Abstract
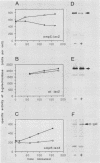
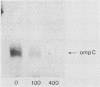
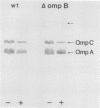
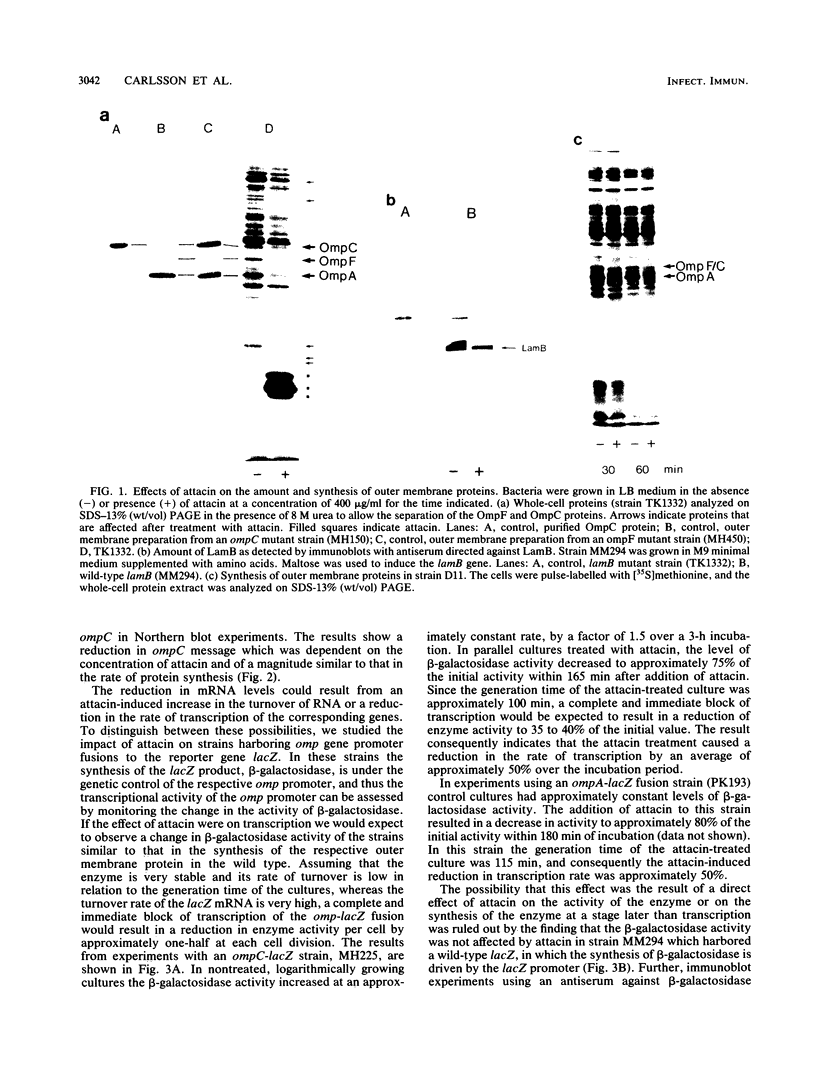
Attacins are antibacterial proteins synthesized by pupae of the giant silk moth, Hyalophora cecropia, in response to a bacterial infection. In this report we show that the previously described, attacin-induced alteration in the structure and the permeability of the outer membrane of Escherichia coli is associated with a specific inhibition of the synthesis of several outer membrane proteins, including OmpC, OmpF, OmpA, and LamB. The inhibition is expressed as a reduction in the steady-state mRNA levels and is at least in part the results of a block in transcription of the corresponding genes. Transcription directed by the promoter of ompR, the positive regulator of ompC and ompF expression in response to environmental conditions, is also affected by attacin. The effects on mutant strains show that the primary activity of attacin is not mediated by the ompR-envZ regulatory system. Instead our data suggest the existence in E. coli of a previously unknown system for the transcriptional regulation of a large set of outer membrane proteins previously not known to be coordinately regulated. We propose that the activity of attacin is directed towards this system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K., Natori S. Inhibitory effect of sarcotoxin IIA, an antibacterial protein of Sarcophaga peregrina, on growth of Escherichia coli. J Biochem. 1988 Apr;103(4):735–739. doi: 10.1093/oxfordjournals.jbchem.a122337. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Eriksson-Grennberg K. G., Normark S., Matsson E. Resistance of Escherichia coli to penicillins. IV. Genetic study of mutants resistant to D,L-ampicillin concentrations o 100 mu-g-ml. Genet Res. 1968 Oct;12(2):169–185. doi: 10.1017/s0016672300011782. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Engström A., Engström P., Tao Z. J., Carlsson A., Bennich H. Insect immunity. The primary structure of the antibacterial protein attacin F and its relation to two native attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2065–2070. doi: 10.1002/j.1460-2075.1984.tb02092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström A., Xanthopoulos K. G., Boman H. G., Bennich H. Amino acid and cDNA sequences of lysozyme from Hyalophora cecropia. EMBO J. 1985 Aug;4(8):2119–2122. doi: 10.1002/j.1460-2075.1985.tb03901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström P., Carlsson A., Engström A., Tao Z. J., Bennich H. The antibacterial effect of attacins from the silk moth Hyalophora cecropia is directed against the outer membrane of Escherichia coli. EMBO J. 1984 Dec 20;3(13):3347–3351. doi: 10.1002/j.1460-2075.1984.tb02302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Comeau D., Norioka S., Inouye M. Localization and membrane topology of EnvZ, a protein involved in osmoregulation of OmpF and OmpC in Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16433–16438. [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Transcriptional regulation of Escherichia coli K-12 major outer membrane protein 1b. J Bacteriol. 1979 Nov;140(2):342–350. doi: 10.1128/jb.140.2.342-350.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holak T. A., Engström A., Kraulis P. J., Lindeberg G., Bennich H., Jones T. A., Gronenborn A. M., Clore G. M. The solution conformation of the antibacterial peptide cecropin A: a nuclear magnetic resonance and dynamical simulated annealing study. Biochemistry. 1988 Oct 4;27(20):7620–7629. doi: 10.1021/bi00420a008. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström A., Andersson K., Steiner H., Bennich H., Boman H. G. Insect immunity. Attacins, a family of antibacterial proteins from Hyalophora cecropia. EMBO J. 1983;2(4):571–576. doi: 10.1002/j.1460-2075.1983.tb01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Jo Y. L., Nara F., Ichihara S., Mizuno T., Mizushima S. Purification and characterization of the OmpR protein, a positive regulator involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1986 Nov 15;261(32):15252–15256. [PubMed] [Google Scholar]
- Kockum K., Faye I., Hofsten P. V., Lee J. Y., Xanthopoulos K. G., Boman H. G. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2071–2075. doi: 10.1002/j.1460-2075.1984.tb02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lång H. A., Palva E. T. A major outer membrane protein in Vibrio cholerae is maltose-inducible. Microb Pathog. 1987 Aug;3(2):143–147. doi: 10.1016/0882-4010(87)90072-6. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Elsbach P., Frangione B., Mannion B. A 25-kDa NH2-terminal fragment carries all the antibacterial activities of the human neutrophil 60-kDa bactericidal/permeability-increasing protein. J Biol Chem. 1987 Nov 5;262(31):14891–14894. [PubMed] [Google Scholar]
- Palva E. T., Silhavy T. J. lacZ fusions to genes that specify exported proteins: a general technique. Mol Gen Genet. 1984;194(3):388–394. doi: 10.1007/BF00425549. [DOI] [PubMed] [Google Scholar]
- Powning R. F., Davidson W. J. Studies on insect bacteriolytic enzymes--II. Some physical and enzymatic properties of lysozyme from haemolymph of Galleria mellonella. Comp Biochem Physiol B. 1976;55(2):221–228. doi: 10.1016/0305-0491(76)90234-0. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Schnaitman C. A. Identification of three genes controlling production of new outer membrane pore proteins in Escherichia coli K-12. J Bacteriol. 1978 Sep;135(3):1118–1129. doi: 10.1128/jb.135.3.1118-1129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Hultmark D., Engström A., Bennich H., Boman H. G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981 Jul 16;292(5820):246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]