this setting, ~ 75% of the renal tubular branches are formed by symmetric or asymmetric bifurcation of the tips (ampullae) of the renal tubules and ~ 7% are formed by lateral branching. The remaining 18% or so, however, are formed by trifurcation, a process in which an ampulla simultaneously branches in three separate directions. However, had it not been witnessed by time-lapse imaging, this process might have been mistaken for two independent bifurcation events. Whether similar trifurcation events occur with any regularity during mammary development is still unclear, although some still images from instantaneously collected glands suggest that they may.
Array-based expression proffling has identified transcripts of numerous genes that are specifically enriched in TEBs and their immediate adjacent stroma as well as ~ 200 other genes that appear to be primarily or exclusively expressed in the more proximal ducts (H. Kouros-Mehr and Z. Werb, unpublished results). Thus, another unresolved question concerns the respective fates of cells located in TEBs and ducts. That is, how much do these two morphologically and molecularly distinct entities—ducts and TEBs—contribute to the formation of new branches? One possibility is that the ducts alone give rise to the entire ductal tree from behind as they push the bifurcating and invading TEBs onward. Alternatively, the TEBs alone may give rise to the trailing ducts—a scenario supported by the observation that most of the proliferation in the developing gland occurs within the TEBs. Or, finally, the TEBs and ducts may both contribute to each new primary branch.
While it is not yet clear which scenario actually occurs during mammary development, recent analyses provide insight into what occurs during the development of another branched system, the kidney collecting system (Shakya et al., 2005). Branching of the ureteric bud that goes on to form this system requires that soluble glial-derived neurotrophic factor (Gdnf) from the metanephric mesenchyme bind to the receptor tyrosine kinase Ret and its co-receptor Gfrα1, both of which are exclusively expressed on the tip cells of the renal ampullae. To explore this requirement further, Shakya et al. (2005) generated chimeric kidneys by injecting ES cells lacking Ret into normal blastocysts. In these embryos, the tubular trunks contained GFP-expressing mutant cells, as they neither express nor require Ret, whereas the mutant cells were excluded from the ampullae, where Ret is required. Therefore, the fates of cells in the ampullae and trunks could be monitored by time-lapse microscopy. The result: the ampullae branched and gave rise to the trailing tubular trunks, and the trunks actively elongated and provided cells to each new branch. Thus, both the tips and the trunks contribute to each new branch. Whether mammary TEBs and ducts behave in a similar manner and each contribute to the developing ductal tree, however, remains to be seen.
Global executives: endocrine directors of mammary branching
Of all the cues that regulate mammary development, one set—long ago recognized to be hormonal—directs all others. Indeed, ovariectomy and hypophysectomy experiments show that ovarian and pituitary hormones are absolutely essential for post-pubertal mammary development. So which hormones are involved? Notably, exogenous estrogens can rescue mammary development in ovariectomized mice (Daniel et al., 1987), implying that ovarian estrogens act as an essential “on-switch.” But just as a light switch fails to function in the absence of electricity, estrogens alone cannot rescue mammary development in hypophysectomized animals (Kleinberg et al., 2000). They can, however, restore TEB and duct development in rats that have been both hypophy-sectomized and ovariectomized if GH or insulin-like growth factor-1 (Igf1) is also provided (Kleinberg et al., 2000). By contrast, the administration of estrogen plus pituitary prolactin does not rescue their development. This implies that GH is the master pituitary hormone—or electricity—that is lost in hypophysectomized animals, and further suggests that its effects are elicited through Igf1. In support of this idea, adolescent mammary development is also impaired in mice lacking GH receptor (Gallego et al., 2001), Igf1 (Kleinberg et al., 2000), ERα (Curtis Hewitt et al., 2000) or the aromatase that is responsible for estrogen biosynthesis (Fisher et al., 1998). On the other hand, it occurs normally in mice lacking ERβ, PR or prolactin receptor (Curtis Hewitt et al., 2000), thus confirming the importance of GH, Igf1, ovarian estrogens and their respective receptors in post-pubertal branching morphogenesis.
Now consider the position of Igf1 in this cascade. As Igf1 rescues ductal development in hypophysectomized (GH-deficient) animals, yet excess GH and estrogen fail to rescue the development of Igf1-null glands, locally produced Igf1 probably acts downstream of GH and/or estrogen (Kleinberg et al., 2000). The importance of locally produced rather than systemic Igf1 is further supported by the observation that mammary branching proceeds normally in mice with a liver-specific deletion of Igf1 that causes a 75% reduction in circulating—but not mammary-specific—Igf1 expression (Richards et al., 2004). By contrast, branching is significantly diminished in mutant mice with a global reduction in both systemic and local Igf1 production. Igf1 receptor (Igf1r) deficient mammary transplants also show significantly reduced growth potential in surgically cleared (i.e., epithelium-free) wild-type fat pads (Bonnette and Hadsell, 2001). This suggests that Igf1r is required in the epithelium, as it was otherwise present in the host fat pads. On the other hand, similar experiments show that the GH receptor is specifically required in the stroma (Gallego et al., 2001). Moreover, GH induces Igf1 and ER expression in epithelium-free fat pads, the induction of Igf1 by GH is enhanced by estradiol, and only GH-treated glands express stromal ER, further indicating that GH regulates mammary development through its stromal receptor (Kleinberg et al., 2000). Taken together, these data support a model in which pituitary GH, which is already present before the pubertal surge in ovarian estrogens, acts via its receptor on mammary stromal cells to elicit the expression of stromal Igf1, which then interacts with its receptor on mammary epithelial cells to stimulate TEB formation and epithelial branching in a paracrine manner.
Moreover, the data indicate that ovarian estrogens act in concert with GH and IGF1 to stimulate mammary branching. However, data concerning precisely where ERα is required have been rather conflicted. Initial embryonic tissue recombination studies suggested that ERα was only required in the stroma (Cunha et al., 1997), whereas adult tissue transplants suggested that it was needed in both the stroma and the epithelium (Mueller et al., 2002). More recent data, however, indicate that these prior studies were compromised by incomplete inactivation of ERα and suggest that it is only required for normal mammary development within the epithelium and not within the mammary stroma (Mallepell et al., 2006). Nevertheless, ERα-null mammary epithelial cells can still contribute to all portions of the developing mammary tree if they are rescued by nearby wild-type cells, thus indicating that ERα affects mammary development in a non-cell-autonomous paracrine manner.
Although estrogens can induce PR expression, and although progesterone can enhance the ability of Igf1 to stimulate mammary ductal morphogenesis in ovariectomized Igf1-null mice (Ruan et al., 2005), the lack of ductal development in ERα-null mice is probably not because of diminished PR function. Indeed, ablation of the PR gene that encodes both PR isoforms PR-A and PR-B has no effect on adolescent ductal development (Soyal et al., 2002). However, this does not necessarily preclude the possibility that progesterone still influences primary and secondary branching in a non-essential manner. In either case, the absence of both PR-A and PR-B does block tertiary side-branching and lobuloalveolar development in adult mice and in pregnancy. Moreover, the selective ablation of PR-B suggests that it alone is necessary and sufficient to elicit tertiary branching. Tissue localization and recombination data also indicate that epithelial rather than stromal PRs stimulate lobuloalveolar development, although stromal PR-B may play a role in tertiary branching (Humphreys et al., 1997; Brisken et al., 1998). So how does PR activation affect tertiary branching? Notably, Wnt4 is also required for tertiary side-branching and it is regulated by progesterone, suggesting that it acts downstream of PR (Brisken et al., 2000). However, the consequences of its absence fade in late pregnancy, suggesting that other parallel pathways also participate. Indeed, receptor activator of NFκB (Rank) ligand may also contribute, as it too is regulated by progesterone (Conneely et al., 2003). Moreover, pregnancy-associated alveolar development is impaired in null mutant mice that harbor mutations in the NFκB activator IκB kinase-α as well as in mice that lack Rank or its ligand (Fata et al., 2000). Otherwise, the precise mechanisms by which progesterone regulates tertiary branching are not yet clear.
Local branch managers
Regulators of embryonic branching
Although some mechanisms may only affect the formation of the embryonic mammary tree, others may only affect its postnatal development, and still others may affect both its pre- and post-natal development. However, the postnatal role of those signaling pathways that are essential for initial mammary development may be difficult to ascertain if, in their absence, the embryonic mammary rudiment fails to form in the first place. For instance, mammary buds form in mice lacking the estrogen-regulated homeobox transcription factor, Msx2, but fail to undergo any further embryonic branching, thus concealing any role Msx2 may have later on (Hens and Wysolmerski, 2005). Likewise, instructive paracrine crosstalk between parathyroid hormone-related protein (PTHrP) from the embryonic mammary bud epithelium and its receptor PTHR1 on adjacent mesenchymal cells is required for forming the mammary-specific mesenchyme, which in turn is required to form a rudimentary ductal tree (Hens and Wysolmerski, 2005). Thus, it is unclear whether PTHrP participates in subsequent branching steps, although gain-of-function data suggest that it does. Specifically, the overexpression of PTHrP during adolescent development slows ductal elongation by increasing apoptosis in TEBs and its overexpression during embryogenesis diminishes ductal branching later in life, although the mechanism is unknown (Dunbar et al., 2001).
Embryonic mammary glands are also arrested at the bud stage in mice that lack the transcription factor LEF1 that lies downstream of the canonical (i.e., β-catenin-dependent) Wnt signaling cascade, and mammary buds fail to form at all in transgenic mice that express the diffusible Wnt inhibitor Dickkopf-1 under the control of an epidermal keratin14 gene promoter (Hens and Wysolmerski, 2005). Confounding matters further, the genetic absence of a number of individual Wnt genes results in early embryonic lethality. Thus, once again, any understanding of Wnt signaling at later times is compromised by requirements earlier in development. Nevertheless, Wnts have been shown to participate in the branching of other tissues, such as kidney; their transgenic and virus-mediated overexpression influences mammary branching; and mammary-targeted expression of an inhibitory form of a Wnt receptor that blocks both canonical and non-canonical Wnt signaling delays adolescent ductal development (A.M.C. Brown, Weill Medical College, personal communication). This suggests that at least one canonical and/or non-canonical Wnt signaling pathway plays a role in post-pubertal mammary development.
Compelling data also indicate that fibroblast growth factor (Fgf) signaling plays a pivotal role in the development of most, if not all, branched systems. Nevertheless, its role in mammary development remains obscure in part because of an early requirement for Fgf signaling in embryonic placode formation. Specifically, four of the five different murine placode pairs fail to appear at all in the absence of either Fgf receptor 2-IIIb (Fgfr2b) or its ligand, Fgf10 (Veltmaat et al., 2003). The only pair that does develop is the abdominal (#4) placode pair, which develops normally until embryonic day E16 in the absence of Fgf10 and which forms, but regresses at ~ E12 in the absence of Fgfr2b. Interestingly, Fgfr2b is expressed in the nascent placode epithelium at E11, whereas Fgf10 is expressed at E10.5 not in the underlying mesenchyme but in the somitederived dermatomyotome. This suggests that Fgf10 acts indirectly, for example by inducing the expression of yet another Fgfr2b ligand. Indeed, Fgf7 is expressed in the early mammary mesenchyme, yet no mammary phenotype has been reported in its absence. Regardless of how Fgfs initiate mammary development, however, the fact that they are indispensable early on complicates efforts to understand what else they do during later development. For this reason, we have used Cre-mediated mosaic analysis and have found that epithelial Fgfr2 is indeed required within TEBs for proper ductal development following puberty (P. Lu, G.R. Martin and Z. Werb, unpublished results).
The broad systemic or embryonic lethal effects of ablating a given gene may also obscure its local role in both embryonic and adolescent branching, although the latter can often be addressed using various transplantation techniques. Unraveling the role of a particular molecule in embryonic branching may be more problematic, however, if it has other pleiotropic effects. For example, branching of the embryonic mammary tree was significantly impaired in a recent analysis of mice that lack either the a disintegrin metalloproteinase 17 (Adam17) or the epidermal growth factor (Egf) receptor (Egfr/ErbB1), yet a prior examination of Egfr-null neonates revealed no such impairment (Sternlicht et al., 2005). There are several possible mechanisms that may account for the observed delay in embryonic branching. For example, it may reflect the generalized runting that results from Egfr or Adam17 ablation, or strain-specific genetic background effects, or a lack of local Adam17-Egfr signaling, or a combination of these different mechanisms. However, although the role of Adam17 and Egfr in embryonic mammary development remains unclear, their role in post-pubertal mammary development has been addressed by mammary gland rescue, as we now discuss.
Receptor tyrosine kinases in mammary branching
Egfr is a receptor tyrosine kinase that activates multiple intracellular pathways that are often associated with cell proliferation and survival. Its activity is elicited when it binds one of seven EGF-related ligands and then dimerizes with another Egfr monomer or one of three related receptors—ErbB2, ErbB3, or ErbB4. Notably, exogenous Egfr ligands rescue ductal development in both ovariectomized (Coleman et al., 1988) and ERα-deficient mice (Kenney et al., 2003), and exogenous estradiol elicits Egfr activation in ovariectomized mice (Sebastian et al., 1998), suggesting that Egfr promotes mammary branching downstream of ERα. Although several Egfr ligands can promote mammary development if given exogenously, amphiregulin (Areg) is the only one that is up-regulated at puberty, when its expression is strongly induced by estrogens. Moreover, Areg is the only EGFR agonist that is absolutely required for mammary development, as ductal outgrowth is impaired in Areg-null mutant mice, but not in mice lacking one or more alternative Egfr ligands (e.g., Egf and transforming growth factor α (Tgfα) or heparin-binding Egf-like growth factor and betacellulin) (Luetteke et al., 1999; Sternlicht et al., 2005). Thus, rather than forming a competent ductal tree that fills the entire fat pad, Areg-deficient mice form what could be called an inadequate bush—a small ductal outgrowth of insufficient size to nourish pups.
Egfr is also required for mammary branching, but only in the stroma, whereas its critical ligand, Areg, is exclusively expressed and solely required in the epithelium (Coleman et al., 1988; Sebastian et al., 1998; Luetteke et al., 1999; Sternlicht et al., 2005). In other words, Areg is only expressed on mammary epithelial cells, yet it has to bind and activate Egfr on nearby—but separate—stromal cells. Moreover, Areg is expressed as a transmembrane precursor. So, for Areg to activate adjacent stromal cells, it has to be shed from the epithelial cell surface. Notably, the transmembrane metalloproteinase Adam17 (Tnfα converting enzyme (TACE)) can release Areg and other Egfr ligands from cells in culture. In addition, Adam17-null mice resemble Egfr-null mice in many ways, including their perinatal lethality and the failure of their mammary glands to develop when transplanted to viable hosts (Sternlicht et al., 2005). In addition, as recombination experiments show, Adam17 is only required in the same epithelial location as its putative substrate, Areg. Which makes sense, as one would generally expect a cell surface enzyme and its cell surface substrate to be required on the same cell surface. Indeed, Egfr activation (i.e., its phosphorylation) only occurs when Adam17 and Areg are present on mammary epithelial cells and Egfr is present in the stroma, whereas Egfr activation proceeds normally in the absence of stromal Adam17, stromal Areg or epithelial Egfr. And most notably, local Areg administration rescues the development of Adam17-null transplants, firmly establishing that it acts downstream of Adam17—i.e., as its substrate. Thus, Adam17 plays a critical role in the epithelial–stromal crosstalk that regulates mammary development by liberating an essential cell surface ligand (Areg) that is solely expressed on epithelial cells so that it can activate its receptor (Egfr) on nearby stromal cells.
So what lies upstream of this paracrine interaction? Undoubtedly, the potent induction of Areg by ovarian estrogens is involved. However, it is not yet clear how else this pathway is regulated. Like Areg-deficient mammary glands, Adam17-deficient glands fail to catch up with wild-type glands over time and thus never fill their fat pads, indicating that other related enzymes are unable to compensate for its absence. However, unlike Areg, which is essential for mammary development, in part, because it is the only Egfr ligand that is expressed at the right time, place and amount in vivo, many Adams and other enzymes that can process Egfr ligands are strongly expressed during mammary development. What this means then, is that either Adam17 is the only physiologic “sheddase” for Areg, that it is independently regulated as compared with these other enzymes, or that it is both independently regulated and the only enzyme that can process Areg in the mammary gland. It is significant that the only endogenous inhibitor of Adam17—tissue inhibitor of metalloproteinases 3 (Timp3)—is specifically down-regulated in invading TEBs (but not in trailing ducts), whereas Timp1 is specifically up-regulated in the TEBs (Sternlicht et al., 2005). This inverse regulation of Timp1 and Timp3 would tend to de-constrain Adam17 in TEBs and thus foster the processing of Areg in a functionally appropriate location while simultaneously limiting the activity of other Timp1-inhibitable enzymes. It is also possible—indeed, likely—that other inputs control the local activity of Adam17. For instance, G-protein-coupled receptors, integrin α5β1 and the cytoplasmic accessory protein Eve1/Sh3d19 can each influence the Adam17-mediated processing of EGFR ligands in culture. Still, the precise physiologic inputs that regulate Adam17 activity during mammary development—or during any other in vivo process for that matter—are not yet known.
Nor do we know what lies downstream of Egfr. We do know, however, that TIMP1 inhibits mammary branching both in culture and in vivo, even though it does not inhibit Adam17 (Wiseman et al., 2003). This means that at least one other metalloproteinase must be involved in mammary development. And as metal-loproteinase inhibitors block branching in culture in response to Egfr agonists, they are probably inhibiting enzymes that act downstream of Egfr. In contrast, the lack of Adam17 does not preclude branching in response to exogenous Egfr agonists, as Adam17 acts upstream of Egfr. In addition, Egfr activation can stimulate the expression of MMP2/gelatinase-A as well as its activator MMP14/MT1-MMP in other systems, including the developing lung, where the Egfr-dependent induction of MMP14 and the resulting activation of MMP2 have been shown to regulate branching morphogenesis (Kheradmand et al., 2002). In the mammary gland, moreover, in vivo data indicate that MMP2 promotes ductal elongation, while MMP3/stromelysin-1, which may or may not be linked to Egfr signaling, promotes side-branching (Wiseman et al., 2003). Moreover, MMP14 promotes mammary ductal development by activating and collaborating with MMP2 to degrade type I collagen (M. Egeblad, M.D. Sternlicht, B.S. Wiseman and Z. Werb, unpublished results). MMP14 is also the only collagenolytic MMP that is not inhibited by Timp1, which is specifically up-regulated in and around TEBs, whereas MMP14 is inhibited by Timp3, which is specifically down-regulated in the same location. And as further evidence that MMP14 acts downstream of EGFR, MMP14 expression is specifically induced in the presumably activated stromal cells that sit immediately in front of the invading TEBs, and re-combination studies suggest that MMP14 is only required in the stromal compartment (M.D. Sternlicht and Z. Werb, unpublished results). Thus, it seems that one of the critical consequences of Egfr activation in mammary development is the induction of stromal MMP14.
In addition, Fgfr signaling may regulate mammary branching in response to Egfr or in parallel with it. This idea is supported by the observation that Fgf2 and Fgf7 elicit the growth and branching of Egfr-null mammary organoids in culture (Sternlicht et al., 2005), whereas Egfr agonists and Fgfs fail to support the growth of organoids lacking Fgf receptor 2 (Fgfr2) (Lu et al., 2005). As indicated in the prior section, Fgfr2b is expressed on mammary epithelial cells and, together with stromal Fgf10, is required for forming embryonic mammary placodes (Hens and Wysolmerski, 2005). In addition, mammary-targeted conditional ablation of Fgfr2 causes a severe delay in adolescent ductal development (Lu et al., 2005). Furthermore, analysis of the mosaic glands obtained due to incomplete ablation of Fgfr2 in all cells reveals that epithelia without Fgfr2 are eliminated from the ducts that do develop, suggesting that they are at a selective disadvantage compared with wild-type cells and/or that Fgfr2 is required in a cell-autonomous way. On the other hand, no mammary phenotype has been described in Fgf7-deficient mice, possibly due to compensatory mechanisms, and it remains unclear whether other FGF receptors or receptor isoforms are involved.
In addition, stromal Fgfs and their epithelial receptors play key roles in vertebrate lung, salivary gland, and kidney branching as well as in patterning of the Drosophila tracheal system, suggesting that similar mechanisms may also influence mammary branching (Affolter et al., 2003). For instance, primary lung buds fail to form in the absence of mesenchymal Fgf10 or epithelial Fgfr2, and renal tubular branching is significantly reduced in the absence of epithelial Fgfr2. Moreover, function-perturbing Fgfr2 antibodies suppress submandibular gland branching (Steinberg et al., 2005) and even Fgf10 haploinsufficiency inhibits normal lacrimal and salivary gland branching, suggesting that the precise levels of local ligand concentration are extremely important (Entesarian et al., 2005). Considerable evidence also suggests that a major role of Fgf signaling is to affect cell migration through chemotaxis, although it may also affect cell proliferation. For instance, Fgf10-soaked beads promote both proliferation and epithelial cell migration toward the source of Fgf in embryonic lung cultures—effects that are blocked by the Tgfβ superfamily member BMP4 (Weaver et al., 2000), thus suggesting growth factor interplay in establishing the branching pattern.
Similar mechanisms appear to be at play in Drosophila tracheal development. In flies, the stereotyped tracheal branching pattern is determined by Branchless (Bnl/Fgf), which is expressed by distinct cell clusters adjacent to the tracheal epithelia, and by its cognate receptor Breathless (Btl/Fgfr), which resides on the epithelium (Affolter et al., 2003). By activating Btl/Fgfr, Bnl/Fgf acts as an external chemoattractant, thereby providing guidance and patterning information. Interestingly, however, once the lead cell of a tracheal branch is determined based on its having bound more of the available Bnl/Fgf than its neighbors, it becomes the only cell in that branch that requires Bnl/Fgf (Ghabrial and Krasnow, 2006). That is, the trailing cells are able to follow the lead cell in a Bnl/Fgf-independent manner. However, contrary to what occurs during mammary branching, tracheal branching in the fly occurs after cell proliferation has ceased. In contrast, branching outgrowth of the fly air sac involves both proliferation and migration. In this setting, Btl/Fgfr signaling again directs the migration of the air sac tip, whereas Egfr signaling is required for cell proliferation and survival (Cabernard and Affolter, 2005). However, both pathways require Ras and mitogen-activated protein kinase (MAPK) function, thus raising the dilemma of how they carry out their distinct roles while using the same intracellular pathways. Part of the answer to this dilemma seems to involve their differential activation of MAPK, which is more highly activated by Fgf signals in cells of the leading edge. Another contributing mechanism appears to be the recruitment of distinct co-factors that initiate independent downstream events.
Although similar Fgfr-mediated migratory and Egfr-mediated proliferation and survival signals could also conceivably apply to mammary development, other aspects appear to differ. Thus in the mammary gland, Egfr and Fgfr2 may carry out their separate functions in part because they are required on different cell types and in part because Fgfr2 may act downstream of Egfr in a reciprocal paracrine cascade. It is also possible that stromal Egfr signals initiate multiple independent pathways—for instance, Fgf-mediated guidance cues, MMP14-mediated path clearing and signaling cues, and other cues that promote cell division and cell survival. However, not only is this or any other bi-directional interaction still speculative, but it remains unclear what intracellular pathways Egfr and Fgfr activate to carry out their effects or even what their specific effects in the mammary gland are. Indeed, similar predicaments exist in other branched systems. For instance, inhibition of intracellular PI3 kinase signaling blocks both tubular elongation and branching in kidney organ cultures (Tang et al., 2002), whereas the inhibition of MAPK activity affects renal tubular branching but not elongation, suggesting that the two processes are differentially regulated (Watanabe and Costantini, 2004). Given its central importance in multiple receptor-mediated pathways, Erk/MAPK signaling is almost certainly involved in mammary branching in one way or another, and yet the combined absence of Areg, Egf, and Tgfα has no apparent effect on either proliferation, apoptosis or Erk activation within mammary TEBs in vivo, even though their absence clearly inhibits mammary development (Luetteke et al., 1999). Thus, unraveling precisely how a central cell-signaling component like MAPK contributes to branching or any other in vivo process seems particularly daunting when that component almost certainly has numerous critical roles.
Evidence also indicates that the potential EGFR partner, ErbB2, a related but ligand-less transmembrane tyrosine kinase that plays a causal role in many breast cancers, influences mammary ductal morphogenesis. Specifically, TEB defects and delays in ductal penetration are seen when mammary glands isolated from ErbB2-null embryos that have been genetically rescued by a cardiac muscle-targeted ErbB2 transgene are transplanted to cleared wild-type fat pads (Jackson-Fisher et al., 2004) as well as after the selective ablation of ErbB2 in mammary epithelial cells (Andrechek et al., 2005). Thus, both studies indicate that ErbB2 is required in the epithelium for normal ductal development to occur. However, ErbB2 exerts its signaling activity not after binding a ligand of its own, as it has none, but after heterodimerizing with another ligand-bound ErbB receptor. This raises the question of which other ErbB receptor interacts with epithelial ErbB2 during mammary development. However, epithelial Egfr and ErbB4 are expendable during ductal development and ErbB3 is only weakly expressed in the developing gland (Sebastian et al., 1998; Tidcombe et al., 2003; Sternlicht et al., 2005). Therefore, it is unclear how ErbB2 regulates ductal development or whether epithelial Egfr-ErbB2 heterodimers participate in ways that have not yet been explicitly addressed.
Unlike Egfr and ErbB2, ErbB4 only appears to influence lobuloalveolar development. For instance, mice that express a mammary-targeted dominant-negative ErbB4 or that lack the ErbB4 ligand, neuregulin-1α exhibit normal ductal development, but impaired alveolar development (Jones et al., 1999; Li et al., 2002). Likewise, ErbB4-null mice that have been rescued from lethality by a cardiac myosin promoter-driven ErbB4 transgene exhibit alveolar defects during pregnancy and lactation (Tidcombe et al., 2003). Surprisingly, however, the timing of their post-pubertal ductal development often actually surpasses that of their wild-type siblings. One possible explanation for their accelerated ductal morphogenesis is that in the absence of ErbB4, additional Egfr may be freed up to concentrate on its task of promoting ductal development.
Regulators of duct and TEB morphology
Although it is reasonable to expect that mechanisms that affect the morphology of TEBs and ducts should also affect their branching and elongation, this may not always be soff Netrin-1, which acts as a diffusible attractant and repellent during neuronal guidance, is also secreted by the inner body cells of mammary TEBs, while its receptor, neogenin, is expressed on adjacent outer cap cells (Srinivasan et al., 2003). Absence of either netrin-1 or neogenin causes a dissociation of the cap and body cell compartments and inappropriate migration of cap cells into the pre-lumenal body cell compartment. Moreover, neogenin mediates netrin-dependent cell clustering in culture, further indicating that netrin–neogenin interactions stabilize the cap cell layer and mediate its adhesion to body cells. Nevertheless, absence of either netrin-1 or neogenin has no effect on overall branching, suggesting that ductal development and TEB morphology may not depend on one another in this setting (L. Hinck, University College of Santa Cruz, personal communication). It may also mean, however, that other adhesive molecules compensate for the absence of netrin and negenin outside of the TEBs.
Hedgehog signaling, which is elicited by the binding of Indian, Sonic, or Desert hedgehog to cell surface patched receptors, can influence many of the signaling pathways that appear to regulate mammary branching, including Fgf, Wnt, Notch, Tgfβ, and PTHrP pathways (Lewis, 2001). Interestingly, Fgf10 is up-regulated in the lung mesenchyme of mice lacking Sonic hedgehog, yet despite its up-regulation, the developing lungs fail to branch beyond the primary bud stage (Pepicelli et al., 1998). Likewise, comparable interactions have been seen during brain and limb bud development (Ohkubo et al., 2002). Moreover, sonic hedgehog supplementation augments the branching of salivary gland organ cultures, whereas a steroidal alkaloid (cyclopamine) that specifically inhibits sonic hedgehog signaling suppresses their branching (Jaskoll et al., 2004). On the other hand, mammary transplants that lack Indian or Sonic hedgehog branch normally in cleared wild-type fat pads, thus indicating that neither ligand alone is essential within the epithelium. However, conditional haploinsufficiency of their receptor, Patched-1, causes defects in duct and TEB histology that disappear following transplantation to wild-type fat pads, suggesting that Patched-1 is required, but only within the stroma. Still, the overall branching pattern of Patched-1 heterozygous glands is unaffected. Moreover, transplants lacking Gli2, a transcription factor that lies downstream of Patched, also display normal ductal branching despite their abnormal intra-ductal morphology, again suggesting that ductal patterning and ductal morphology may not be entirely interdependent.
Conversely, some molecules, such as ErbB2, do appear to influence both duct morphology and branching (Jackson-Fisher et al., 2004). For example, the cell surface morphogen, epimorphin, is required for growth factor-induced branching of organotypic mammary cultures and affects ductal diameter when it is provided in an apolar manner in culture or as a mammarytargeted transgene in vivo (Radisky et al., 2003). The metalloproteinase-dependent release of epimorphin from stromal fibroblasts appears to be required for epimorphin to promote the branching of mammary epithelial cells, although myoepithelial epimorphin could still act in a juxtacrine manner without being shed. As a downstream consequence, epimorphin stimulates the expression of MMP2 and MMP3, both of which are also required for proper mammary branching. In addition, epimorphin induces expression of the transcription factor C/EBPβ—which is also required for mammary morphogenesis—and increases the relative expression of the shorter of two C/EBPβ isoforms. Indeed, experimental manipulation of the relative expression of these two isoforms in the absence of epimorphin signaling has the same morphogenic effect in culture as epimorphin itself, suggesting that C/EBPβ acts downstream of epimorphin.
Another fundamental aspect of the development of any organ or tissue is the specification of its constituent cell types from multipotent stem and progenitor cells, as well as their subsequent maintenance. Initial cell fate decisions are determined in part by hierarchical networks of transcription factors, yet it is unclear whether these factors also contribute to the maintenance of an established cell fate. What then are the constituent cell types of the mammary gland? What determines their fate? And how do they persist as such? Mammary ducts and alveoli contain two principal cell types: inner (luminal) epithelial cells that produce milk, and outer (basal) myoepithelial cells that contract in response to oxytocin to promote milk ejection during suckling. These two cell types arise from a recently characterized mammary stem cell that can regenerate an entire mammary gland from a single cell (Shackleton et al., 2006; Stingl et al., 2006). As regards their maintenance, our own data indicate that the transcription factor Gata3 plays a pivotal role in maintaining the luminal cell fate (H. Kouros-Mehr and Z. Werb, unpublished results). Notably, GATA transcription factors—so named for binding (A/T)GATA(A/G) DNA sequences—have also been shown to specify erythroid and myeloid cell fates (Patient and McGhee, 2002). In the mammary gland, Gata3 is highly and exclusively expressed in the body cells of invading TEBs and the luminal cells of nascent and mature ducts. Moreover, early mammary glandspecific (i.e., MMTV-Cre-mediated) ablation of Gata3 results in an absence of TEB formation at puberty and in severe defects in ductal outgrowth. This indicates that Gata3 is essential for post-pubertal development. Moreover, its late (i.e., WAP-rtTA-Cre, doxycycline-induced) deletion in adult mammary glands leads to acute expansion of an undifferentiated luminal cell population followed by cellular detachment from the basement membrane and caspase-mediated cell death. These results further suggest that Gata3 is crucial for maintaining luminal cell differentiation in mature ducts. In addition, its requirement for proper TEB and duct development suggests that Gata3 may also function to drive luminal differentiation in the first place. All these findings raise new questions concerning the mechanisms that control Gata3 and what critical target genes are activated or repressed by it.
Macrophage- and eosinophil-derived signals
Of the various stromal cells that affect mammary ductal elongation and branching, macrophages and eosinophils are particularly important (Gouon-Evans et al., 2002). Macrophage recruitment to the stroma surrounding TEBs is severely impaired in myelosuppressed (γ-irradiated) mice and in mice that lack macrophage colony-stimulating factor 1 (Csf1) or its receptor (c-fms). As a presumed consequence of their absence, TEB formation and adolescent ductal outgrowth are also severely impaired. On the other hand, macrophage recruitment, TEB formation and ductal development are rescued by bone marrow transplantation in irradiated mice and by the administration of exogenous Csf1 or the expression of a mammary-targeted Csf1 transgene in Csf1-null mice. Thus, the essential effects of Csf1 on macrophage behavior are local rather than systemic. Indeed, in the developing gland, ductal cells produce Csf1, whereas c-fms is exclusively expressed on macrophages. Nevertheless, it remains unclear precisely how macrophages influence ductal development—that is, whether it is their phagocytic, trophic, angiogenic or extracellular matrix (ECM) remodeling activities that are important.
In the case of eosinophils, their recruitment to the TEB stroma coincides with the local up-regulation of their chemoattractant eotaxin, which is in turn recognized by their Ccr3 receptor (Gouon-Evans et al., 2002). Indeed, such recruitment is severely diminished in eotaxin- deficient mice, as is ductal branching, but not ductal elongation. On the other hand, absence of the eosinophil chemotactic factor interleukin-5 causes a deficiency in circulating eosinophils, but has no effect on the number of mammary eosinophils, nor does it affect mammary development, further supporting the importance of eotaxin as a local chemoattractant for eosinophils during mammary development. Although it is not entirely clear how eosinophils promote ductal branching, mammary eosinophils secrete the chemokine C10, which appears to further enhance macrophage recruitment. Thus, eosinophils and macrophages may collaborate with one another to bring about proper ductal morphogenesis.
Negative regulators of branching morphogenesis
Although controls against precocious, accelerated or excess branching undoubtedly exist, a full understanding of their individual importance is difficult to obtain in the face of redundant or even unrelated mechanisms. For example, the endogenous MMP inhibitor Timp1 defies ductal development in a gain-of-function setting, yet its absence has little or no effect on branching in a loss-of-function setting (Wiseman et al., 2003). Thus, it is unclear whether Timp1 is truly involved or compensated for by other Timps, as other constraints as basic as limits on the rate of cell division undoubtedly continue to exert their own rate-limiting effects. Thus, just as a car that loses its brakes does not necessarily speed up, the loss of a bona fide negative regulator of mammary development may not reveal itself in the face of other rate-limiting factors.
Nevertheless, each of the branching agonists outlined in this review have their own negative regulators, some of which might accelerate branching morphogenesis if lost. And, indeed, some examples of accelerated ductal development following gene inactivation have been seen—the most notable examples being the effects of deleting Tgfβ1 and Sprouty2 (Spry2). The Drosophila Spry gene was initially discovered in a genetic screen for mutations that affect tracheal branching. In this case, Spry mutant flies were identified because they “sprouted” extra secondary tracheal branches, and it was later determined that this was because Spry normally inhibits Btl/Fgfr signaling within the tracheal epithelium in a cell-autonomous manner (Kim and Bar-Sagi, 2004). Thus, in its absence, the so-called “lead cells” of the primary tracheal branches received essentially unrestrained Bnl-Btl (Fgf-Fgfr) stimulation and, as a result, formed ectopic secondary sprouts. Moreover, consistent with the role of Bnl-Btl signals in specifying which particular cell of a primary branch becomes the lead cell, Spry mutant cells were more apt to adopt the lead cell fate than their neighboring wild-type cells in a mosaic setting (Ghabrial and Krasnow, 2006).
Significantly, Spry and its four vertebrate homologs are not specific Fgfr antagonists. Rather, they can also inhibit Egfr signaling and presumably the activity of other receptor tyrosine kinases as well (Kim and Bar-Sagi, 2004). Thus, it is hardly surprising that murine Spry genes have been implicated in the branching morphogenesis of organs other than the fly tracheal system. Mice lacking Spry1, for instance, develop polycystic kidneys due to the formation of ectopic ureteric buds—a phenotype comparable with that seen with excess Gdnf-Ret signaling (Basson et al., 2005). So, if Spry1 normally acts to constrain Gdnf-Ret signaling during ureteric bud formation, then the artificial down-regulation of Ret activity should negate the effects of Spry1 loss. And indeed, the genetic down-regulation of Gdnf-Ret signaling afforded by ablating a single Gdnf allele—thus lowering the overall Gdnf dosage—rescues the renal phenotype of Spry1-deficient mice. Moreover, Spry1 inhibits the formation of ectopic renal tubular branches at later stages of kidney development, presumably in the same way—that is, by inhibiting Gdnf-Ret signaling (M.A. Basson and J.D. Licht, Mount Sinai School of Medicine, personal communication). Likewise, Spry2, which is specifically expressed at the distal tips of branching epithelial lung buds, appears to negatively control lung branching morphogenesis. Its antisense down-regulation stimulates lung branching, whereas a gain of Spry2 function has exactly the opposite effect and instead defies branching (Tefft et al., 1999; Mailleux et al., 2001). In the mammary gland, increased ductal invasion occurs when Spry2 is inactivated in the epithelium, suggesting its possible importance in controlling Fgfr2 or perhaps Igfr1 signaling (Lu et al., 2005). Still, it is not yet known which receptor tyrosine kinase pathway is affected in lung development, nor is it entirely clear how Spry genes affect mammary branching.
Considerable evidence also indicates that Tgfβ1—which is regulated by ovarian hormones—acts as a key negative regulator of mammary branching by limiting epithelial proliferation and by stimulating ECM production (Daniel et al., 1996; Ewan et al., 2002). Indeed, Tgfβ inhibits the branching of both lung and kidney cultures (Serra et al., 1994; Bush et al., 2004). Therefore, epithelial-generated Tgfβ gradients may dictate where adjacent branches form. That is, by keeping adjacent branches apart, Tgfβ may generate and maintain an open branched architecture that leaves space for other functional structures, such as salivary acini or pulmonary alveoli, to develop. So, is this the case in the mammary gland? Consider what happens when Tgfβ signaling is altered in vivo In gain-of-function experiments, mammary-targeted overexpression of activated Tgfβ1 slows ductal development, resulting in the formation of a hypomorphic mammary tree, while slow-release Tgfβ1 implants locally inhibit epithelial cell proliferation, TEB formation and ductal elongation. Conversely, heterozygous Tgfβ1-deficient mice, which have <10% of normal Tgfβ1 levels due to an absence of positive feedback, exhibit two to four times more ductal proliferation than normal, 15-fold more proliferation in response to exogenous ovarian hormones, and significantly accelerated but morphologically normal ductal development. In addition, the Tgfβ1 heterozygous glands exhibit accelerated outgrowth in wild-type fat pads, indicating that the growth inhibitory effects of Tgfβ1 are epithelial in origin. Nevertheless, these effects appear to be carried out through both autocrine feedback mechanisms and paracrine interactions that may involve stromal type II Tgfβ receptors (TgfβRII) and reciprocal stromal responses. Indeed, as in Tgfβ1 haploinsufficient mice, mammary side-branching is accelerated when stromal TgfβRII function is blocked by expressing a dominant-negative TgfβRII transgene in the stroma under the zinc sulfate-inducible metallothionine promoter (Joseph et al., 1999; Crowley et al., 2005) or by conditionally ablating the TgfβRII gene in stromal fibroblasts (Cheng et al., 2005).
Thus, Tgfβ1 may aid in the maintenance of proper ductal spacing by enabling adjacent ducts to avoid one another. But how? As to this question, there are at least four distinct—though not necessarily mutually exclusive—possibilities. Epithelial Tgfβ may act as an autocrine inhibitor that (1) suppresses the expression of an epithelial protein that promotes ductal development or that (2) induces the expression of another inhibitor. By the same token, Tgfβ may act in a paracrine manner to (3) induce the production of a stromal antagonist to ductal development or (4) inhibit the expression of a stromal agonist. For instance, Tgfβ1 suppresses Fgf10 expression in fibroblast, lung, and prostate cultures (Beer et al., 1997; Lebeche et al., 1999; Tomlinson et al., 2004). It also down-regulates MMP3 expression, which would tend to inhibit secondary side-branching without affecting ductal elongation. In contrast, Tgfβ1 up-regulates MMP2 expression, which would foster ductal elongation and thus increase the distance between secondary branch-points (Sternlicht and Werb, 2001; Wiseman et al., 2003). In addition, Tgfβ1 is known to induce the production and remodeling of numerous ECM components in the mammary gland and elsewhere, including fibronectin and several collagens (Silberstein et al., 1990). And this too could influence mammary development, as we now discuss.
ECM-mediated effects
The dated notion that the ECM acts as a mere barrier or foundation for cells has been revised to reflect the fact that the ECM also conveys its own contextual information and that it actively rather than passively affects cell behavior (Lukashev and Werb, 1998). Indeed, virtually all aspects of cell behavior (and misbehavior)—their shape, polarity, organization, motility, invasion, growth, differentiation, and survival, for instance—can be influenced by ECM-derived signals. In general, ECM components affect cell behavior by acting as fixed ligands for adhesion molecules, such as integrins, that transduce ECM-derived signals to the cytoskeletal machinery and cell interior, as well as by sequestering other signaling molecules, such as growth factors and their binding proteins. Indeed, if the ECM influences cell behavior, and adhesion molecules transmit its cues to the cell interior, then adhesion molecules must lie at the very nexus of how the structural environment affects cell behavior. By extension, enzymes that remodel the ECM can also affect cell behavior by altering the content and organization of the ECM-associated information a cell sees. For instance, ECM-degrading MMPs appear to play a path-clearing role during branching morphogenesis as well as an indirect cell signaling role that may reflect their ability to alter ECM inputs, generate bioactive ECM fragments, and/or release ECM-bound growth factors (Sternlicht and Werb, 2001; Ortega and Werb, 2002; Fata et al., 2004). Indeed, ECM remodeling can alter existing ECM signals and remove physical barriers that might otherwise defy ductal branching and elongation. ECM remodeling can also unmask hidden structural cues, such as cryptic integrin-binding sites on fibrillar collagens, a bioactive laminin-5 fragment that promotes epithelial motility, or the anti-angiogenic fragments tumstatin and endostatin from collagens IV and XVIII, respectively. And ECM remodeling can release ECM-sequestered signaling molecules, such as Areg, Fgfs, Wnts, and Tgfβ, each of which have been shown to influence branching morphogenesis. In addition, spatial differences in ECM deposition rather than ECM degradation may elicit transitions between different modes of adhesion that, in turn, influence ductal branch-point selection and patterning. For instance, localized fibronectin deposition is required for cleft formation in salivary gland cultures and is associated with a switch from Ecadherin-mediated (cell–cell) adhesion to α5β1 integrin-mediated (cell–matrix) adhesion, a transition that may, in effect, stabilize the points at which bifurcation takes place (Sakai et al., 2003).
It is not surprising, therefore, that the ECM, its receptors and its remodeling enzymes affect branching morphogenesis (Fata et al., 2004). However, because multiple ECM receptors may be involved, the genetic ablation of a single receptor may have subtle or completely hidden rather than severe consequences. Nevertheless, the absence of discoidin domain receptor-1 (Ddr1), a tyrosine kinase receptor that elicits signals upon binding fibrillar collagens, results in delayed and abnormal mammary development (Vogel et al., 2001). This suggests that fibrillar collagens provide more than just structural support, and may partly explain the delayed mammary branching seen in mice that are treated with MMP inhibitors, that lack the collagenolytic enzymes MMP2 or MMP14, or that express cleavage-resistant or mutant type I collagen (M. Egeblad, M.D. Sternlicht, B.S. Wiseman and Z. Werb, unpublished results). Yet another role for MMP14 that appears to involve collagen degradation and that may impact mammary development is its regulation of the 3-D organization of white adipose tissue, which is precisely the type of stroma within which the murine mammary gland resides (Chun et al., 2006).
Several lines of evidence also support a role for integrin-mediated signaling in mammary morphogenesis. However, the data also remain somewhat conflicted. Mice that lack integrin α2β1 collagen/laminin receptors due to the genetic deletion of α2 integrin exhibit diminished, but otherwise normal, mammary branching and are entirely able to nurse their pups (Chen et al., 2002). Similarly, antibodies against β1 integrin cause diminished ductal elongation, as do antibodies against the γ1 chain found in several laminins, which in turn are the main ECM ligands for β1 integrins (Klinowska et al., 1999). However, these defects are far milder than those that occur when α2β1 integrin function is blocked or absent in culture. Likewise, α3, α6, and β4 integrins can affect branching in culture, but are entirely dispensable in vivo (Klinowska et al., 2001). Moreover, the mammary-targeted conditional deletion of all β1 integrins has no overt effect on mammary development (White et al., 2004). Although this latter result may reflect selective pressures that favor the development of non-recombined wild-type cells or their non-cell-autonomous rescue of null cells, it may also reflect the existence of redundant or compensatory interactions. Indeed, overexpression of another laminin receptor, β-1,4-galactosyltransferase, elicits abnormal and diminished mammary development (Hathaway and Shur, 1996), and yet another laminin receptor, dystroglycan, has been shown to regulate cell polarity in cultured mammary epithelial cells (Muschler et al., 2002). Thus these non-integrin receptors may also participate and may mitigate the effects of losing β1 integrin.
Another aspect of ductal development that depends, in part, on the matrix microenvironment is lumen formation—the assembly of hollow polarized cysts (alveoli) and tubes (ducts). In the developing mammary gland, the leading portion of the TEB is largely a lumen-less structure covered by a cap cell layer and filled by so-called body cells, whereas the trailing ducts are essentially hollow bi-layered tubes. The process of lumen formation for any epithelial cyst or tube appears to be driven in part by an intrinsic program wherein adherent epithelial cells seek to establish basal, lateral and free apical surfaces via cell–cell, cell–matrix, and corresponding cytoskeletal interactions (O’Brien et al., 2002). In addition, canalization of the lumen may involve anti-adhesive mechanisms that promote the separation of otherwise apposed membranes and the removal of unwanted pre-luminal body cells by apoptosis or autophagy. There is absolutely no evidence, however, that single cell protrusions or discontinuities occur as mam-mary branches form. If anything, early time-lapse images suggest that the cells of mammary TEBs undergo considerable movement relative to one another, but that they bulge or expand outward as an otherwise cohesive mass as they branch and elongate (Williams and Daniel, 1983). In addition, the tunneling process that continuously follows the invading TEBs appears to involve the clearance of centrally located body cells by apoptosis. Thus the formation of mammary ducts appears to involve both continuous sheet-like budding and the apoptotic removal of pre-luminal cells rather than any of the other mechanisms by which tubular structures are thought to develop (O’Brien et al., 2002; Lubarsky and Krasnow, 2003).
However they form, mammary ducts end up as bilayered tubes composed of inner luminal epithelial cells surrounded by myoepithelial cells, which are in turn surrounded by an extracellular basement membrane. Although myoepithelial cells are best known for promoting oxytocin-induced milk ejection by virtue of their contractile activity, they are also the cells that actually contact the basement membrane directly and are required to produce many of its components, including laminins. Thus myoepithelial cells are ideally situated to transmit structural morphogenetic cues from the basement membrane to the luminal epithelia. Indeed, isolated luminal epithelial cells (which do not synthesize their own basement membranes) fail to form properly polarized hollow spheres when they are cultured in type I collagen gels, and instead form solid lumen-less structures with inverse polarity. However, if myoepithelial cells are also added, they do form correctly polarized, hollow, bi-layered acinar-like structures (Gudjonsson et al., 2002). Furthermore, laminin-1 or minor amounts of reconstituted laminin-rich basement membrane are also able to rescue the polarity of pure luminal cell cultures, despite the absence of myoepithelial cells, whereas laminins 5 and 10/11 are unable to do soff In addition, the proper positioning of luminal and myoepithelial cells is blocked by peptides that interfere with desmosomal cell–cell interactions (Runswick et al., 2001). Thus myoepithelial cells, their desmosomal adhesion to adjacent luminal cells, and the basement membrane components they produce (in particular laminin-1) appear to act as key mediators in establishing proper duct polarity and lumen formation.
Perspectives
Many tissues undergo branching morphogenesis and there are clear differences in how branching proceeds in each setting. Nevertheless, many of the basic mechanisms that control branching in one situation are likely to be evolutionarily conserved among all branched organs and organisms. Indeed, many mechanistic similarities have been seen in comparative analyses of diverse branched tissues and in organisms as different from one another as flies and mice (Affolter et al., 2003). However, these organs also clearly differ from one another. Thus there must also be unique tissue-and species-specific mechanisms that elicit their obvious differences in form and function. The challenge, of course, is to characterize and decipher these ubiquitous and unique mechanisms. In addition, various modes of branching within any given system—the primary, secondary, and tertiary branching of mammary ducts, for instance—appear to utilize distinct mechanisms both in terms of the cues that initiate and orchestrate each process and in terms of the cellular events that distinguish one mode from another—for example, primary TEB bifurcation from secondary or tertiary side-branching. Nevertheless, despite considerable headway, our understanding of the cues that pass back and forth between neighboring cells, their over-riding systemic regulation, and the role of the stromal microenvironment remains largely incomplete, thus leaving the field ripe for further progress.
Certain aspects of mammary branching—the consequences of cell movements and neighbor–neighbor rearrangements, for instance, or the relative contribution of the leading edge of the TEB versus any other portion of the TEB or duct to branch point formation and duct advancement—seem poised to yield intriguing new insights. Indeed, such paths of inquiry are now particularly accessible given recent advances in a host of complimentary approaches, from the mapping of cell fates by prolonged intra-vital and culture-based timelapse imaging of lineage-specific reporters to the inducible and conditional ablation of putative regulatory genes in specific cell types to assess their impact. Other facets, however, may require more creative strategies owing to their inherent complexity—such as determining what multi-pathway signaling molecules, such as MAPK, do in vivo and how they distinguish between various intersecting pathways; or determining, for instance, how a single pleiotropic factor contributes to one process if it affects many other confounding processes from guidance to survival; or resolving what negative regulators do if they have redundant, compensatory or entirely unrelated rate-limiting factors that make it difficult to determination if they are even involved in the first place, let alone how they function. There are, of course, two sides to this dilemma: the purely conceptual problem that complex systems pose to unraveling the role of an otherwise non-essential participant for the sake of scientific insight alone, versus the evolutionary advantages they provide to ensure proper development of the organ or organism itself. Nevertheless, the same basic processes, from proliferation to invasion, that occur during normal mammary development also occur during various disease states, and most of the pathways that influence normal branching morphogenesis have also been associated, in one way or another, with the onset or evolution of cancer. Thus a better understanding of the mechanisms that control mammary branching per se should provide important new insight into additional processes, both normal and otherwise.
Acknowledgments
This work was supported by grants from the National Cancer Institute (CA57621 and CA58207), a grant jointly funded by the National Institute of Environmental Health Sciences and National Cancer Institute (ES012801), an institutional NRSA post-doctoral training grant (HL07731 to P.L.) and a pre-doctoral fellowship from the California Breast Cancer Research Program (to H.K.-M.).
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. doi: 10.1016/s1534-5807(02)00410-0. [DOI] [PubMed] [Google Scholar]
- Andrechek ER, White D, Muller WJ. Targeted disruption of ErbB2/Neu in the mammary epithelium results in impaired ductal outgrowth. Oncogene. 2005;24:932–937. doi: 10.1038/sj.onc.1208230. [DOI] [PubMed] [Google Scholar]
- Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell. 2005;8:229–239. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Beer HD, Florence C, Dammeier J, McGuire L, Werner S, Duan DR. Mouse fibroblast growth factor 10:cDNA cloning, protein characterization, and regulation of mRNA expression. Oncogene. 1997;15:2211–2218. doi: 10.1038/sj.onc.1201383. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, Korach KS. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA. 1998;95:5076–5081. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush KT, Sakurai H, Steer DL, Leonard MO, Sampogna RV, Meyer TN, Schwesinger C, Qiao J, Nigam SK. TGF-beta superfamily members modulate growth, branching, shaping, and patterning of the ureteric bud. Dev Biol. 2004;266:285–298. doi: 10.1016/j.ydbio.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP-and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Coleman S, Silberstein GB, Daniel CW. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988;127:304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Jericevic BM, Lydon JP. Progesterone receptors in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:205–214. doi: 10.1023/a:1025952924864. [DOI] [PubMed] [Google Scholar]
- Crowley MR, Bowtell D, Serra R. TGF-beta, c-Cbl, and PDGFR-alpha the in mammary stroma. Dev Biol. 2005;279:58–72. doi: 10.1016/j.ydbio.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Christov K, Guzman R, Nandi S, Talamantes F, Thordarson G. Mammary phenotypic expression induced in epidermal cells by embryonic mammary mesenchyme. Acta Anat (Basel) 1995;152:195–204. doi: 10.1159/000147698. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Hom YK, Cooke PS, Taylor JA, Lubahn DB. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- Curtis Hewitt S, Couse JF, Korach KS. Estrogen receptor transcription and transactivation: Estrogen receptor knockout mice: what their phenotypes reveal about mechanisms of estrogen action. Breast Cancer Res. 2000;2:345–352. doi: 10.1186/bcr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Robinson S, Silberstein GB. The role of TGF-b in patterning and growth of the mammary ductal tree. J Mammary Gland Biol Neoplasia. 1996;1:331–341. doi: 10.1007/BF02017389. [DOI] [PubMed] [Google Scholar]
- Daniel CW, Silberstein GB, Strickland P. Direct action of 17 beta-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res. 1987;47:6052–6057. [PubMed] [Google Scholar]
- Dunbar ME, Dann P, Brown CW, Van Houton J, Dreyer B, Philbrick WP, Wysolmerski JJ. Temporally regulated overexpression of parathyroid hormone-related protein in the mammary gland reveals distinct fetal and pubertal phenotypes. J Endocrinol. 2001;171:403–416. doi: 10.1677/joe.0.1710403. [DOI] [PubMed] [Google Scholar]
- Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI, Jonsson R, Wahren-Herlenius M, Dahl N. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125–128. doi: 10.1038/ng1507. [DOI] [PubMed] [Google Scholar]
- Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, Barcellos-Hoff MH. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am J Pathol. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. [DOI] [PubMed] [Google Scholar]
- Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol. 2001;229:163–175. doi: 10.1006/dbio.2000.9961. [DOI] [PubMed] [Google Scholar]
- Ghabrial A, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway HJ, Shur BD. Mammary gland morphogenesis is inhibited in transgenic mice that overexpress cell surface beta1,4-galactosyltransferase. Development. 1996;122:2859–2872. doi: 10.1242/dev.122.9.2859. [DOI] [PubMed] [Google Scholar]
- Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 2005;7:220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. Key stages of mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- Humphreys RC, Lydon J, O’Malley BW, Rosen JM. Mammary gland development is mediated by both stromal and epithelial progesterone receptors. Mol Endocrinol. 1997;11:801–811. doi: 10.1210/mend.11.6.9891. [DOI] [PubMed] [Google Scholar]
- Jackson-Fisher AJ, Bellinger G, Ramabhadran R, Morris JK, Lee KF, Stern DF. ErbB2 is required for ductal morphogenesis of the mammary gland. Proc Natl Acad Sci USA. 2004;101:17138–17143. doi: 10.1073/pnas.0407057101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P, Jr, Carlsson P, Melnick M. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004;229:722–732. doi: 10.1002/dvdy.10472. [DOI] [PubMed] [Google Scholar]
- Jones FE, Welte T, Fu XY, Stern DF. ErbB4 signaling in the mammary gland is required for lobuloalveolar development and Stat5 activation during lactation. J Cell Biol. 1999;147:77–88. doi: 10.1083/jcb.147.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph H, Gorska AE, Sohn P, Moses HL, Serra R. Overexpression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching. Mol Biol Cell. 1999;10:1221–1234. doi: 10.1091/mbc.10.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney NJ, Bowman A, Korach KS, Barrett JC, Salomon DS. Effect of exogenous epidermal-like growth factors on mammary gland development and differentiation in the estrogen receptor-alpha knockout (ERKO) mouse. Breast Cancer Res Treat. 2003;79:161–173. doi: 10.1023/a:1023938510508. [DOI] [PubMed] [Google Scholar]
- Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–848. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Bar-Sagi D. Modulation of signalling by Sprouty: a developing story. Nat Rev Mol Cell Biol. 2004;5:441–450. doi: 10.1038/nrm1400. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]
- Klinowska TC, Alexander CM, Georges-Labouesse E, Van der Neut R, Kreidberg JA, Jones CJ, Sonnenberg A, Streuli CH. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol. 2001;233:449–467. doi: 10.1006/dbio.2001.0204. [DOI] [PubMed] [Google Scholar]
- Klinowska TC, Soriano JV, Edwards GM, Oliver JM, Valentijn AJ, Montesano R, Streuli CH. Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Dev Biol. 1999;215:13–32. doi: 10.1006/dbio.1999.9435. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Schwartz P. Tissue interaction in androgen response of embryonic mammary rudiment of mouse: identification of target tissue for testosterone. Proc Natl Acad Sci USA. 1976;73:4041–4044. doi: 10.1073/pnas.73.11.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe M, Sakakura T, Sano M, Nishizuka Y. A pituitary-salivary mixed gland induced by tissue recombination of embryonic pituitary epithelium and embryonic submandibular gland mesenchyme in mice. Dev Biol. 1985;110:382–391. doi: 10.1016/0012-1606(85)90097-1. [DOI] [PubMed] [Google Scholar]
- Lebeche D, Malpel S, Cardoso WV. Fibroblast growth factor interactions in the developing lung. Mech Dev. 1999;86:125–136. doi: 10.1016/s0925-4773(99)00124-0. [DOI] [PubMed] [Google Scholar]
- Lewis MT. Hedgehog signaling in mouse mammary gland development and neoplasia. J Mammary Gland Biol Neoplasia. 2001;6:53–66. doi: 10.1023/a:1009516515338. [DOI] [PubMed] [Google Scholar]
- Li L, Cleary S, Mandarano MA, Long W, Birchmeier C, Jones FE. The breast proto-oncogene, HRGalpha regulates epithelial proliferation and lobuloalveolar development in the mouse mammary gland. Oncogene. 2002;21:4900–4907. doi: 10.1038/sj.onc.1205634. [DOI] [PubMed] [Google Scholar]
- Lu P, Ewald A, Martin G, Werb Z. Essential function of FGF signaling pathway during branching morphogenesis of mammary gland (abstract #515) Dev Biol. 2005;283:680–681. [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger RJ, Krasnow MA. Genetic control of branching morphogenesis. Science. 1999;284:1635–1639. doi: 10.1126/science.284.5420.1635. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- Naylor MJ, Ormandy CJ. Mouse strain-specific patterns of mammary epithelial ductal side branching are elicited by stromal factors. Dev Dyn. 2002;225:100–105. doi: 10.1002/dvdy.10133. [DOI] [PubMed] [Google Scholar]
- Oakes SR, Hilton HN, Ormandy CJ. Key stages in mammary gland development - The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res. 2006;8:207. doi: 10.1186/bcr1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MMP, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Chiang C, Rubenstein JL. Coordinate regulation and synergistic actions of BMP4, SHH and FGF8 in the rostral prosencephalon regulate morphogenesis of the telencephalic and optic vesicles. Neuroscience. 2002;111:1–17. doi: 10.1016/s0306-4522(01)00616-9. [DOI] [PubMed] [Google Scholar]
- Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Young P, Emerman JT, Neve RM, Dairkee S, Cunha GR. A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology. 2002;143:4886–4896. doi: 10.1210/en.2002-220570. [DOI] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–1086. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Propper A, Gomot L. Control of chick epidermis differentiation by rabbit mammary mesenchyme. Experientia. 1973;29:1543–1544. doi: 10.1007/BF01943907. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Hirai Y, Bissell MJ. Delivering the message: epimorphin and mammary epithelial morphogenesis. Trends Cell Biol. 2003;13:426–434. doi: 10.1016/s0962-8924(03)00146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, Klotz DM, Walker MP, Diaugustine RP. Mammary gland branching morphogenesis is diminished in mice with a deficiency of insulin-like growth factor-I (IGF-I), but not in mice with a liver-specific deletion of IGF-I. Endocrinology. 2004;145:3106–3110. doi: 10.1210/en.2003-1112. [DOI] [PubMed] [Google Scholar]
- Ruan W, Monaco ME, Kleinberg DL. Progesterone stimulates mammary gland ductal morphogenesis by synergizing with and enhancing insulin-like growth factor-I action. Endocrinology. 2005;146:1170–1178. doi: 10.1210/en.2004-1360. [DOI] [PubMed] [Google Scholar]
- Runswick SK, O’Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. [DOI] [PubMed] [Google Scholar]
- Sakakura T, Nishizuka Y, Dawe CJ. Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science. 1976;194:1439–1441. doi: 10.1126/science.827022. [DOI] [PubMed] [Google Scholar]
- Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, Hom YK, Cunha GR, DiAugustine RP. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–785. [PubMed] [Google Scholar]
- Serra R, Pelton RW, Moses HL. TGF beta 1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development. 1994;120:2153–2161. doi: 10.1242/dev.120.8.2153. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Silberstein GB, Strickland P, Coleman S, Daniel CW. Epithelium-dependent extracellular matrix synthesis in transforming growth factor-beta 1-growth-inhibited mouse mammary gland. J Cell Biol. 1990;110:2209–2219. doi: 10.1083/jcb.110.6.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyal S, Ismail PM, Li J, Mulac-Jericevic B, Conneely OM, Lydon JP. Progesterone’s role in mammary gland development and tumorigenesis as disclosed by experimental mouse genetics. Breast Cancer Res. 2002;4:191–196. doi: 10.1186/bcr451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, Larsen M, Hoffman MP. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005;132:1223–1234. doi: 10.1242/dev.01690. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–3933. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metal-loproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol. 2002;243:128–136. doi: 10.1006/dbio.2001.0557. [DOI] [PubMed] [Google Scholar]
- Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson DC, Grindley JC, Thomson AA. Regulation of Fgf10 gene expression in the prostate: identification of transforming growth factor-beta1 and promoter elements. Endocrinology. 2004;145:1988–1995. doi: 10.1210/en.2003-0842. [DOI] [PubMed] [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Costantini F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev Biol. 2004;271:98–108. doi: 10.1016/j.ydbio.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Williams JM, Daniel CW. Mammary ductal elongation: differentiation of myoepithelium and basal lamina during branching morphogenesis. Dev Biol. 1983;97:274–290. doi: 10.1016/0012-1606(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Sternlicht MD, Lund LR, Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S, Werb Z. Site-specific inductive and inhibitory activities of MMP-2 and MMP-3 orchestrate mammary gland branching morphogenesis. J Cell Biol. 2003;162:1123–1133. doi: 10.1083/jcb.200302090. [DOI] [PMC free article] [PubMed] [Google Scholar]