Abstract
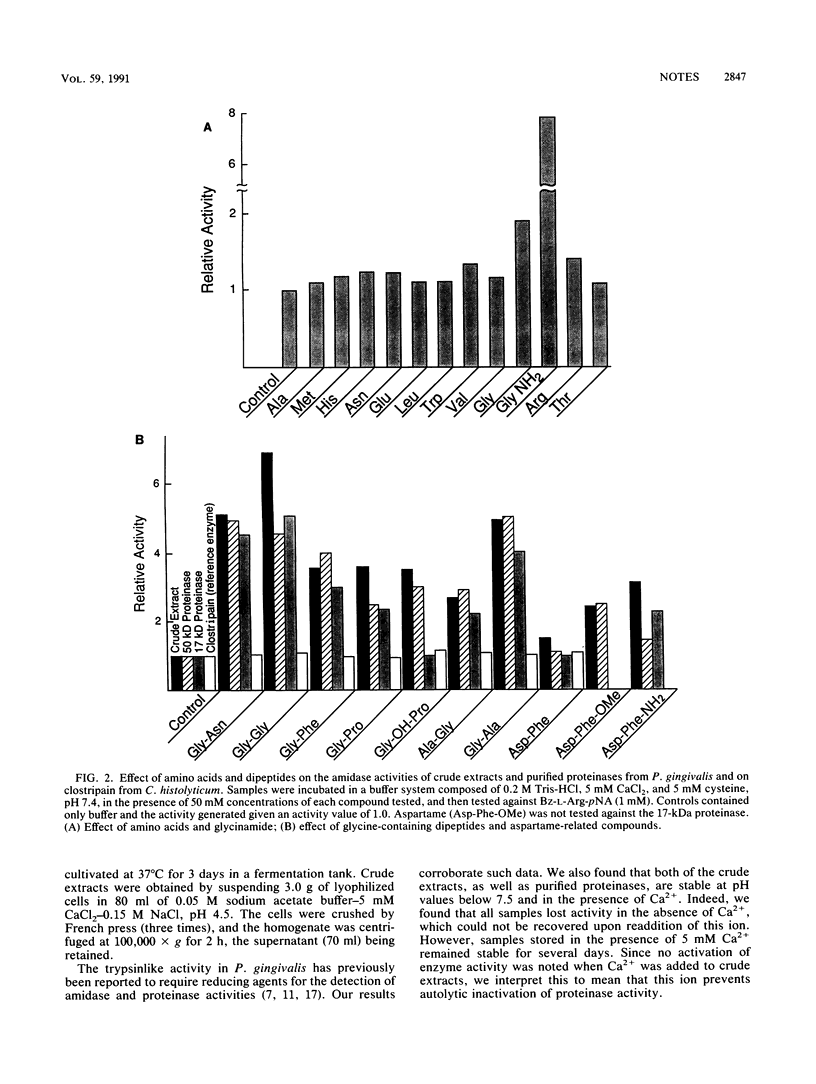
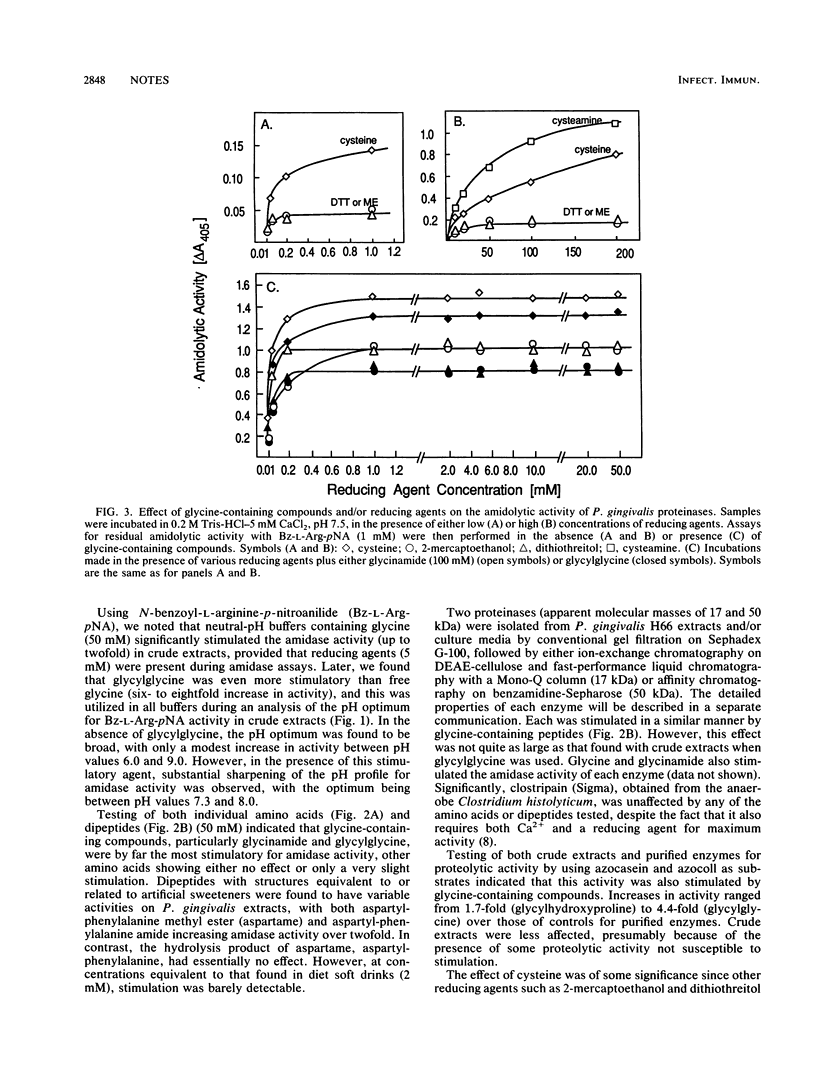
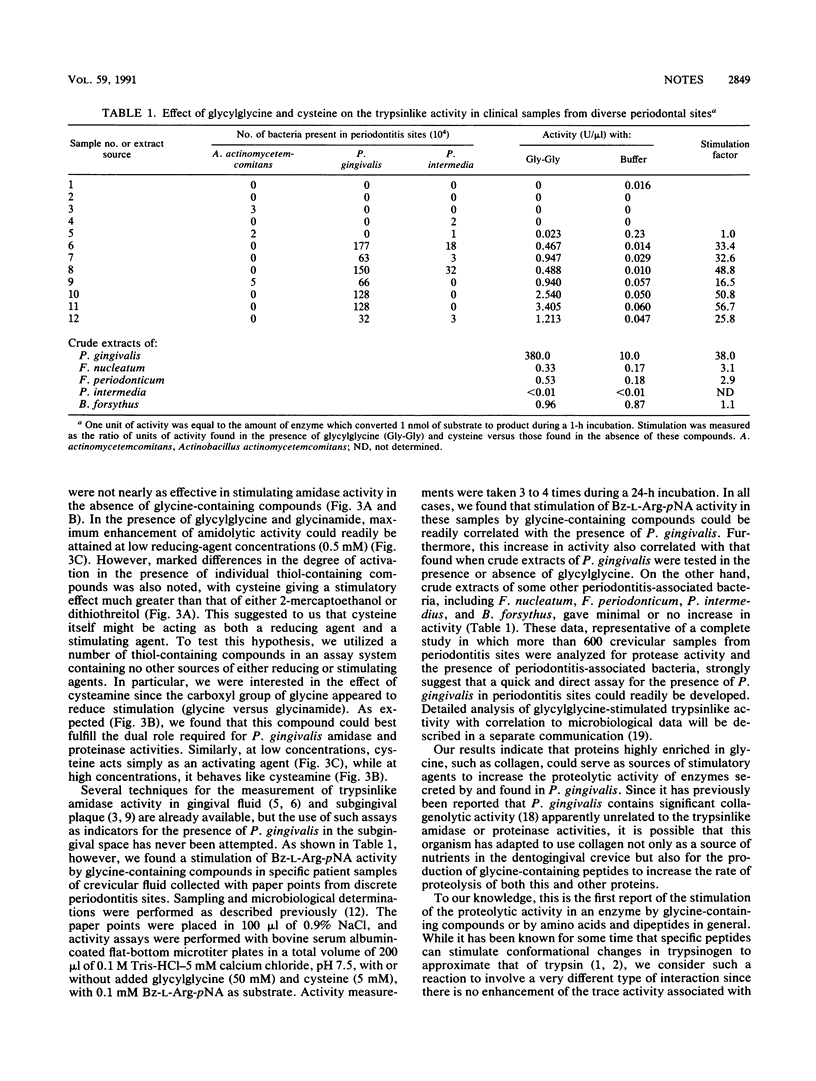
Proteolytic enzymes from the organism Porphyromonas gingivalis are believed to be involved in the development of periodontitis. Studies on both crude extracts and purified trypsinlike enzymes from this organism indicate that substantial stimulation of both amidase and proteinase activities can be obtained during incubation with glycine-containing compounds. We postulate that P. gingivalis may have developed this unusual property to take advantage of the glycine-rich environment which occurs during the periodontitis-associated degradation of gingival collagen. The finding of such a stimulation in crevicular fluids from discrete periodontal sites has been correlated with the presence of P. gingivalis and could be utilized for the early detection of infection by this organism during the onset of periodontitis.
Full text
PDF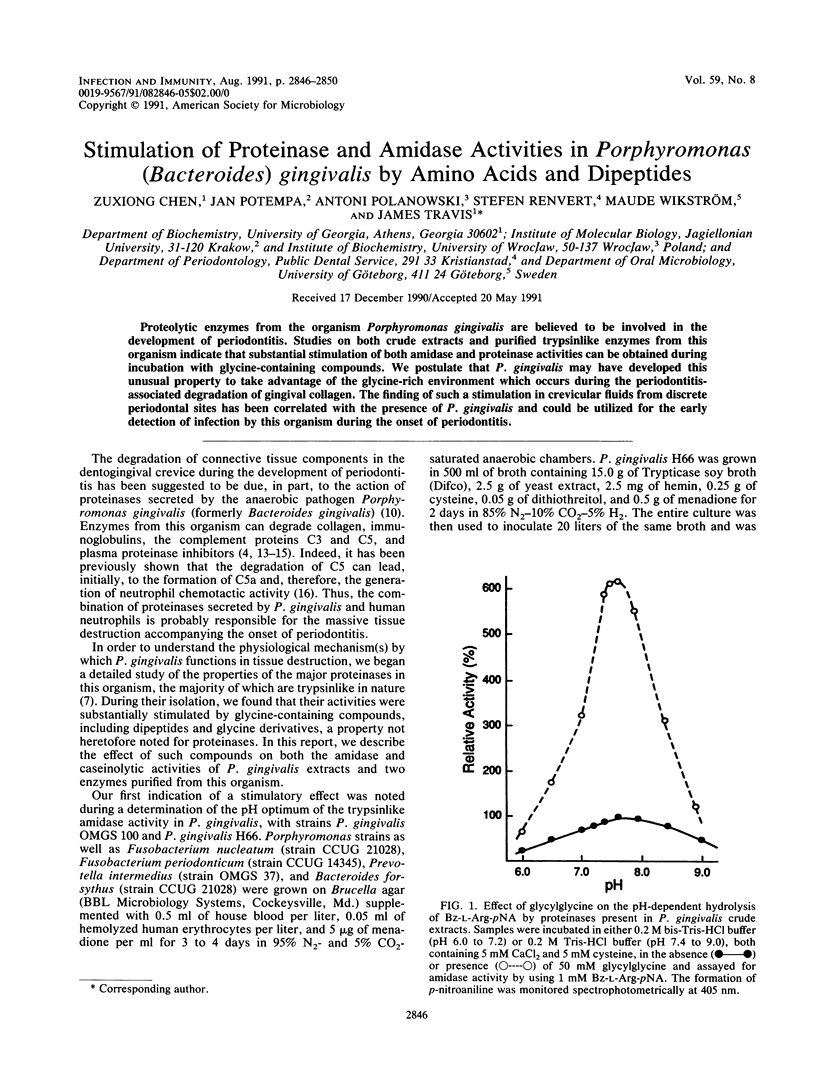

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode W., Huber R. Induction of the bovine trypsinogen-trypsin transition by peptides sequentially similar to the N-terminus of trypsin. FEBS Lett. 1976 Oct 1;68(2):231–236. doi: 10.1016/0014-5793(76)80443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W. The transition of bovine trypsinogen to a trypsin-like state upon strong ligand binding. II. The binding of the pancreatic trypsin inhibitor and of isoleucine-valine and of sequentially related peptides to trypsinogen and to p-guanidinobenzoate-trypsinogen. J Mol Biol. 1979 Feb 5;127(4):357–374. doi: 10.1016/0022-2836(79)90227-4. [DOI] [PubMed] [Google Scholar]
- Bretz W. A., Loesche W. J. Characteristics of trypsin-like activity in subgingival plaque samples. J Dent Res. 1987 Nov;66(11):1668–1672. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Herrmann B. F., Höfling J. F., Sundqvist G. K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984 Feb;43(2):644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S. W., Eley B. M. Detection of cathepsin B- and L-, elastase-, tryptase-, trypsin-, and dipeptidyl peptidase IV-like activities in crevicular fluid from gingivitis and periodontitis patients with peptidyl derivatives of 7-amino-4-trifluoromethyl coumarin. J Periodontal Res. 1989 Nov;24(6):353–361. doi: 10.1111/j.1600-0765.1989.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Cox S. W., Eley B. M. Tryptase-like activity in crevicular fluid from gingivitis and periodontitis patients. J Periodontal Res. 1989 Jan;24(1):41–44. doi: 10.1111/j.1600-0765.1989.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles A. M., Imhoff J. M., Keil B. alpha-Clostripain. Chemical characterization, activity, and thiol content of the highly active form of clostripain. J Biol Chem. 1979 Mar 10;254(5):1462–1468. [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Stoll J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease? J Periodontol. 1987 Apr;58(4):266–273. doi: 10.1902/jop.1987.58.4.266. [DOI] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Detection of specific antibody in adult human periodontitis sera to surface antigens of Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):832–834. doi: 10.1128/iai.55.3.832-834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Okuda K., Takazoe I. Purification and characterization of a thiol-protease from Bacteroides gingivalis strain 381. Oral Microbiol Immunol. 1987 Jun;2(2):77–81. doi: 10.1111/j.1399-302x.1987.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Renvert S., Wikström M., Dahlén G., Slots J., Egelberg J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J Clin Periodontol. 1990 Jul;17(6):345–350. doi: 10.1111/j.1600-051x.1990.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Robertson P. B., Lantz M., Marucha P. T., Kornman K. S., Trummel C. L., Holt S. C. Collagenolytic activity associated with Bacteroides species and Actinobacillus actinomycetemcomitans. J Periodontal Res. 1982 May;17(3):275–283. doi: 10.1111/j.1600-0765.1982.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Sato M., Otsuka M., Maehara R., Endo J., Nakamura R. Degradation of human secretory immunoglobulin A by protease isolated from the anaerobic periodontopathogenic bacterium, Bacteroides gingivalis. Arch Oral Biol. 1987;32(4):235–238. doi: 10.1016/0003-9969(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A., Berry C. R. Production of chemotactic factors for neutrophils following the interaction of Bacteroides gingivalis with purified C5. J Periodontal Res. 1988 Sep;23(5):308–312. doi: 10.1111/j.1600-0765.1988.tb01422.x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. The effect of periodontal proteolytic Bacteroides species on proteins of the human complement system. J Periodontal Res. 1988 May;23(3):187–192. doi: 10.1111/j.1600-0765.1988.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Haapasalo M., Laakso T., Salo T. Degradation of basement membrane collagen by proteases from some anaerobic oral micro-organisms. Oral Microbiol Immunol. 1988 Sep;3(3):97–102. doi: 10.1111/j.1399-302x.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]


