Abstract
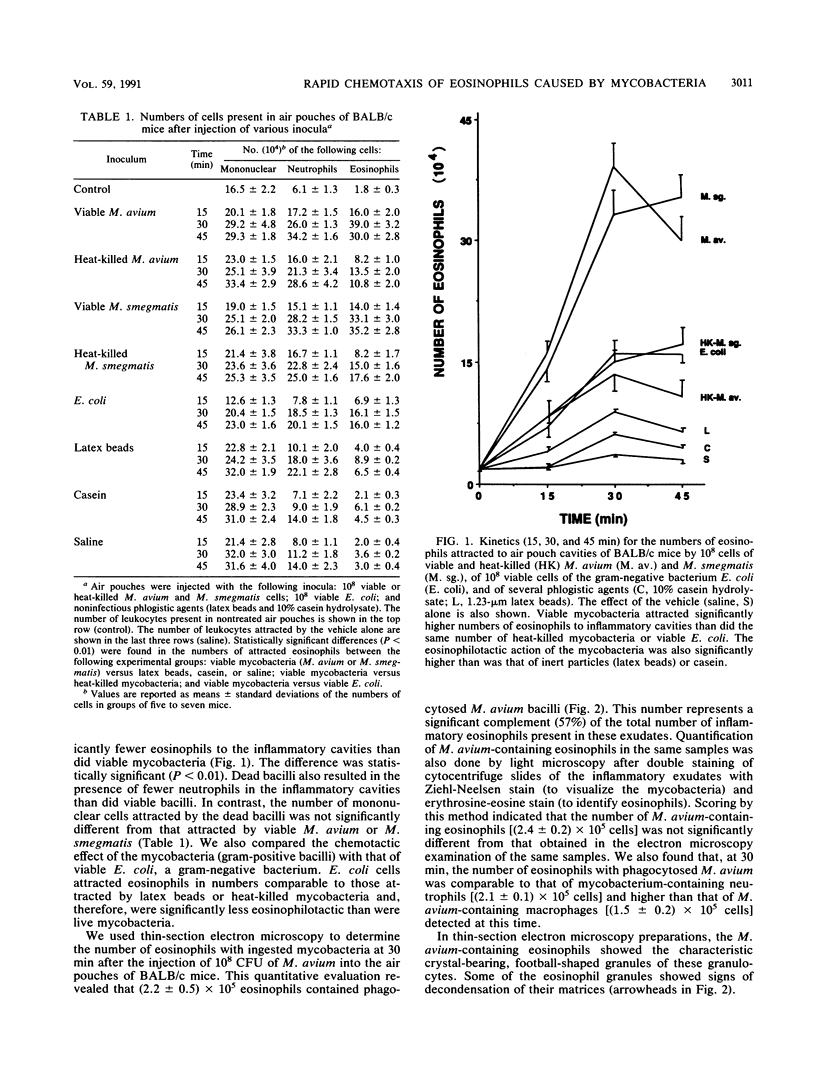
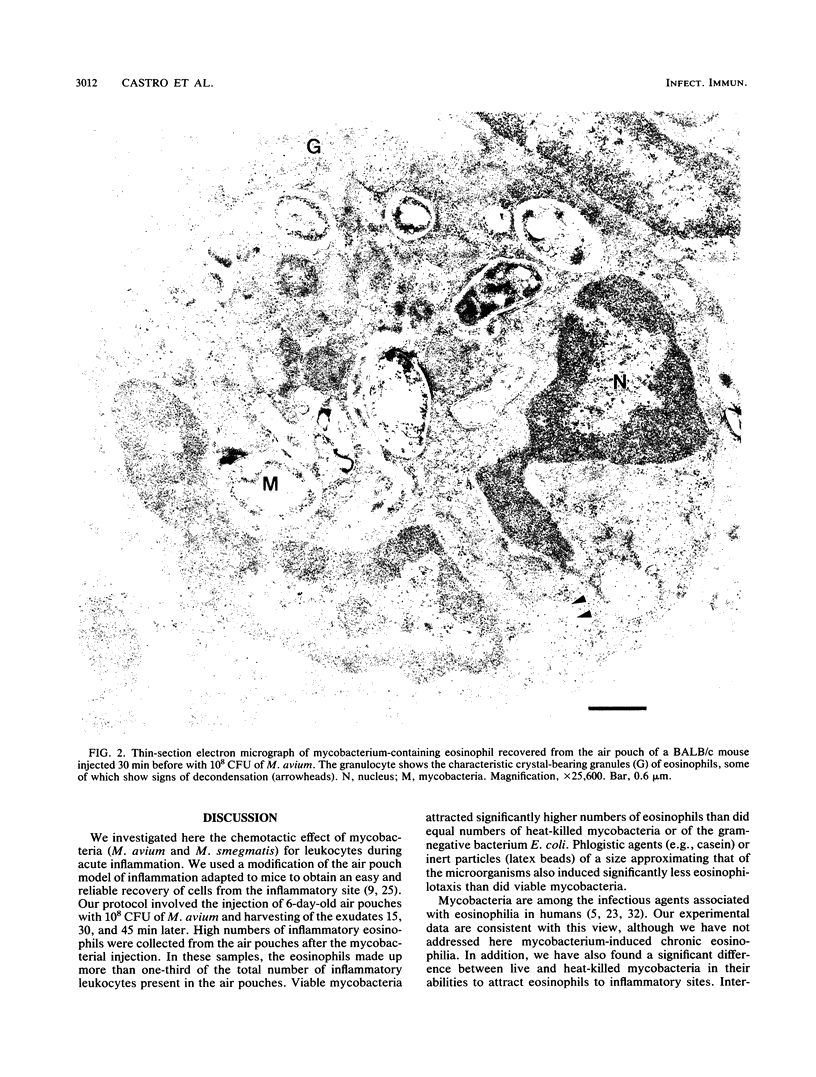
We studied leukocyte chemotaxis triggered by a local injection of mycobacteria (Mycobacterium avium and M. smegmatis) in BALB/c and C57BL/6 mice. Our experimental model consisted of the induction of a subcutaneous air pouch in the dorsal area of mice and inoculation 6 days later of 10(8) CFU of myocobacteria. Inflammatory exudates were harvested from the air pouch cavities 15, 30, and 45 min after the injection of the inocula. Injection of the microorganisms resulted in the migration of an elevated number of eosinophilic granulocytes into the inflammatory cavities. At 30 min after the inoculation of the mycobacteria, the air pouches contained between (3.9 +/- 0.3) x 10(5) (M. avium) and (3.3 +/- 0.3) x 10(5) (M. smegmatis) eosinophils, corresponding to more than one-third (41.4 to 38.3%) of the leukocytes present in the inflammatory cavities. Less than one-half of the eosinophils were attracted to the air pouches when the same number of heat-killed mycobacteria were inoculated [(1.3 +/- 0.2) x 10(5) cells for M. avium and (1.5 +/- 0.2) x 10(5) cells for M. smegmatis]. Injection of gram-negative bacteria (Escherichia coli), of latex beads, or of casein resulted in the attraction of inflammatory eosinophils in numbers that were comparable to those attracted by the heat-killed mycobacteria. Our data document the fact that live mycobacteria exert a rapid chemotactic effect on eosinophils. We therefore postulate that mycobacteria either contain or induce the production of an eosinophilotactic factor. Because this chemotactic effect occurs during the acute inflammatory response to mycobacteria, it cannot be due to the formation of immune complexes (a major infection-associated chemotactic factor for eosinophils). The attracted eosinophils had an important role in the local phagocytosis of mycobacteria, as indicated by our finding, derived from thin-section electron microscopy quantifications, that at 30 min after M. avium inoculation the inflammatory exudates contained (2.2 +/- 0.5) x 10(5) mycobacterium-bearing eosinophils (corresponding to 57% of the total eosinophils), as compared with (2.1 +/- 0.1) x 10(5) neutrophils and (1.5 +/- 0.2) x 10(5) macrophages with ingested bacilli. We conclude that mycobacteria induce the attraction of eosinophils to inflammatory sites and that these granulocytes have the capacity to phagocytize these bacilli in situ.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Silva M. T. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989 Dec;78(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Basten A., Boyer M. H., Beeson P. B. Mechanism of eosinophilia. I. Factors affecting the eosinophil response of rats to Trichinella spiralis. J Exp Med. 1970 Jun 1;131(6):1271–1287. doi: 10.1084/jem.131.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. E., Holzer T. J., Andersen B. R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987 Dec;156(6):985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- Colley D. G. Variations in peripheral blood eosinophil levels in normal and Schistosoma mansoni-infected mice. J Lab Clin Med. 1974 Jun;83(6):871–876. [PubMed] [Google Scholar]
- Contreras M. A., Cheung O. T., Sanders D. E., Goldstein R. S. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis. 1988 Jan;137(1):149–152. doi: 10.1164/ajrccm/137.1.149. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981 Jun;134(2):147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Geertsma M. F., Nibbering P. H., Pos O., Van Furth R. Interferon-gamma-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than normal granulocytes. Eur J Immunol. 1990 Apr;20(4):869–873. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Adolphson C. R. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Ottesen E. A., Leiferman K. M., Ackerman S. J. Eosinophils and human disease. Int Arch Allergy Appl Immunol. 1989;88(1-2):59–62. doi: 10.1159/000234749. [DOI] [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Isaji M., Momose Y., Naito J. Enhancement of inflammatory reactions in a non-immunological air pouch model in rats. Br J Exp Pathol. 1989 Dec;70(6):705–716. [PMC free article] [PubMed] [Google Scholar]
- Jones G. S., Amirault H. J., Andersen B. R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990 Sep;162(3):700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- LITT M. EOSINOPHILS AND ANTIGEN-ANTIBODY REACTIONS. Ann N Y Acad Sci. 1964 Aug 27;116:964–985. doi: 10.1111/j.1749-6632.1964.tb52562.x. [DOI] [PubMed] [Google Scholar]
- Laster C. E., Gleich G. J. Chemotaxis of eosinophils and neutrophils by aggregated immunoglobulins. J Allergy Clin Immunol. 1971 Nov;48(5):297–304. doi: 10.1016/0091-6749(71)90031-5. [DOI] [PubMed] [Google Scholar]
- Modilevsky T., Sattler F. R., Barnes P. F. Mycobacterial disease in patients with human immunodeficiency virus infection. Arch Intern Med. 1989 Oct;149(10):2201–2205. [PubMed] [Google Scholar]
- Nunes J. F., Soares J. O., Alves de Matos A. P. Micro-buffy coats of whole blood: a method for the electron microscopic study of mononuclear cells. Stain Technol. 1979 Sep;54(5):257–260. doi: 10.3109/10520297909110681. [DOI] [PubMed] [Google Scholar]
- Prince D. S., Peterson D. D., Steiner R. M., Gottlieb J. E., Scott R., Israel H. L., Figueroa W. G., Fish J. E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989 Sep 28;321(13):863–868. doi: 10.1056/NEJM198909283211304. [DOI] [PubMed] [Google Scholar]
- Proudfoot A. T., Akhtar A. J., Douglas A. C., Horne N. W. Miliary tuberculosis in adults. Br Med J. 1969 May 3;2(5652):273–276. doi: 10.1136/bmj.2.5652.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RILEY J. F. Functional significance of histamine and heparin in tissue mast cells. Ann N Y Acad Sci. 1963 Feb 26;103:151–163. doi: 10.1111/j.1749-6632.1963.tb53695.x. [DOI] [PubMed] [Google Scholar]
- Sedgwick A. D., Sin Y. M., Edwards J. C., Willoughby D. A. Increased inflammatory reactivity in newly formed lining tissue. J Pathol. 1983 Dec;141(4):483–495. doi: 10.1002/path.1711410406. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Appelberg R., Silva M. N., Macedo P. M. In vivo killing and degradation of Mycobacterium aurum within mouse peritoneal macrophages. Infect Immun. 1987 Sep;55(9):2006–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. Ultrastructure of Mycobacterium leprae and other acid-fast bacteria as influenced by fixation conditions. Ann Microbiol (Paris) 1982 Jul-Aug;133(1):59–73. [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- TWOMEY J. J., LEAVELL B. S. LEUKEMOID REACTIONS TO TUBERCULOSIS. Arch Intern Med. 1965 Jul;116:21–28. doi: 10.1001/archinte.1965.03870010023005. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Tsukamura M., Blacklock Z. M., Silcox V. A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988 Jul;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]