Abstract
The lipid component present in high-molecular-mass fractions with molecular masses of greater than 200 kDa derived from Mycobacterium tuberculosis extracts passaged through Sephacryl S.200 columns activate CD8+ lymphocytes to suppress lymphocyte blastogenesis. Suppression is mediated by the release of suppressor molecules by these CD8+ lymphocytes. Release of suppressor molecules occurs as early as 2 h following pulsing with the high-molecular-mass mycobacterial components and is maximal at 24 h, after which their release declines rapidly. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western immunoblotting indicates that the active components are carbohydrate moieties with approximate molecular masses of 122 to 148 kDa. Our results suggest a mechanism of interaction between mycobacteria and host mononuclear cells such that mycobacterial lipids, once exposed, activate CD8+ suppressor lymphocytes. Activation of these lymphocytes results in the release of carbohydrate-containing molecules that ultimately inhibit the blastogenesis of other lymphocytes.
Full text
PDF


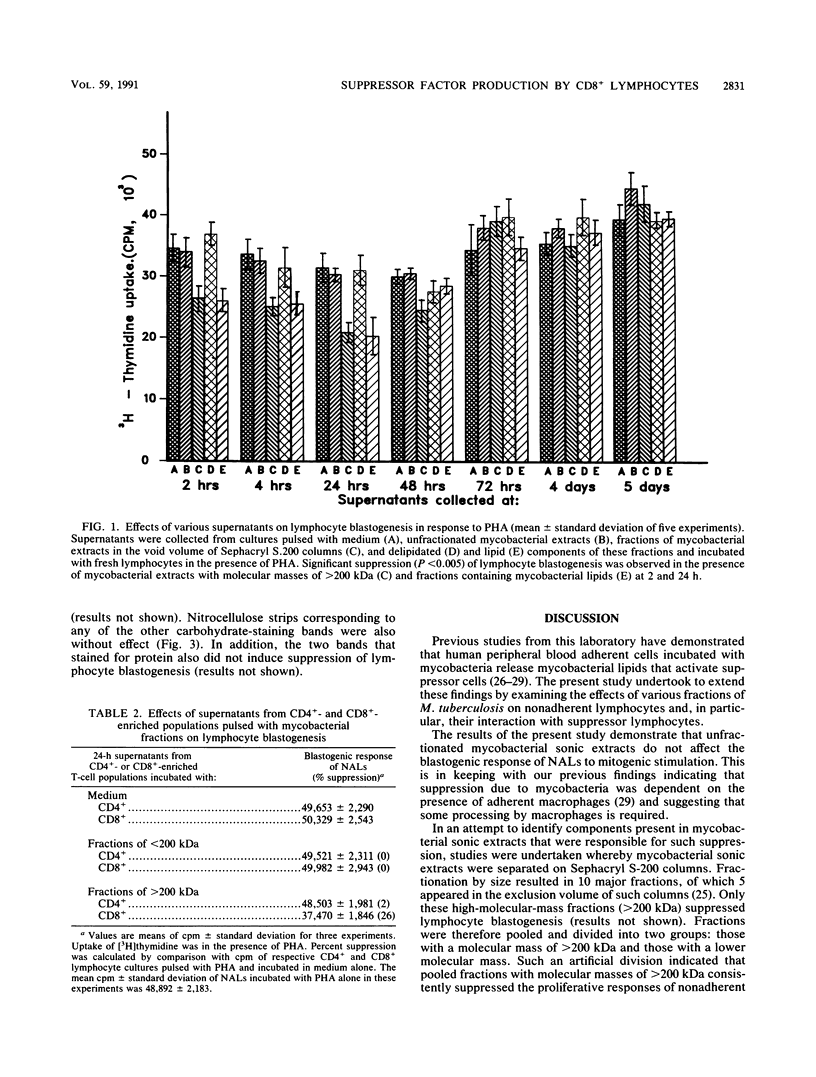
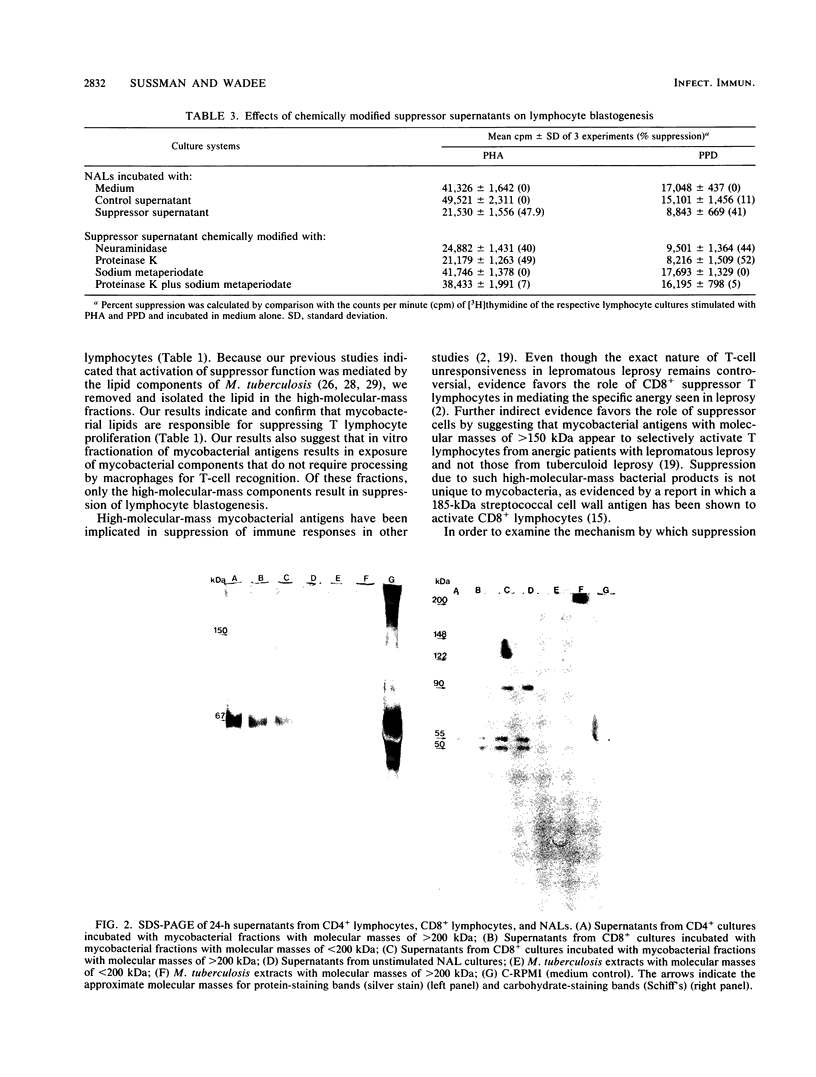
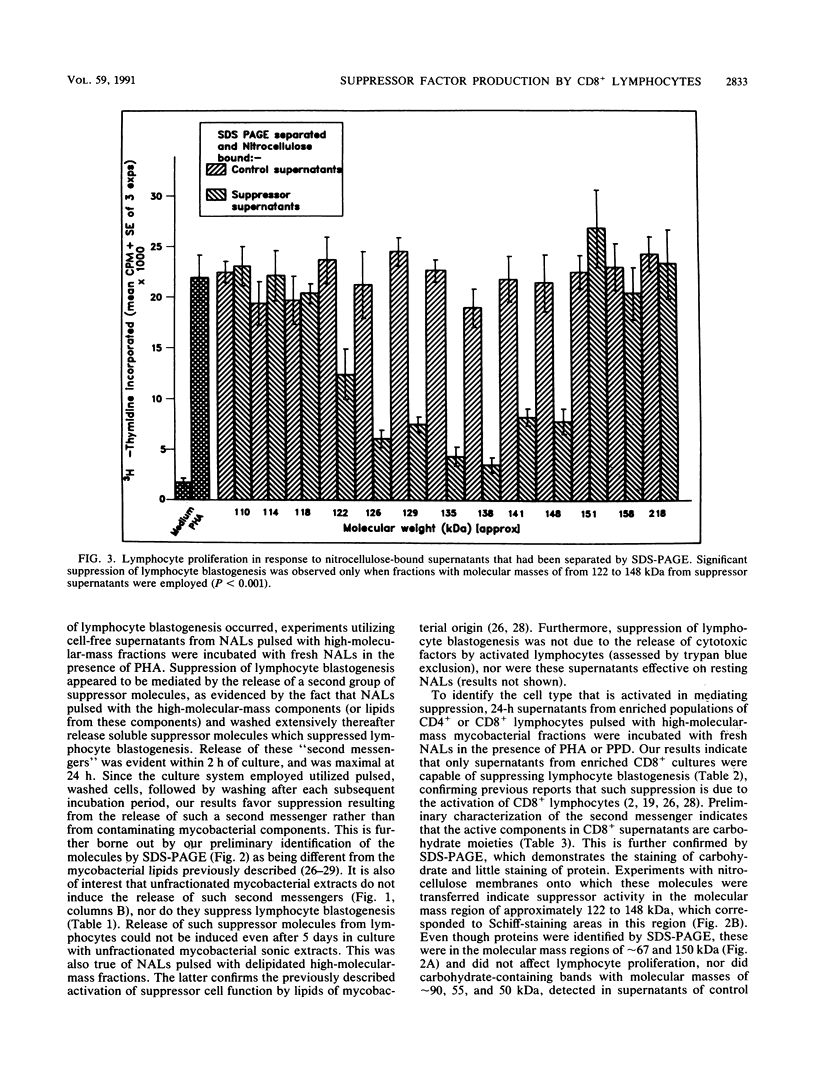


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Ferluga J., Garreau F., Malkovsky M., Asherson G. L. Suppressor cells induced by BCG release non-specific factors in vitro which inhibit DNA synthesis and interleukin-2 production. Immunology. 1984 Jan;51(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Deal H., Steele J. K., Stammers A. T., Singhai R., Levy J. G. Preadministration of a T-suppressor factor enhances tumor immunity in DBA/2 mice. Cancer Immunol Immunother. 1989;28(3):193–198. doi: 10.1007/BF00204988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie J. J., Adams E., Mullins R. J., Basten A. Inhibition of human lymphoproliferative responses by mycobacterial phenolic glycolipids. Infect Immun. 1989 Nov;57(11):3653–3659. doi: 10.1128/iai.57.11.3653-3659.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene W. C., Fleisher T. A., Waldmann T. A. Soluble suppressor supernatants elaborated by concanavalin A-activated human mononuclear cells. I. Characterization of a soluble suppressor T cell proliferation. J Immunol. 1981 Mar;126(3):1185–1191. [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Suppression of LIF production but not blastogenesis in patients with tuberculous meningitis. Clin Immunol Immunopathol. 1981 Feb;18(2):245–253. doi: 10.1016/0090-1229(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Klimpel G. R., Henney C. S. BCG-induced suppressor cells. I. Demonstration of a macrophage-like suppressor cell that inhibits cytotoxic T cell generation in vitro. J Immunol. 1978 Feb;120(2):563–569. [PubMed] [Google Scholar]
- Lehner T., Mehlert A., Avery J., Jones T., Caldwell J. The helper and suppressor functions of primate T cells elicited by a 185K streptococcal antigen, as compared with the helper function elicited by a 4K streptococcal antigen. J Immunol. 1985 Aug;135(2):1437–1442. [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Muchmore A. V., Shifrin S., Decker J. M. In vitro evidence that carbohydrate moieties derived from uromodulin, an 85,000 dalton immunosuppressive glycoprotein isolated from human pregnancy urine, are immunosuppressive in the absence of intact protein. J Immunol. 1987 Apr 15;138(8):2547–2553. [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Owhashi M., Horii Y., Ishii A. Eosinophil chemotactic factor in schistosome eggs: a comparative study of eosinophil chemotactic factors in the eggs of Schistosoma japonicum and S. mansoni in vitro. Am J Trop Med Hyg. 1983 Mar;32(2):359–366. doi: 10.4269/ajtmh.1983.32.359. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Mishra R. S., Nath I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J Exp Med. 1987 Jan 1;165(1):239–244. doi: 10.1084/jem.165.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokura Y., Miyachi Y., Takigawa M., Yamada M. Ultraviolet-induced suppressor T cells and factor(s) in murine contact photosensitivity. I. Biological and immunochemical characterization of factor(s) extracted from suppressor T cells. Cell Immunol. 1987 Dec;110(2):305–320. doi: 10.1016/0008-8749(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Evidence for two distinct populations of suppressor cells in the spleens of Mycobacterium bovis BCG-Sensitized mice. Infect Immun. 1981 Nov;34(2):315–322. doi: 10.1128/iai.34.2.315-322.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Cohen J. D., Rabson A. R. A 180-kilodalton protein from Mycobacterium tuberculosis defined by a human T cell clone. J Clin Lab Immunol. 1989 May;29(1):25–32. [PubMed] [Google Scholar]
- Wadee A. A., Mendelsohn D., Rabson A. R. Characterization of a suppressor cell-activating factor (SCAF) released by adherent cells treated with M. tuberculosis. J Immunol. 1983 May;130(5):2266–2270. [PubMed] [Google Scholar]
- Wadee A. A., Rabson A. R. Binding of phosphatidylethanolamine and phosphatidylinositol to OKT8+ lymphocytes activates suppressor cell activity. J Immunol. 1983 May;130(5):2271–2276. [PubMed] [Google Scholar]
- Wadee A. A., Rabson A. R. Production of a suppressor factor by adherent cells from Mycobacterium tuberculosis-infected guinea-pigs. Clin Exp Immunol. 1981 Aug;45(2):427–432. [PMC free article] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]
- Yee G. K., Levy J. G., Kripke M. L., Ullrich S. E. The role of suppressor factors in the regulation of immune responses by ultraviolet radiation-induced suppressor T lymphocytes. III. Isolation of a suppressor factor with the B16G monoclonal antibody. Cell Immunol. 1990 Apr 1;126(2):255–267. doi: 10.1016/0008-8749(90)90319-m. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]