Abstract
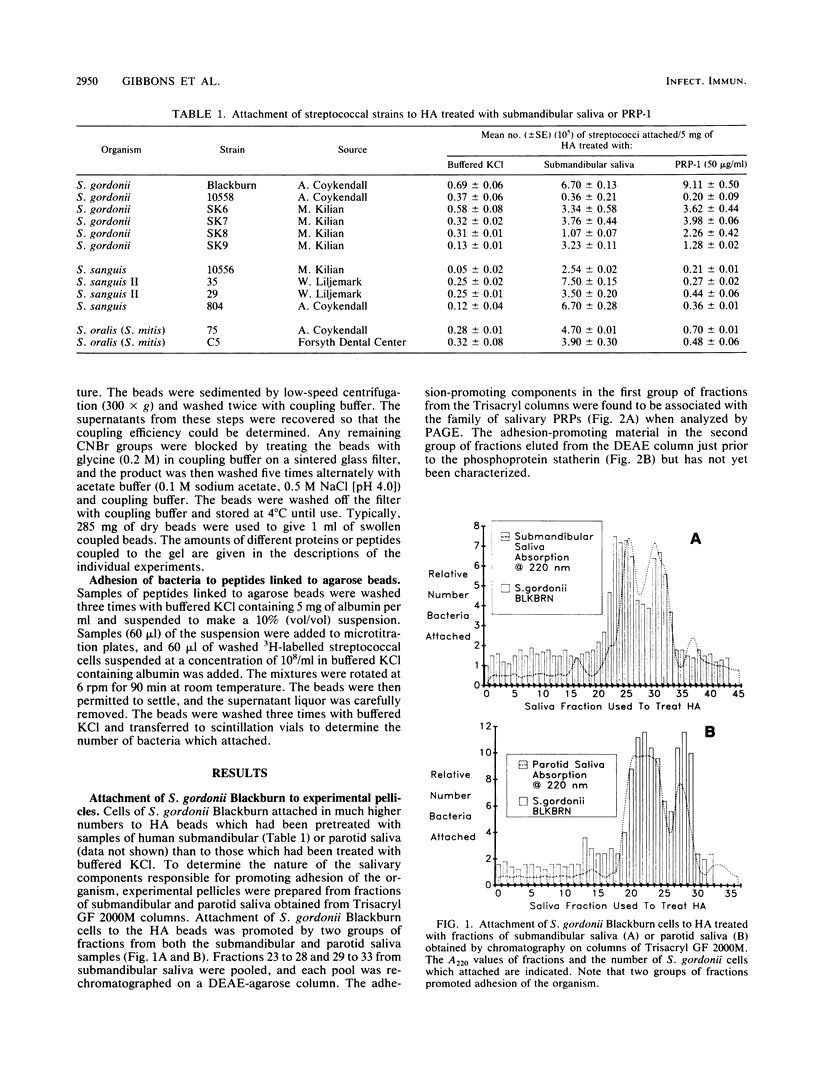
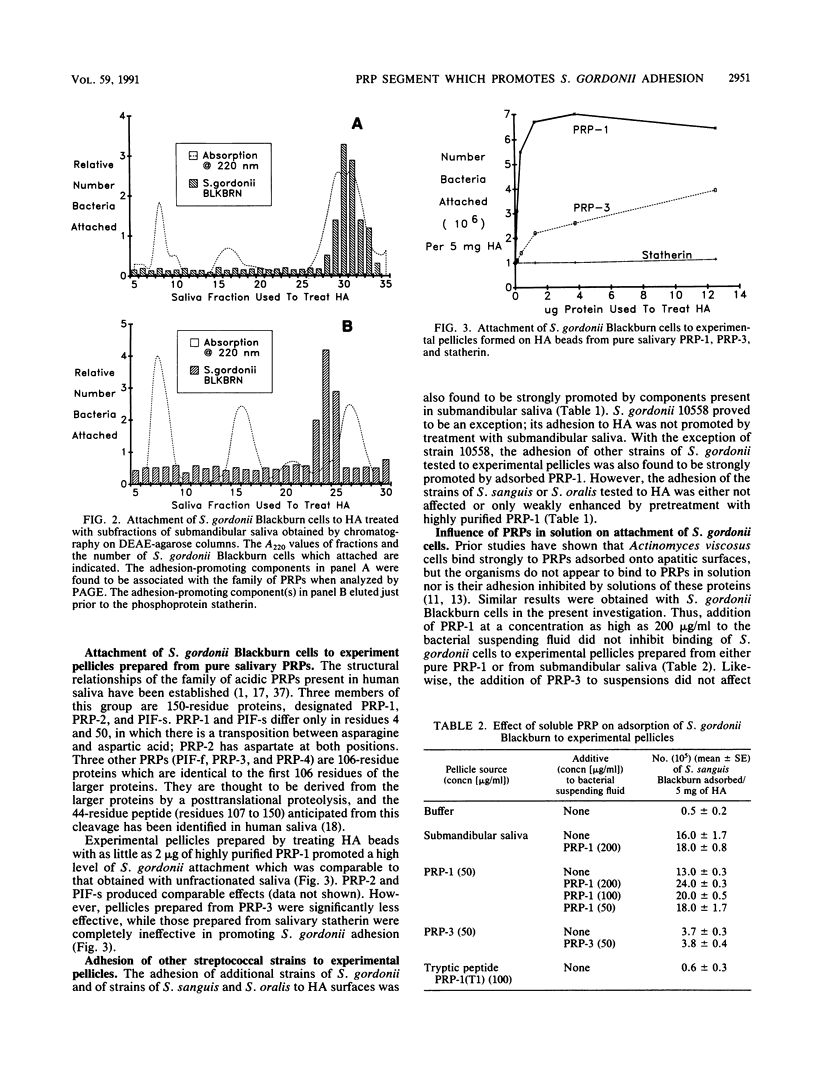
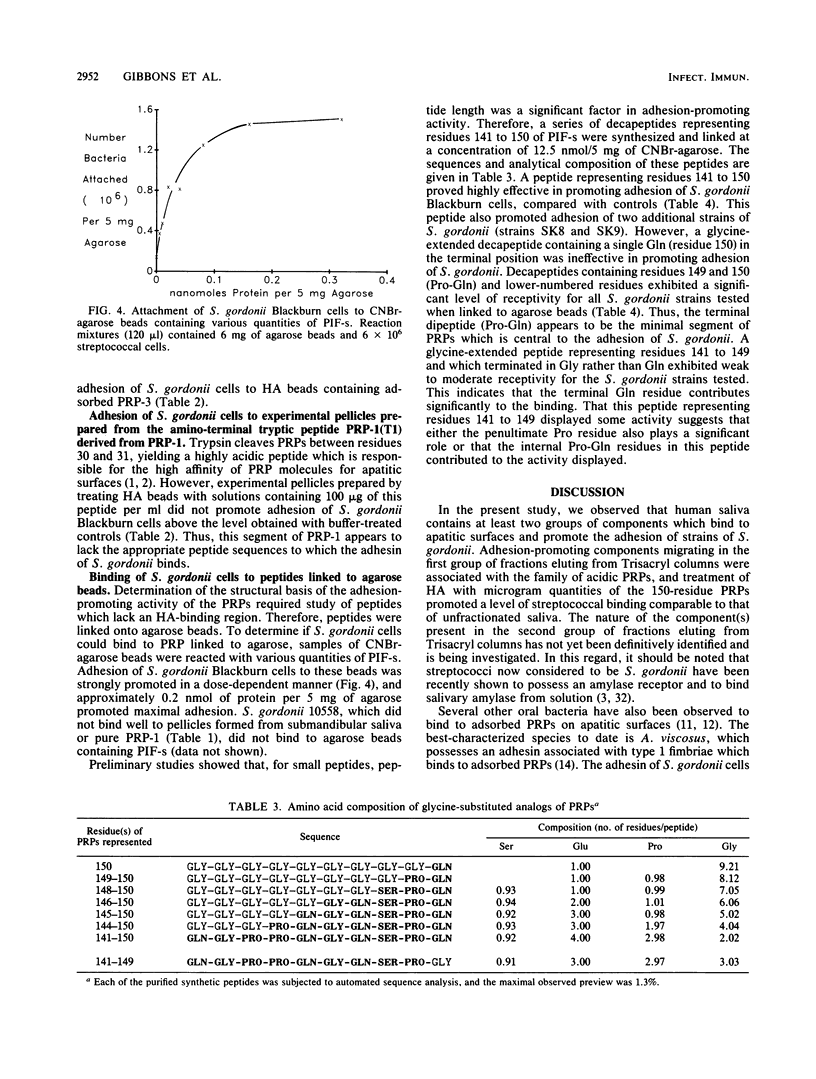
Cells of several strains of Streptococcus gordonii attached in much higher numbers to experimental pellicles formed from samples of submandibular or parotid saliva on hydroxyapatite (HA) beads than to buffer controls. The nature of the salivary components responsible were investigated by preparing experimental pellicles from chromatographic fractions of submandibular saliva obtained from Trisacryl GF 2000M columns. Adhesion of S. gordonii Blackburn was promoted by two groups of fractions. The adhesion-promoting activity in the first group of fractions was associated with the family of acidic proline-rich proteins (PRPs), while that of the second group is as yet unidentified. Experimental pellicles prepared by treating HA with 2 micrograms of pure 150-amino-acid-residue PRPs (PRP-1, PRP-2, and PIF-s) promoted adhesion of S. gordonii Blackburn cells to an extent comparable to that obtained with unfractionated saliva. However, pellicles prepared from a 106-residue PRP (PRP-3) were significantly less effective, and those prepared from the amino-terminal tryptic peptide (residues 1 to 30) of the PRP and the salivary phosphoprotein statherin were completely ineffective in promoting adhesion. Although adhesion of several strains of S. gordonii was promoted by adsorbed PRP-1, the adhesion of several strains of Streptococcus sanguis or Streptococcus oralis was either not affected or only weakly enhanced by this protein. S. gordonii cells bound avidly to PRPs adsorbed onto HA beads, but the streptococci did not appear to bind PRPs in solution, since concentrations of PRP as high as 200 micrograms/ml did not inhibit binding of bacterial cells to pellicles prepared from pure PRP. S. gordonii cells also attached well to PRP or a synthetic decapeptide representing residues 142 to 150 of the PRP when the peptide was linked to agarose beads. Studies with a series of synthetic decapeptides indicated that the minimal segment of PRP which promoted high levels of S. gordonii adhesion was the carboxy-terminal dipeptide Pro-Gln (residues 149 and 150).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennick A., Cannon M., Madapallimattam G. The nature of the hydroxyapatite-binding site in salivary acidic proline-rich proteins. Biochem J. 1979 Oct 1;183(1):115–126. doi: 10.1042/bj1830115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Structural and genetic aspects of proline-rich proteins. J Dent Res. 1987 Feb;66(2):457–461. doi: 10.1177/00220345870660021201. [DOI] [PubMed] [Google Scholar]
- Douglas C. W. Characterization of the alpha-amylase receptor of Streptococcus gordonii NCTC 7868. J Dent Res. 1990 Nov;69(11):1746–1752. doi: 10.1177/00220345900690110701. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Ericson T. Adsorption to hydroxyapatite of proteins and conjugated proteins from human saliva. Caries Res. 1967;1(1):52–58. doi: 10.1159/000259499. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I. Albumin as a blocking agent in studies of streptococcal adsorption to experimental salivary pellicles. Infect Immun. 1985 Nov;50(2):592–594. doi: 10.1128/iai.50.2.592-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Childs W. C., 3rd, Davis G. Role of cryptic receptors (cryptitopes) in bacterial adhesion to oral surfaces. Arch Oral Biol. 1990;35 (Suppl):107S–114S. doi: 10.1016/0003-9969(90)90139-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I., Cisar J. O., Clark W. B. Adsorbed salivary proline-rich protein 1 and statherin: receptors for type 1 fimbriae of Actinomyces viscosus T14V-J1 on apatitic surfaces. Infect Immun. 1988 Nov;56(11):2990–2993. doi: 10.1128/iai.56.11.2990-2993.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Hay D. I. Human salivary acidic proline-rich proteins and statherin promote the attachment of Actinomyces viscosus LY7 to apatitic surfaces. Infect Immun. 1988 Feb;56(2):439–445. doi: 10.1128/iai.56.2.439-445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I., Bennick A., Schlesinger D. H., Minaguchi K., Madapallimattam G., Schluckebier S. K. The primary structures of six human salivary acidic proline-rich proteins (PRP-1, PRP-2, PRP-3, PRP-4, PIF-s and PIF-f). Biochem J. 1988 Oct 1;255(1):15–21. doi: 10.1042/bj2550015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay D. I. The adsorption of salivary proteins by hydroxyapatite and enamel. Arch Oral Biol. 1967 Aug;12(8):937–946. doi: 10.1016/0003-9969(67)90088-x. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. The amino acid sequence of a salivary proline-rich peptide, P-C, and its relation to a salivary proline-rich phosphoprotein, protein C. J Biochem. 1980 Apr;87(4):1071–1077. [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Kishimoto E., Hay D. I., Gibbons R. J. A human salivary protein which promotes adhesion of Streptococcus mutans serotype c strains to hydroxyapatite. Infect Immun. 1989 Dec;57(12):3702–3707. doi: 10.1128/iai.57.12.3702-3707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljemark W. F., Bloomquist C. G., Fenner L. J., Antonelli P. J., Coulter M. C. Effect of neuraminidase on the adherence to salivary pellicle of Streptococcus sanguis and Streptococcus mitis. Caries Res. 1989;23(3):141–145. doi: 10.1159/000261167. [DOI] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Reddy M. S., Tabak L. A., Bergey E. J. Preparation of a sialic acid-binding protein from Streptococcus mitis KS32AR. Infect Immun. 1986 Aug;53(2):359–365. doi: 10.1128/iai.53.2.359-365.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. A., Levine M. J., Tabak L. A., Reddy M. S. Specificity of salivary-bacterial interactions: II. Evidence for a lectin on Streptococcus sanguis with specificity for a NeuAc alpha 2, 3Ga1 beta 1, 3Ga1NAc sequence. Biochem Biophys Res Commun. 1982 May 31;106(2):390–396. doi: 10.1016/0006-291x(82)91122-6. [DOI] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24(4):267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- Nyvad B., Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987 Oct;95(5):369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Orstavik D., Kraus F. W. The acquired pellicle: immunofluorescent demonstration of specific proteins. J Oral Pathol. 1973;2(1):68–76. doi: 10.1111/j.1600-0714.1973.tb01675.x. [DOI] [PubMed] [Google Scholar]
- Rölla G., Ciardi J. E., Bowen W. H. Identification of IgA, IgG, lysozyme, albumin, alpha-amylase and glucosyltransferase in the protein layer adsorbed to hydroxyapatite from whole saliva. Scand J Dent Res. 1983 Jun;91(3):186–190. doi: 10.1111/j.1600-0722.1983.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco F. A., Bergey E. J., Reddy M. S., Levine M. J. Characterization of salivary alpha-amylase binding to Streptococcus sanguis. Infect Immun. 1989 Sep;57(9):2853–2863. doi: 10.1128/iai.57.9.2853-2863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger D. H., Hay D. I. Complete covalent structure of a proline-rich phosphoprotein, PRP-2, an inhibitor of calcium phosphate crystal growth from human parotid saliva. Int J Pept Protein Res. 1986 Apr;27(4):373–379. doi: 10.1111/j.1399-3011.1986.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger D. H., Hay D. I. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977 Mar 10;252(5):1689–1695. [PubMed] [Google Scholar]
- Wong R. S., Bennick A. The primary structure of a salivary calcium-binding proline-rich phosphoprotein (protein C), a possible precursor of a related salivary protein A. J Biol Chem. 1980 Jun 25;255(12):5943–5948. [PubMed] [Google Scholar]



