Abstract
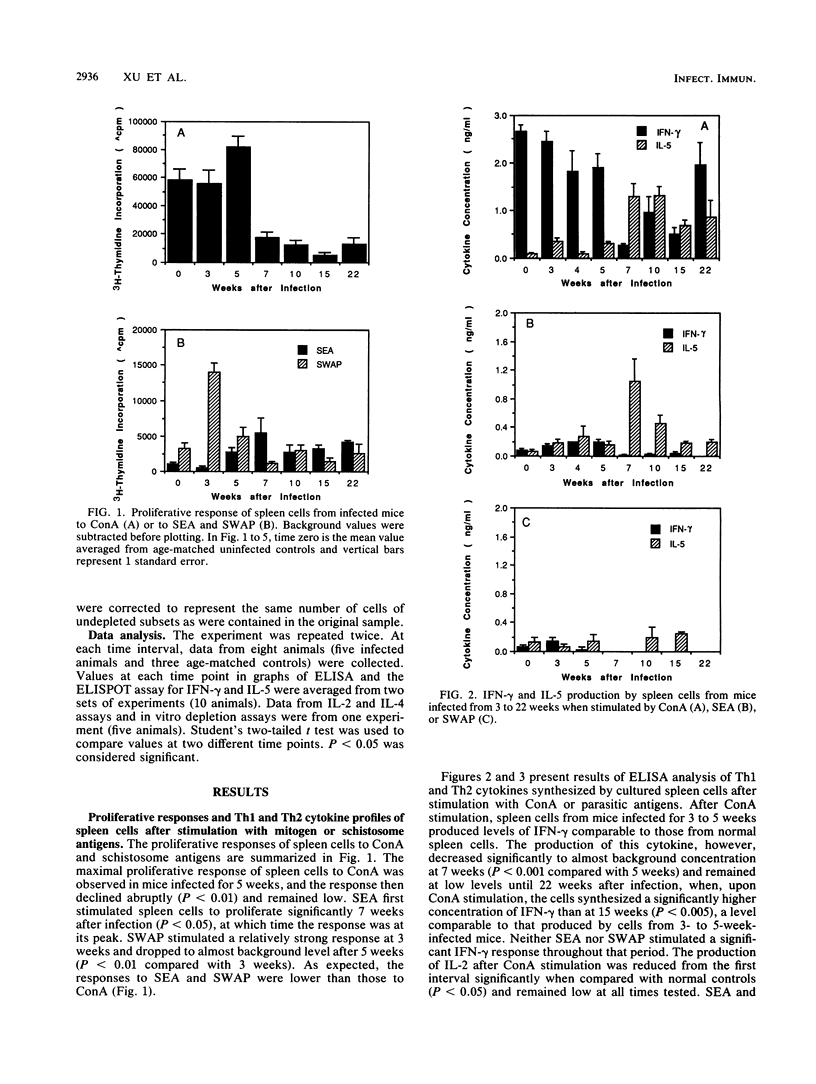
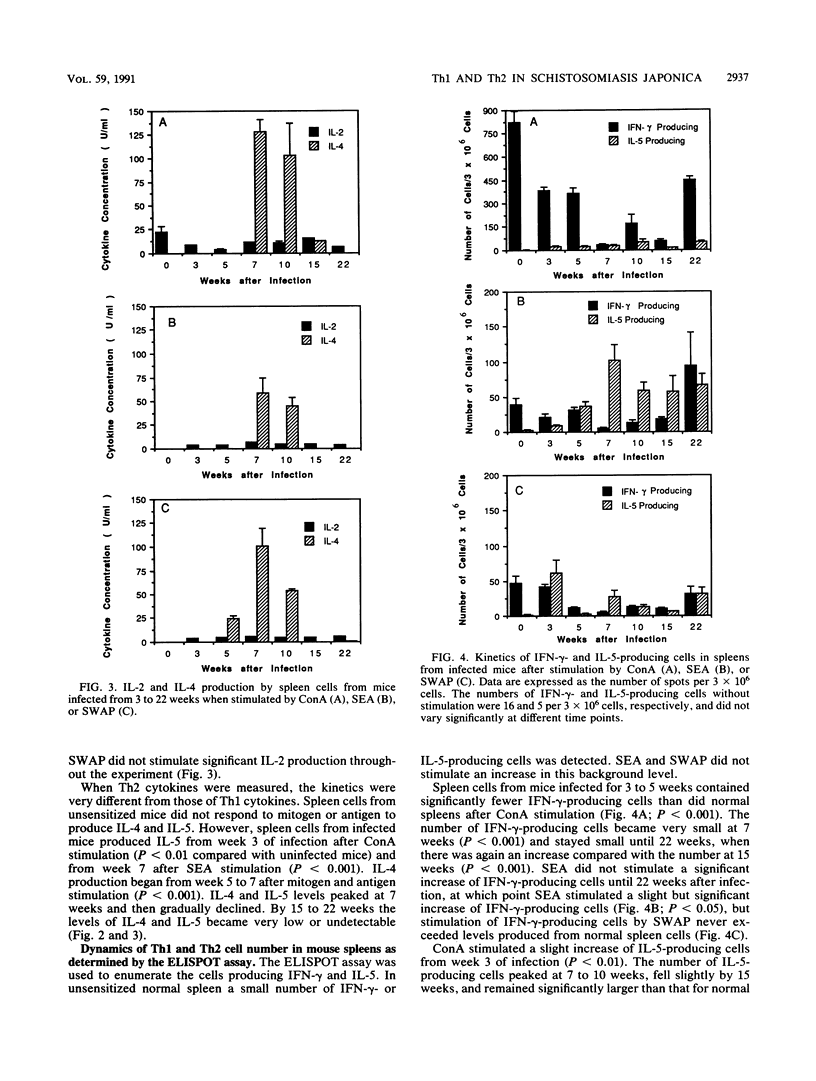
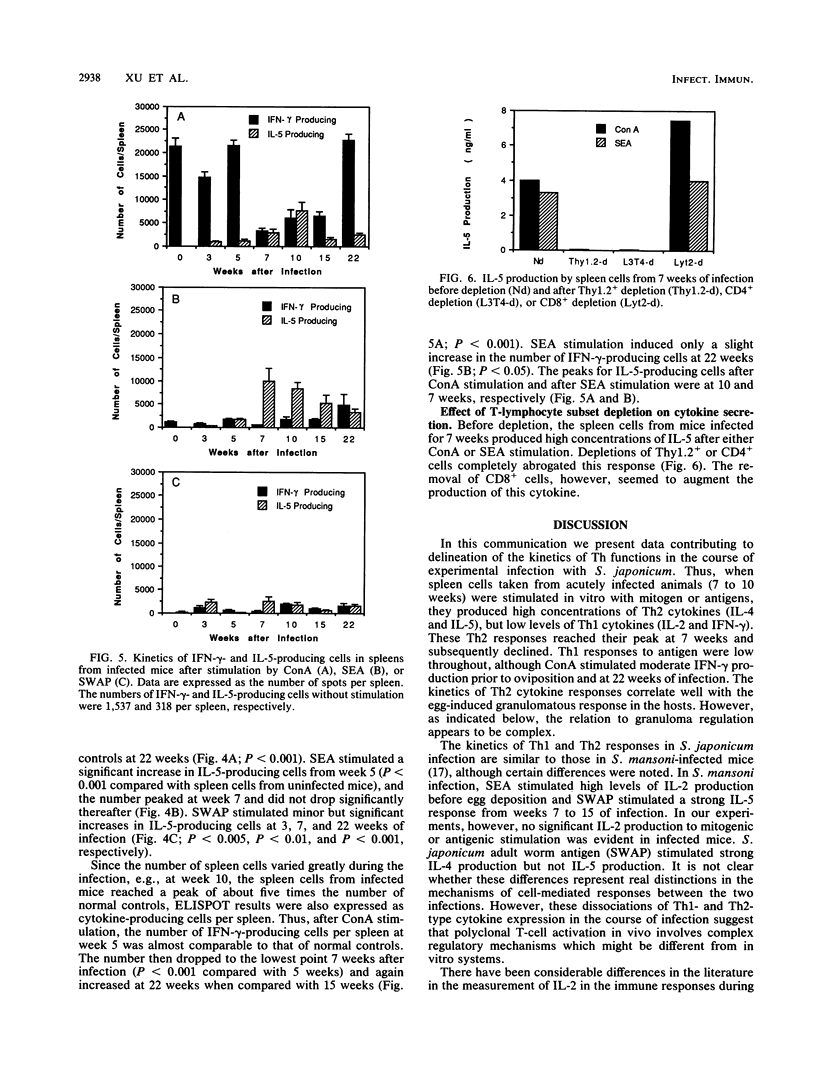
Recent studies indicate that egg granuloma formation in murine Schistosoma mansoni infection is associated with Th2-mediated immune responses. The present study was designed to analyze dynamically the Th1 and Th2 responses in S. japonicum-infected animals and compare them with the results seen with S. mansoni. C3H mice were infected with 10 to 20 cercariae of S. japonicum and sacrificed 3 to 22 weeks later. Spleen cells were stimulated with parasite antigens (egg and adult worm) or the mitogen concanavalin A. Interleukin-2 (IL-2), IL-4, IL-5, and gamma interferon (IFN-gamma) levels were measured in the culture supernatants by enzyme-linked immunosorbent assay (ELISA) or bioassays. Additionally, cytokine-producing cells were enumerated by ELISPOT. The results show that Th2 cytokine production, characterized by IL-4 and IL-5, represents the major response in the first month after egg laying begins, while the Th1 functions of IFN-gamma and IL-2 production are greatly depressed. However, by 22 weeks Th2 responses have diminished and IFN-gamma production in response to concanavalin A is apparent. IL-2 responses are minimal at all times. In vitro depletion of T-cell subsets indicates that CD4+ cells are the major subset responsible for production of IL-5 at 7 weeks of infection. These findings suggest that, as in the case of S. mansoni infection, S. japonicum-induced immunopathology is temporally associated with the host Th2 response, although other experiments indicate that IFN-gamma is also involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheever A. W., Byram J. E., Hieny S., von Lichtenberg F., Lunde M. N., Sher A. Immunopathology of Schistosoma japonicum and S. mansoni infection in B cell depleted mice. Parasite Immunol. 1985 Jul;7(4):399–413. doi: 10.1111/j.1365-3024.1985.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Byram J. E., von Lichtenberg F. Immunopathology of Schistosoma japonicum infection in athymic mice. Parasite Immunol. 1985 Jul;7(4):387–398. doi: 10.1111/j.1365-3024.1985.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Cheever A. W., Deb S., Duvall R. H. Granuloma formation in Schistosoma japonicum infected nude mice: the effects of reconstitution with L3T4+ or Lyt2+ splenic cells. Am J Trop Med Hyg. 1989 Jan;40(1):66–71. doi: 10.4269/ajtmh.1989.40.66. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Colley D. G. Adoptive suppression of granuloma formation. J Exp Med. 1976 Mar 1;143(3):696–700. doi: 10.1084/jem.143.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry R. C., Kiener P. A., Spitalny G. L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987 Nov 23;104(1-2):137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A., Dowell T. A., Huang K., Dudley D. Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med. 1990 Apr 1;171(4):979–996. doi: 10.1084/jem.171.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. E., Ragheb S., Wellhausen S. R., Boros D. L. Interactions between adherent mononuclear cells and lymphocytes from granulomas of mice with schistosomiasis mansoni. Infect Immun. 1990 Jun;58(6):1577–1583. doi: 10.1128/iai.58.6.1577-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Hedlund G., Kalland T., Sjögren H. O., Dohlsten M. Independent regulation of IFN-gamma and tumor necrosis factor by IL-1 in human T helper cells. J Immunol. 1990 Dec 1;145(11):3767–3772. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Olds G. R., Tracy J. W., Mahmoud A. A. Immune regulation in murine schistosomiasis japonica: inhibition of in vitro antigen- and mitogen-induced cellular responses by splenocyte culture supernatants and by purified fractions from serum of chronically infected mice. J Immunol. 1982 Dec;129(6):2752–2758. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- James S. L., Sher A. Cell-mediated immune response to schistosomiasis. Curr Top Microbiol Immunol. 1990;155:21–31. doi: 10.1007/978-3-642-74983-4_2. [DOI] [PubMed] [Google Scholar]
- Kresina T. F., Olds G. R. Concomitant cellular and humoral expression of a regulatory cross-reactive idiotype in acute Schistosoma japonicum infection. Infect Immun. 1986 Jul;53(1):90–94. doi: 10.1128/iai.53.1.90-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Olds G. R., Mahmoud A. A. Kinetics and mechanisms of pulmonary granuloma formation around Schistosoma japonicum eggs injected into mice. Cell Immunol. 1981 May 15;60(2):251–260. doi: 10.1016/0008-8749(81)90267-7. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Owhashi M., Nawa Y., Watanabe N. Granulomatous response in selective IgE-deficient SJA/9 mice infected with Schistosoma japonicum. Int Arch Allergy Appl Immunol. 1989;90(3):310–312. doi: 10.1159/000235044. [DOI] [PubMed] [Google Scholar]
- Pearce E. J., Caspar P., Grzych J. M., Lewis F. A., Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991 Jan 1;173(1):159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J. H., O'Garra A., Shrader B., van Kimmenade A., Bond M. W., Mosmann T. R., Coffman R. L. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988 Sep 1;141(5):1576–1581. [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Harold W. W. Deficiency of interleukin-2 activity upon addition of soluble egg antigen to cultures of spleen cells from mice infected with Schistosoma japonicum. Infect Immun. 1988 Jul;56(7):1778–1784. doi: 10.1128/iai.56.7.1778-1784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Olds G. R., Peterson L. B. Regulation of egg antigen-induced in vitro proliferative response by splenic suppressor T cells in murine Schistosoma japonicum infection. Infect Immun. 1985 Sep;49(3):635–640. doi: 10.1128/iai.49.3.635-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., McKenzie D. T., Weinberg A. D., Hancock W. Characterization of T helper 1 and 2 cell subsets in normal mice. Helper T cells responsible for IL-4 and IL-5 production are present as precursors that require priming before they develop into lymphokine-secreting cells. J Immunol. 1988 Nov 15;141(10):3445–3455. [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Boros D. L., Hang L. M., Mahmoud A. A. The Schistosoma japonicum egg granuloma. Am J Pathol. 1975 Aug;80(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O., Cowan R. B. Granuloma formation around schistosome eggs as a manifestation of delayed hypersensitivity. Am J Pathol. 1967 Nov;51(5):735–756. [PMC free article] [PubMed] [Google Scholar]
- Warren K. S., Domingo E. O. Granuloma formation around Schistosoma mansoni, S. HAEMATOBIUM, AND S. japonicum eggs. Size and rate of development, cellular composition, cross-sensitivity, and rate of egg destruction. Am J Trop Med Hyg. 1970 Mar;19(2):292–304. doi: 10.4269/ajtmh.1970.19.292. [DOI] [PubMed] [Google Scholar]
- Warren K. S., Grove D. I., Pelley R. P. The Schistosoma japonicum egg granuloma. II. Cellular composition, granuloma size, and immunologic concomitants. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):271–275. doi: 10.4269/ajtmh.1978.27.271. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Watanabe T., Sendo F. Studies on the immunological disturbance in murine schistosomiasis japonica from the viewpoint of the interleukin cascade reaction. Immunology. 1987 Oct;62(2):215–221. [PMC free article] [PubMed] [Google Scholar]



