Abstract
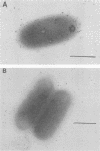
Exoenzyme S from Pseudomonas aeruginosa has been studied as an adhesion for glycosphingolipids and buccal cells. Binding of exoenzyme S to gangliotriosylceramide (GalNAc beta 1-4Gal beta 1-4Glc beta 1-1Cer), gangliotetraosylceramide (Gal beta 1-3 GalNAcT beta 1-4 Gal beta 1-4Glc beta 1-1Cer), and lactosylceramide (Gal beta 1-4Glc beta 1-1Cer) separated on thin-layer chromatograms was observed. Binding curves for exoenzyme S with dilutions of gangliotetraosylceramide immobilized on plastic plates were similar to previously reported results for the intact bacteria. Binding of exoenzyme S to sialylated counterparts of these glycosphingolipids was not seen, indicating that the addition of a sialic acid residue interferes with binding. Exoenzyme S and monoclonal antibody to exoenzyme S inhibit the binding of P. aeruginosa to buccal cells. The presence of exoenzyme S on the surface of P. aeruginosa was detected by immunogold labeling of bacteria with antibodies to exoenzyme S. Results of these studies led us to conclude that exoenzyme S is an important adhesion of P. aeruginosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N., Hansson G. C., Leffler H., Riise G., Svanborg-Edén C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990 Jul;58(7):2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan C. S., Reynolds K. L. Bacteremic nosocomial pneumonia. Analysis of 172 episodes from a single metropolitan area. Am Rev Respir Dis. 1984 May;129(5):668–671. doi: 10.1164/arrd.1984.129.5.668. [DOI] [PubMed] [Google Scholar]
- Coburn J., Wyatt R. T., Iglewski B. H., Gill D. M. Several GTP-binding proteins, including p21c-H-ras, are preferred substrates of Pseudomonas aeruginosa exoenzyme S. J Biol Chem. 1989 May 25;264(15):9004–9008. [PubMed] [Google Scholar]
- Doig P., Paranchych W., Sastry P. A., Irvin R. T. Human buccal epithelial cell receptors of Pseudomonas aeruginosa: identification of glycoproteins with pilus binding activity. Can J Microbiol. 1989 Dec;35(12):1141–1145. doi: 10.1139/m89-189. [DOI] [PubMed] [Google Scholar]
- Doig P., Sastry P. A., Hodges R. S., Lee K. K., Paranchych W., Irvin R. T. Inhibition of pilus-mediated adhesion of Pseudomonas aeruginosa to human buccal epithelial cells by monoclonal antibodies directed against pili. Infect Immun. 1990 Jan;58(1):124–130. doi: 10.1128/iai.58.1.124-130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer J. J., 3rd Pseudomonas in the hospital. Hosp Pract. 1976 Feb;11(2):63–70. doi: 10.1080/21548331.1976.11706501. [DOI] [PubMed] [Google Scholar]
- Godfrey A. J., Shahrabadi M. S., Bryan L. E. Distribution of porin and lipopolysaccharide antigens on a Pseudomonas aeruginosa permeability mutant. Antimicrob Agents Chemother. 1986 Nov;30(5):802–805. doi: 10.1128/aac.30.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. C., Karlsson K. A., Larson G., McKibbin J. M., Strömberg N., Thurin J. Isoglobotriaosylceramide and the Forssman glycolipid of dog small intestine occupy separate tissue compartments and differ in ceramide composition. Biochim Biophys Acta. 1983 Jan 7;750(1):214–216. doi: 10.1016/0005-2760(83)90224-2. [DOI] [PubMed] [Google Scholar]
- Hazlett L. D., Moon M., Berk R. S. In vivo identification of sialic acid as the ocular receptor for Pseudomonas aeruginosa. Infect Immun. 1986 Feb;51(2):687–689. doi: 10.1128/iai.51.2.687-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., Doig P., Lee K. K., Sastry P. A., Paranchych W., Todd T., Hodges R. S. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect Immun. 1989 Dec;57(12):3720–3726. doi: 10.1128/iai.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Habbick B. F., Tumber S. K. Role of sialic acid in saliva-mediated aggregation of Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1987 Oct;55(10):2364–2369. doi: 10.1128/iai.55.10.2364-2369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Ginsburg V., Roberts D. D. Pseudomonas aeruginosa and Pseudomonas cepacia isolated from cystic fibrosis patients bind specifically to gangliotetraosylceramide (asialo GM1) and gangliotriaosylceramide (asialo GM2). Arch Biochem Biophys. 1988 Jan;260(1):493–496. doi: 10.1016/0003-9861(88)90473-0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki L. L., Murphy T. M., Bellanti J. A. Pseudomonas colonization in cystic fibrosis. A study of 160 patients. JAMA. 1978 Jul 7;240(1):30–34. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laraya-Cuasay L. R., Cundy K. R., Huang N. N. Pseudomonas carrier rates of patients with cystic fibrosis and of members of their families. J Pediatr. 1976 Jul;89(1):23–26. doi: 10.1016/s0022-3476(76)80920-1. [DOI] [PubMed] [Google Scholar]
- Leprat R., Michel-Briand Y. Extracellular neuraminidase production by a strain of Pseudomonas aeruginosa isolated from cystic fibrosis. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):209–222. [PubMed] [Google Scholar]
- Lund B., Lindberg F., Marklund B. I., Normark S. The PapG protein is the alpha-D-galactopyranosyl-(1----4)-beta-D-galactopyranose-binding adhesin of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5898–5902. doi: 10.1073/pnas.84.16.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri D. K., Seifert H. S., Ajioka R. S., Karlsson K. A., So M. Identification and characterization of a Neisseria gonorrhoeae gene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci U S A. 1990 Jan;87(1):333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Guay C., Pier G. B. Pseudomonas aeruginosa adhesins for tracheobronchial mucin. Infect Immun. 1987 Mar;55(3):600–603. doi: 10.1128/iai.55.3.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphal R., Sadoff J. C., Pyle M., Silipigni J. D. Role of pili in the adherence of Pseudomonas aeruginosa to injured tracheal epithelium. Infect Immun. 1984 Apr;44(1):38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Hazlett L. D., Berk R. S. Characterization of Pseudomonas aeruginosa adherence to mouse corneas in organ culture. Infect Immun. 1990 May;58(5):1301–1307. doi: 10.1128/iai.58.5.1301-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strömberg N., Karlsson K. A. Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem. 1990 Jul 5;265(19):11251–11258. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Que J. U. Purification of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1987 Mar;55(3):579–586. doi: 10.1128/iai.55.3.579-586.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Sokol P. A. Use of transposon mutants to assess the role of exoenzyme S in chronic pulmonary disease due to Pseudomonas aeruginosa. Eur J Clin Microbiol. 1985 Apr;4(2):163–169. doi: 10.1007/BF02013591. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., To M., Sokol P. A. Pseudomonas aeruginosa exoenzyme S as a pathogenic determinant in respiratory infections. Antibiot Chemother (1971) 1989;42:27–35. doi: 10.1159/000417600. [DOI] [PubMed] [Google Scholar]