Abstract
An improved mammalian two-hybrid system designed for interaction trap screening is described in this paper. CV-1/EBNA-1 monkey kidney epithelial cells expressing Epstein–Barr virus nuclear antigen 1 (EBNA-1) were stably transfected with a reporter plasmid for GAL4-dependent expression of the green fluorescent protein (GFP). A resulting clone, GB133, expressed GFP strongly when transfected transiently with transcriptional activators fused to GAL4 DNA-binding domain with minimal background GFP expression. GB133 cells maintained plasmids containing the OriP Epstein–Barr virus replication origin that directs replication of plasmids in mammalian cells in the presence of the EBNA-1 protein. GB133 cells transfected stably with a model bait expressed GFP when further transfected transiently with an expression plasmid for a known positive prey. When the bait-expressing GB133 cells were transfected transiently with an OriP-containing expression plasmid for the positive prey together with excess amounts of empty vector, cells that received the positive prey were readily identified by green fluorescence in cell culture and eventually formed green fluorescent microcolonies, because the prey plasmid was maintained by the EBNA-1/Ori-P system. The green fluorescent microcolonies were harvested directly from the culture dishes under a fluorescence microscope, and total DNA was then prepared. Prey-encoding cDNA was recovered by PCR using primers annealing to the vector sequences flanking the insert-cloning site. This system should be useful in mammalian cells for efficient screening of cDNA libraries by two-hybrid interaction.
Two-hybrid interaction screening is a promising approach to obtain cDNA clones that encode binding proteins to a known target protein (1–8). Conventional yeast two-hybrid screening has been proven powerful and convenient for this purpose. However, some interactions of mammalian proteins may not occur in the yeast milieu because of possible lack of associating factors, protein modifications (such as signal-induced phosphorylation), or correct protein folding. Therefore, it seemed desirable to develop a mammalian cell two-hybrid screening system to identify interacting proteins that are difficult to detect by the yeast system.
Although several attempts have been made to adapt the principle of two-hybrid screening to mammalian cells, the previously reported mammalian two-hybrid screening systems are relatively inefficient. For example, in the case of selection by interaction-dependent expression of drug-resistant genes (9), efficiency and specificity of selection depends on the features of the selecting drug as well as its concentration in the culture medium. It is therefore important to optimize the drug concentration empirically; too high a drug concentration may suppress background cell growth but, concomitantly, it may result in preferential isolation of strongly interacting preys that render stronger resistance to the selection drug. To date, the low efficiency of stable transfection of a prey cDNA library into mammalian cells has made screening very laborious. Repeated selections (to concentrate positive clones) by interaction-dependent expression of growth-promoting genes (10) or surface marker antigens by using a cell sorter (9) may be prone to preferential isolation of strong interactors, which would eventually dominate the culture. It therefore seemed critical to develop an efficient and convenient method for interaction screening using mammalian cells.
Interaction of the melanocyte-specific gene 1 (MSG1) transcriptional activator and the Smad4 signal transducer/DNA-binding protein in mammalian cells depends on signaling activated by transforming growth factor-β (11, 12). Using MSG1 and Smad4 as model interactors, we attempted to develop such a system. In this paper, we provide proof of principle of this approach to mammalian two-hybrid screening that should be useful for interaction screening.
Materials and Methods
Cells.
CV-1/EBNA-1 cells (13) were purchased from American Type Culture Collections and maintained in DMEM supplemented with 10% FCS. The characterized grade FCS we used in this study (purchased from HyClone) contained 3 to 10 pM of activated transforming growth factor-β, which was sufficient to support the MSG1–Smad4 interaction in mammalian cells (11).
Plasmids.
Expression plasmids for MSG1, MSG1 mutants, and GAL4 DNA-binding domain (GAL4DB) fusion proteins were described previously (11, 12, 14, 15). MSG1 mutants lacking the CR2 transactivating domain or the Smad interaction domain were described (11, 12). A 2.5-kb DNA fragment containing the OriP sequence was excised from pCEP4 (Invitrogen) and inserted to the unique NdeI site of the pM.CR2 plasmid that expresses GAL4DB fusion of the CR2 transcriptional activator domain derived from MSG1 (15). An expression vector pCMV.OriP, which harbors the OriP sequence, was constructed by removing the TK-Hyg (thymidine kinase–hygromycin) cassette (SalI-NruI, 1.9 kb) and the EBNA-1 expression cassette (ClaI-SacII, 2.6 kb) from pCEP4. An expression plasmid for MSG1 harboring the OriP sequence was constructed by inserting hemagglutinin-tagged human MSG1 cDNA (14) into the multiple cloning site of pCMV.OriP. pG5GFP, a GAL4-dependent reporter plasmid for expression of the green fluorescent protein (GFP), contained five repeats of GAL4 binding elements followed by the adenovirus E1b promoter/TATAA box and GFP cDNA; it was constructed by replacing the chloramphenicol acetyltransferase cDNA cassette of pG5CAT (CLONTECH) with a humanized GFP cDNA excised from pEGFP (CLONTECH). The pG5GFP-Hyg reporter plasmid was constructed by inserting a hygromycin resistance gene cassette excised from pCEP4 to pG5GFP. Detailed construction procedures and restriction maps of the plasmids described in this report are available on request.
Two-Hybrid Assays.
Transfection of mammalian cells with Lipofectamine reagent (GIBCO/BRL) and in situ cell staining for expression of β-galactosidase for evaluation of transfection efficiency were described (11). GB133 cells were generated by transfecting CV-1/EBNA-1 cells with linearized pG5GFP-Hyg reporter plasmid followed by selection with 500 μg/ml hygromycin-B (Sigma) for 2 weeks. GB133-DB Smad4 C-terminal domain cells were generated by transfecting GB133 cells with an expression plasmid for the GAL4DB-fusion Smad4 C-terminal domain (amino acids 302–552) (11) constructed on the pcDNA3 vector (Invitrogen), followed by selection with G418 at 1 mg/ml for 2 weeks. Expression of GFP in living mammalian cells was evaluated with an inverted phase-contrast microscope (Nikon Diaphot) equipped with an epifluorescence light source and a fluorescein isothiocyanate (FITC) filter set (Nikon).
PCR.
Total DNA was prepared from GFP-positive microcolonies by using a micro DNA extraction kit from Stratagene. The prey cDNA inserted in the multiple cloning site of pCMV.OriP was amplified from 0.1–1 μg of total DNA template using primers (sense, 5′-GCGTGTACGGTGGGAGGTCT; antisense, 5′-TCACTGCATTCTAGTTGTGGTTTGT) that annealed to the vector sequences flanking the multiple cloning sites. PCR was performed with Advantage-2 polymerase (CLONTECH) with 35 cycles of 95°C for 30 sec, 52.6°C for 30 sec, and 68°C for 90 sec.
Results and Discussion
A GFP-Reporter Mammalian Cell Two-Hybrid System with Extrachromosomal Maintenance of Prey Expression Plasmids.
The Smad4 DNA-binding protein interacts with the MSG1 transcriptional activator in mammalian cells in a manner dependent on transforming growth factor-β signaling (11, 12). Although GAL4DB-fusion Smad4 (bait) and MSG1 (prey) interact in mammalian cells, this bait–prey pair does not interact in yeast, which lacks the transforming growth factor-β signaling pathway (data not shown). Using this bait–prey pair as model interactors, we attempted to develop an efficient mammalian two-hybrid system with the aim of applying it to interaction screening.
We chose as host mammalian cells CV-1/EBNA-1 monkey kidney epithelial cells that express the EBNA-1 (13). This cell line has been used successfully to isolate a cell surface receptor of interleukin-1 by transfection with an expression cDNA library followed by screening with a radiolabeled interleukin-1 probe (13). In the presence of the EBNA-1 antigen, plasmids harboring the Epstein–Barr virus DNA replication origin sequence (the OriP sequence) are maintained stably and extrachromosomally at relatively low copy numbers (16). Thus, transient transfection of CV-1/EBNA-1 cells with OriP-containing prey expression plasmids will efficiently generate cells that stably harbor prey expression plasmids.
To obtain CV-1/EBNA-1 cell variants that express GFP in response to two-hybrid interaction without cotransfecting reporter plasmids, we transfected them with a linearized form of pG5GFP-Hyg reporter plasmid, which expresses the GFP mRNA transcript under the control of five copies of GAL4 binding elements, and selected them with hygromycin. Among the resulting stable transfectants, the GB133 clone was chosen for further characterization because it strongly expressed GFP when transfected with GAL4DB-fusion transactivators, whereas its background GFP expression was scarcely detectable.
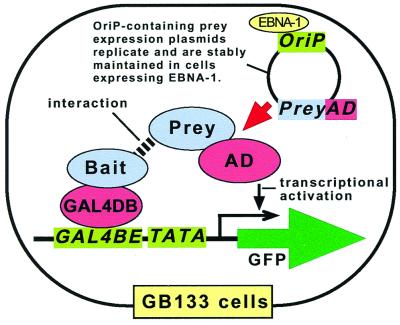
GB133 cells were further transfected stably with an expression plasmid for the model bait (GAL4DB-Smad4) cloned in a NeoR-expressing vector. Cells were selected by using G418 and then transfected transiently with an expression plasmid for the model prey (MSG1) harboring the OriP sequence. When the bait and prey interacted, the GFP gene was activated following the two-hybrid principle. Because the prey expression plasmid (containing the OriP sequence) was maintained in the cells (which expressed EBNA-1), the interaction-positive cells expressed GFP continuously, eventually forming green fluorescent colonies that were readily detectable in, and collectable from, cell culture dishes by fluorescence microscopy of live cells. The prey cDNA insert was recovered from total DNA preparations of the GFP-expressing colonies by PCR using primers annealing to the vector sequences flanking the insert-cloning site. The overall design of the GB133-based mammalian two-hybrid system described above is depicted in Fig. 1.
Figure 1.
Diagram of the GFP-reporter mammalian two-hybrid system with extrachromosomal maintenance of prey expression plasmid. GB133 cells, which express the EBNA-1 protein, harbor a GAL4-dependent GFP reporter plasmid in their chromosomes. For interaction screening, GB133 cells can be stably transfected with an expression plasmid for a GAL4 DNA-binding domain (GAL4DB) fusion protein of bait. Cells can then be transiently transfected with a cDNA library expressing fusion proteins of a transcriptional activating domain (AD) and preys. The two-hybrid interaction will induce transcription of the GFP gene, which is readily detectable by fluorescent microscopy of living cells. Once introduced into GB133 cells by transient transfection protocols, the prey-expressing plasmids harboring the OriP replication origin sequence will be maintained stably because of the presence of the EBNA-1 protein, thus keeping the positive prey-expressing cells permanently green fluorescent. GAL4BE, GAL4 binding element; TATA, TATAA box from adenovirus E1b gene promoter.
Two-Hybrid Regulated GFP Expression in GB133 Cells.
GB133 cells were transfected with an expression plasmid for β-galactosidase to evaluate transfection efficiency. As shown in Fig. 2A, at least 20% of GB133 cells were transfected transiently by a standard lipofection protocol using the Lipofectamine reagent when evaluated by in situ cell staining for β-galactosidase expression.
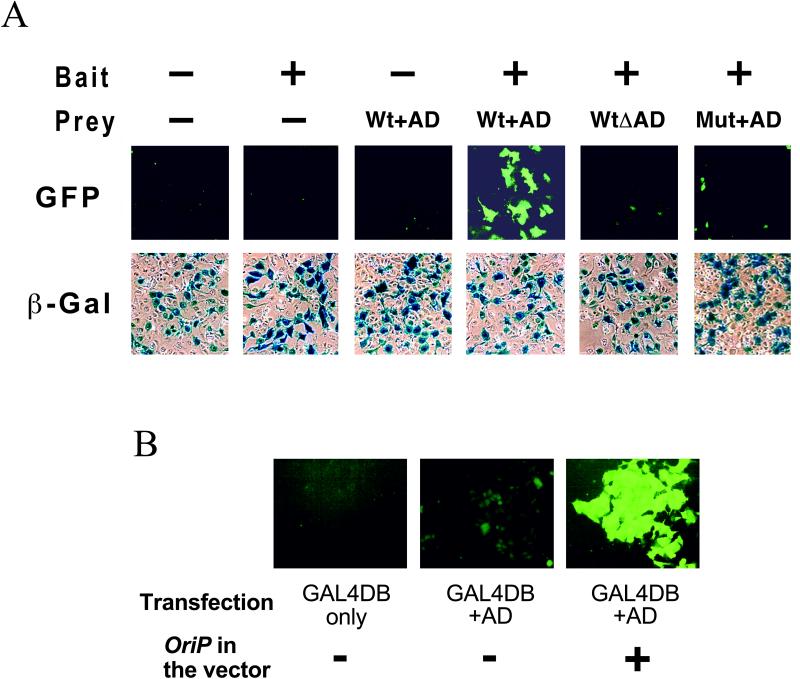
Figure 2.
GFP expression in GB133 cells. (A) Two-hybrid dependent expression of GFP in GB133 cells. Cells were transiently transfected with expression plasmids for a model bait (GAL4DB-fusion Smad4) and prey (MSG1) together with a β-galactosidase (β-Gal) reporter plasmid. Expression of GFP or β-galactosidase was evaluated 48 h after transfection. Wt + AD, wild-type prey with the transactivating domain; WtΔAD, wild-type prey lacking the transactivating domain; Mut + AD, prey mutant with the transactivating domain that does not interact with the bait. (B) GB133 cells maintain OriP-containing plasmids. Cells were transfected transiently with expression plasmids for GAL4DB or a GAL4DB-fusion CR2 transactivating domain (AD) with or without harboring the OriP sequence. GFP expression was evaluated 7 days after transfection, when cells formed confluent monolayers (phase contrast images are not shown).
To evaluate the efficiency and specificity of GB133 cells to detect protein–protein interaction, they were transfected transiently with the model bait (GAL4DB-fusion Smad4) and prey (MSG1), followed by observation by cell fluorescent microscopy of live cells 48 h after transfection. As shown in Fig. 2A, GB133 cells showed strong green fluorescence only when they were transfected with the interacting bait–prey pair. No significant fluorescence was observed when transfected by bait only or prey only. The GFP expression by the two-hybrid interaction depended on the transactivating domain of the prey, because the model prey lacking the transactivating domain (Fig. 2A, WtΔAD) did not induce green fluorescence, even in the presence of the prey. Furthermore, a minimum deletion mutation (30 aa) of the model prey that abolishes interaction with the prey (Mut + AD) also did not induce green fluorescence, even in the presence of the prey. Thus, the GFP reporter gene integrated in the chromosomal DNA of GB133 cells correctly detected the protein–protein interaction between the bait (GAL4DB fusion proteins) and the prey (with a transcriptional activator domain).
GB133 Cells Maintain OriP-Containing Plasmids.
To evaluate whether GB133 cells can maintain OriP-containing plasmids stably and effectively, we transfected them transiently with expression plasmids for a GAL4DB-fusion transcriptional activating domain (from MSG1 prey) harboring or not harboring the OriP sequence. When the expression plasmid lacked the OriP sequence, GFP expression by the transfected cells was diminished to the background level within 7 days after transfection (Fig. 2B). On the other hand, when the OriP sequence was present, cells continued to express GFP strongly, eventually forming green fluorescent colonies on culture dishes within 7 days of transfection. These results indicated that GB133 cells maintain OriP-containing plasmids stably and effectively without significantly damaging the cells by overreplication of the extrachromosomal plasmids.
Recovery of Positive Prey cDNA Inserts from Green Fluorescent GB133 Cells.
We then evaluated the possibility of applying the GB133 cell-based mammalian two-hybrid system to interaction screening. To generate GB133 cells stably expressing the bait, cells were transfected with an expression plasmid for the model bait (GAL4DB-Smad4), which harbored the NeoR gene in its vector backbone, and stable clones were selected by G418. Expression of the bait protein was evaluated by anti-GAL4 Western blotting (data not shown), and a clone expressing the bait protein most strongly was chosen for further characterization.
To evaluate two-hybrid-dependent expression of GFP in the bait-expressing GB133 cells, cells were transfected transiently with an expression plasmid for the prey (MSG1). As shown in Fig. 3B and C, prey-transfected cells expressed GFP, whereas cells transfected with control vector showed no significant fluorescence (Fig. 3A). Importantly, even a single cell weakly expressing GFP was readily identified by living cell fluorescence microscopy (Fig. 3C, arrowhead).
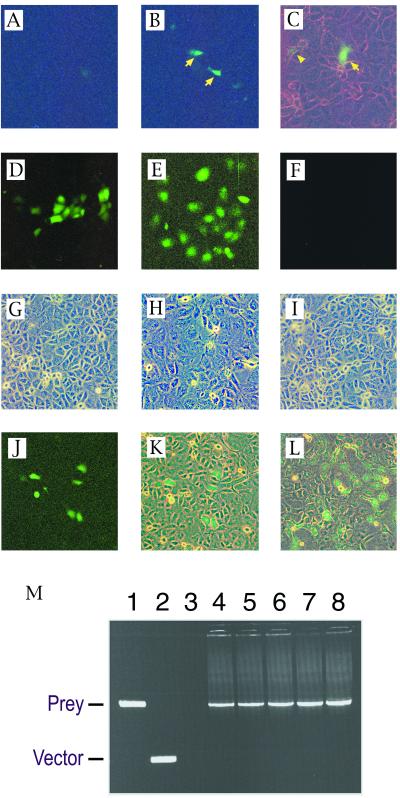
Figure 3.
Recovery of a model prey (MSG1) plasmid from GB133 cells stably expressing a model bait (GAL4DB-Smad4). (A–C) Cells were transiently transfected with a prey (B and C) or empty vector (A), followed by evaluation of GFP expression by fluorescence microscopy at 48 h after transfection (fluorescent light only in A and B; fluorescent light plus weak white light in C). Arrows indicate strongly green fluorescent cells; arrowhead indicates a weakly green fluorescent cell. (D–I) Maintenance of OriP-harboring plasmid in bait-expressing GB133 cells. Expression plasmids for a GAL4DB-fusion transactivating domain with (E) or without (D and F) harboring the OriP replication origin sequence were transiently transfected into the bait-expressing GB133 cells, and expression of GFP was evaluated at 48 h (D) or at 7 days (E and F) after transfection. (G–I) Phase contrast images of cell monolayers corresponding to fluorescence images of D–F are shown in panels G–I, respectively. (J and K) Detection of prey diluted with an excess of empty vectors. Cells were transiently transfected with an OriP-containing prey expression plasmid together with a 2,000-fold molar excess of empty vector, followed by evaluation of GFP expression at 7 days after transfection. Single prey-transfected cells divided three times after transfection, forming green fluorescent cell clusters, each of which consisted of six to eight GFP-expressing cells (fluorescent light only in J; fluorescent light plus weak white light in K). (L) A green fluorescent microcolony of prey-transfected cells 3 days after subculture of the GFP-expressing cell clusters shown in J (fluorescent light plus weak white light). (M) Recovery of prey cDNA from green fluorescent microcolonies by PCR using primers that annealed to the vector sequences flanking the insert-cloning site. Agarose gel electrophoresis of PCR products is shown. Lane 1, positive control amplification from the prey plasmid; lane 2, negative control amplification from empty vector; lanes 3–8, amplification of prey cDNA from total DNA preparations of five independent GFP-expressing microcolonies.
To confirm that the bait-expressing GB133 cells maintain the OriP-containing plasmid, cells were transfected transiently with expression plasmids for GAL4DB-fusion transactivating domain (from MSG1 prey) harboring or not harboring the OriP sequence (Fig. 3 D–I). The bait-expressing GB133 cells maintained the OriP-containing expression plasmid and continued to express GFP strongly for at least 7 days after transfection (Fig. 3E). On the other hand, GFP expression from the expression plasmid without the OriP sequence was strongly diminished within 2–3 days of transfection (Fig. 3F). These results indicate that the bait-expressing GB133 cells maintained OriP-harboring plasmids by extrachromosomal replication in the presence of the EBNA-1 protein.
The bait-expressing GB133 cells then were transfected transiently with a mixture of an OriP-containing expression plasmid for a model positive prey (MSG1) and an empty vector with the prey/vector ratio of 1/2,000. When subconfluent cells on 10-cm dishes were transfected with this plasmid mixture, 48 h later we observed about five green fluorescent cell clusters per dish, each of which consisted of five to eight GFP-expressing cells (Fig. 3 J and K). Each cell cluster apparently was derived from a single prey-transfected cell that had two to three rounds of cell division after transfection and that migrated in the vicinity until the cells formed confluent monolayers. To facilitate the growth of the GFP-positive cells, adjacent cells on culture dishes were removed by using a cell scraper under the fluorescence microscope, and green fluorescent microcolonies were obtained 1 week later (Fig. 3L). GFP-positive cells then were recovered directly from culture dishes manually under a fluorescence microscope, and total DNA was isolated by a DNA microextraction protocol. The total cellular DNA was then subjected to amplification of the prey cDNA insert by PCR using primers that annealed to the vector sequence flanking the cDNA insertion site. From five independent DNA preparations obtained from GFP-expressing microcolonies, we recovered the prey cDNA insert with 100% success (Fig. 3M). These results demonstrate that the prey cDNA sequences are easily recovered from GFP-expressing cells that are readily identifiable by fluorescence microscopy.
The observed efficiency of this system in detecting the positive prey clones, which was five clones per 10-cm dish for 1/2,000 diluted prey plasmid, corresponded to an expected detection of a single positive clone after transfecting 100 dishes of cells when the positive prey was diluted with empty vector by 1/1 × 106. The efficiency of this system thus seems acceptable for application to interaction screening, provided the screening conditions are optimal. Although we have not tested the system with real cDNA expression libraries, the present results provide strong support for the principle of the GB133-based mammalian two-hybrid system.
Once GB133 cells stably expressing GAL4DB-fusion baits are generated, the whole procedure described above, from transfection to recovery of positive prey cDNA sequences, should be fast, taking less than 2 weeks for the small-scale demonstration using the model bait and prey. This system should also be useful for quick verification of candidate clones isolated by yeast two-hybrid screening.
In summary, we have demonstrated a mammalian two-hybrid approach that should be applicable to interaction screening. The two-hybrid interaction was detected by expression of the GFP reporter gene integrated stably into the chromosome of a newly generated cell line. Prey expression plasmids were maintained stably in this cell line after transfection by the OriP/EBNA-1 extrachromosomal plasmid replication system, allowing formation of green fluorescent colonies of cells transfected with positive preys. The prey cDNA sequence was readily isolated by PCR of microextracted DNA from the green fluorescent colonies by using vector-annealing primers. This approach should prove to be an efficient method to perform mammalian two-hybrid assays for the purpose of interaction screening.
Acknowledgments
This work was supported by the Alexander and Margaret Stewart Trust Fund to T.S.
Abbreviations
- EBNA-1
Epstein–Barr virus nuclear antigen 1
- GFP
green fluorescent protein
- MSG1
melanocyte-specific gene 1
- GAL4DB
GAL4 DNA-binding domain
References
- 1.Allen J, Walberg M, Edwards M, Elledge S. Trends Biochem Sci. 1995;20:511–516. doi: 10.1016/s0968-0004(00)89119-7. [DOI] [PubMed] [Google Scholar]
- 2.Brachmann R, Boeke J. Curr Opin Biotechnol. 1997;8:561–568. doi: 10.1016/s0958-1669(97)80029-8. [DOI] [PubMed] [Google Scholar]
- 3.Brent R, Finley R J. Annu Rev Genet. 1997;31:663–704. doi: 10.1146/annurev.genet.31.1.663. [DOI] [PubMed] [Google Scholar]
- 4.Young K. Biol Reprod. 1998;58:302–311. doi: 10.1095/biolreprod58.2.302. [DOI] [PubMed] [Google Scholar]
- 5.Frederickson R. Curr Opin Biotechnol. 1998;9:90–96. doi: 10.1016/s0958-1669(98)80090-6. [DOI] [PubMed] [Google Scholar]
- 6.Colas P, Brent R. Trends Biotechnol. 1998;16:355–363. doi: 10.1016/s0167-7799(98)01225-6. [DOI] [PubMed] [Google Scholar]
- 7.Vidal M, Legrain P. Nucleic Acids Res. 1999;27:919–929. doi: 10.1093/nar/27.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dress B. Curr Opin Chem Biol. 1999;3:64–70. doi: 10.1016/s1367-5931(99)80012-x. [DOI] [PubMed] [Google Scholar]
- 9.Fearon E, Finkel T, Gillson M, Kennedy S, Casella J, Tomaselli G, Morrow J, Dang C. Proc Natl Acad Sci USA. 1992;89:7958–7962. doi: 10.1073/pnas.89.17.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasavada H, Ganguly S, Germino F, Wang Z, Weissman S. Proc Natl Acad Sci USA. 1991;88:10686–10690. doi: 10.1073/pnas.88.23.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shioda T, Lechleider R J, Dunwoodie S L, Li H, Yahata T, de Caestecker M P, Fenner M H, Roberts A B, Isselbacher K J. Proc Natl Acad Sci USA. 1998;95:9785–9790. doi: 10.1073/pnas.95.17.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yahata T, de Caestecker M, Lechleider R, Andriole S, Roberts A, Isselbacher K, Shioda T. J Biol Chem. 2000;275:8825–8834. doi: 10.1074/jbc.275.12.8825. [DOI] [PubMed] [Google Scholar]
- 13.McMahan C, Slack J, Mosley B, Cosman D, Lupton S, Bruton L, Grubin C, Wignall J, Jenkins N, Brannan C, et al. EMBO J. 1991;10:2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shioda T, Fenner M H, Isselbacher K J. Proc Natl Acad Sci USA. 1996;93:12298–12303. doi: 10.1073/pnas.93.22.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shioda T, Fenner M H, Isselbacher K J. Gene. 1997;204:235–241. doi: 10.1016/s0378-1119(97)00551-9. [DOI] [PubMed] [Google Scholar]
- 16.Chittenden T, Lupton S, Levine A. J Virol. 1989;63:3016–3025. doi: 10.1128/jvi.63.7.3016-3025.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]