Abstract
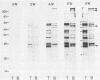
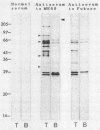
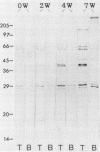
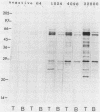
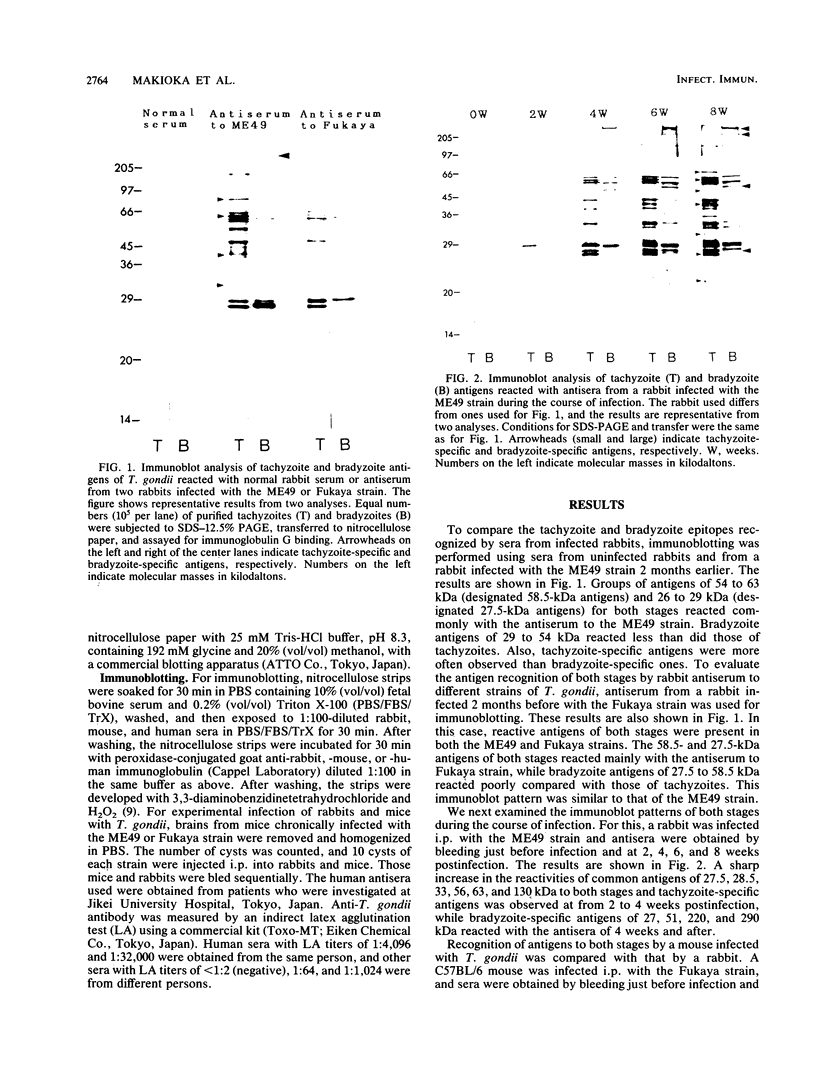
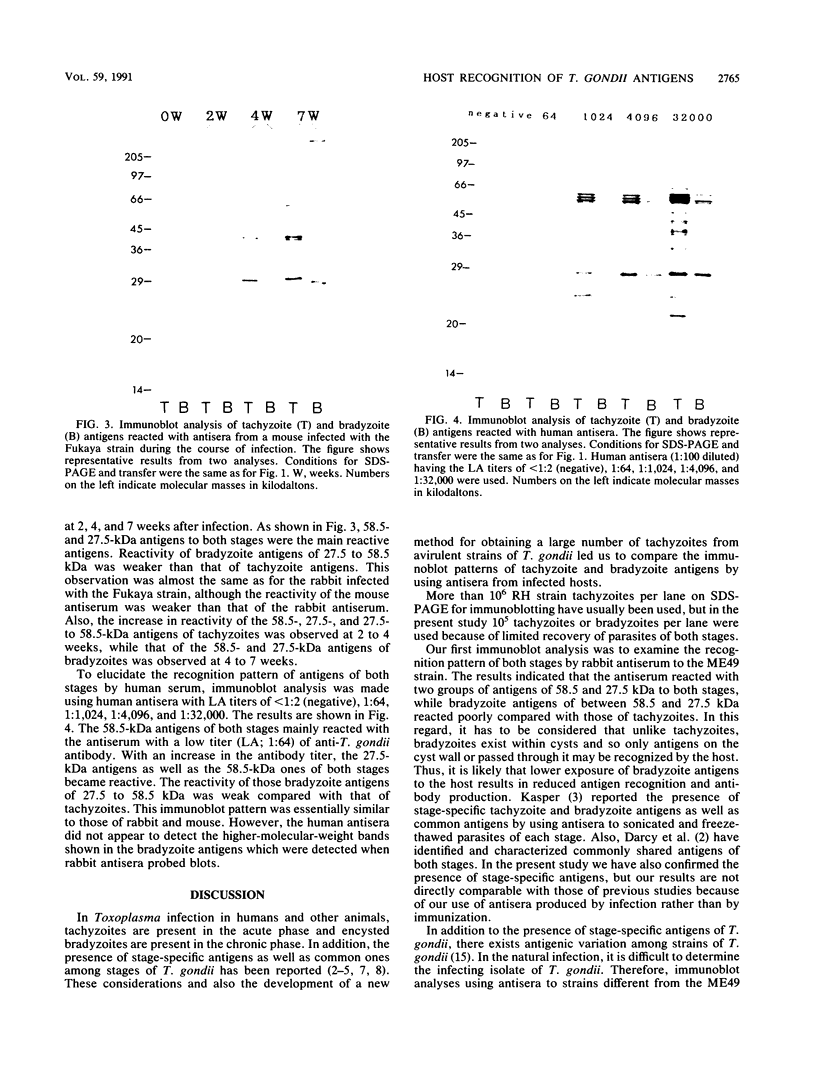
Western immunoblots of tachyzoite and bradyzoite antigens of Toxoplasma gondii were probed with antisera from rabbits and mice at intervals between 2 and 8 weeks after infection and with human antisera with various titers of antibody. With rabbit and mouse antisera, two groups of antigens with molecular masses of 54 to 63 kDa (designated 58.5-kDa antigens) and 26 to 29 kDa (designated 27.5-kDa antigens) were demonstrated commonly for both stages, while those antisera reacted strongly with tachyzoite (but not bradyzoite) antigens with molecular masses of 29 to 54 kDa. Tachyzoite antigens of 21.5, 26.5, 31, 38, 40, 49, and 58 kDa reacted with antisera 2 to 4 weeks after infection, while bradyzoite antigens of 27, 51, 220, and 290 kDa reacted with antisera obtained 4 or more weeks after infection. The 58.5-kDa antigens of both stages reacted primarily with human antisera that had low titers of anti-T. gondii antibodies. Human (as well as rabbit and mouse) sera with high antibody titers reacted with the 27.5-kDa antigens as well as the 58.5-kDa antigens, but the reactivity of the 27.5- to 58.5-kDa antigens of tachyzoites was greater than that of bradyzoites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornelissen A. W., Overdulve J. P., Hoenderboom J. M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981 Aug;83(Pt 1):103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- Darcy F., Charif H., Caron H., Deslée D., Pierce R. J., Cesbron-Delauw M. F., Decoster A., Capron A. Identification and biochemical characterization of antigens of tachyzoites and bradyzoites of Toxoplasma gondii with cross-reactive epitopes. Parasitol Res. 1990;76(6):473–478. doi: 10.1007/BF00931052. [DOI] [PubMed] [Google Scholar]
- Kasper L. H., Bradley M. S., Pfefferkorn E. R. Identification of stage-specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol. 1984 Jan;132(1):443–449. [PubMed] [Google Scholar]
- Kasper L. H. Identification of stage-specific antigens of Toxoplasma gondii. Infect Immun. 1989 Mar;57(3):668–672. doi: 10.1128/iai.57.3.668-672.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper L. H., Ware P. L. Recognition and characterization of stage-specific oocyst/sporozoite antigens of Toxoplasma gondii by human antisera. J Clin Invest. 1985 May;75(5):1570–1577. doi: 10.1172/JCI111862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunde M. N., Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol. 1983 Oct;69(5):806–808. [PubMed] [Google Scholar]
- Omata Y., Igarashi M., Ramos M. I., Nakabayashi T. Toxoplasma gondii: antigenic differences between endozoites and cystozoites defined by monoclonal antibodies. Parasitol Res. 1989;75(3):189–193. doi: 10.1007/BF00931274. [DOI] [PubMed] [Google Scholar]
- Partanen P., Turunen H. J., Paasivuo R. T., Leinikki P. O. Immunoblot analysis of Toxoplasma gondii antigens by human immunoglobulins G, M, and A antibodies at different stages of infection. J Clin Microbiol. 1984 Jul;20(1):133–135. doi: 10.1128/jcm.20.1.133-135.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen P., Turunen H. J., Paasivuo R., Forsblom E., Suni J., Leinikki P. O. Identification of antigenic components of Toxoplasma gondii by an immunoblotting technique. FEBS Lett. 1983 Jul 25;158(2):252–254. doi: 10.1016/0014-5793(83)80589-4. [DOI] [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Desmonts G., Remington J. S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986 Oct;154(4):650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Mullenax J., Araujo F. G., Erlich H. A., Remington J. S. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983 Aug;131(2):977–983. [PubMed] [Google Scholar]
- Suzuki Y., Remington J. S. A method for obtaining large numbers of trophozoites of avirulent strains of Toxoplasma gondii using an antibody to interferon-gamma. J Parasitol. 1989 Feb;75(1):174–176. [PubMed] [Google Scholar]
- Verhofstede C., Van Gelder P., Rabaey M. The infection-stage-related IgG response to Toxoplasma gondii studied by immunoblotting. Parasitol Res. 1988;74(6):516–520. doi: 10.1007/BF00531628. [DOI] [PubMed] [Google Scholar]
- Ware P. L., Kasper L. H. Strain-specific antigens of Toxoplasma gondii. Infect Immun. 1987 Mar;55(3):778–783. doi: 10.1128/iai.55.3.778-783.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]