Abstract
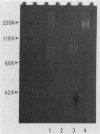
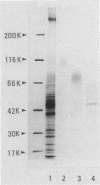
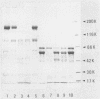
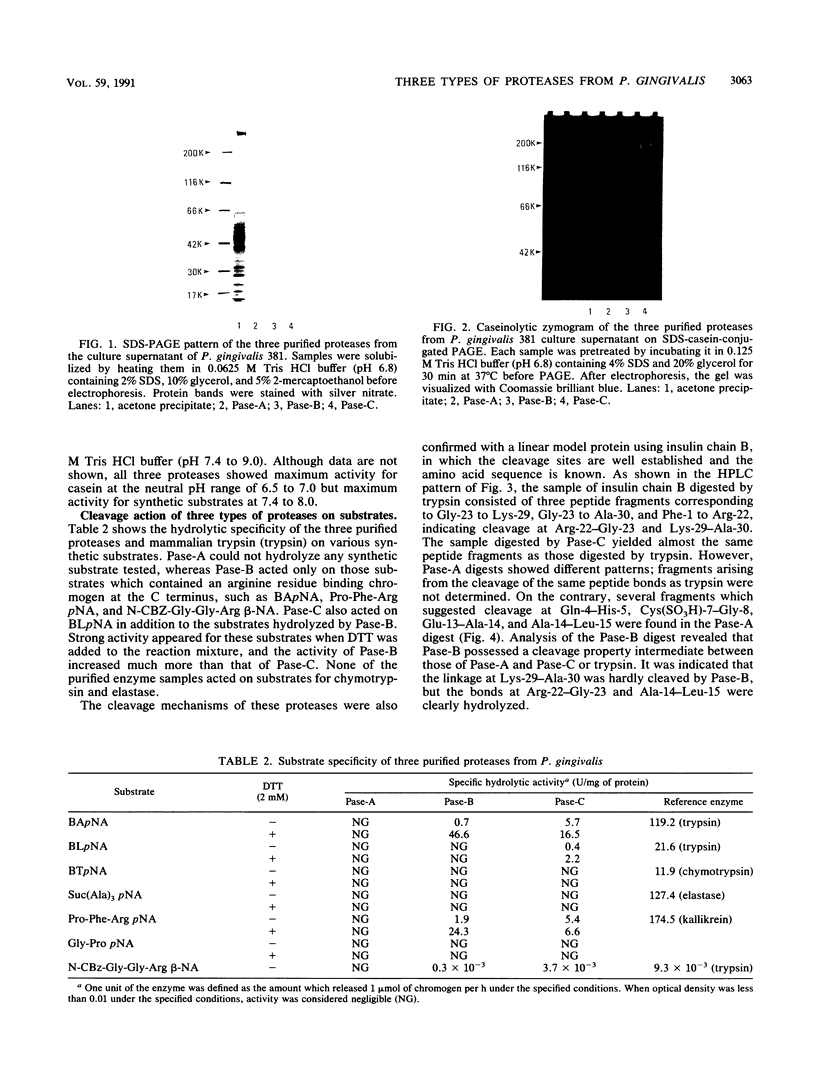
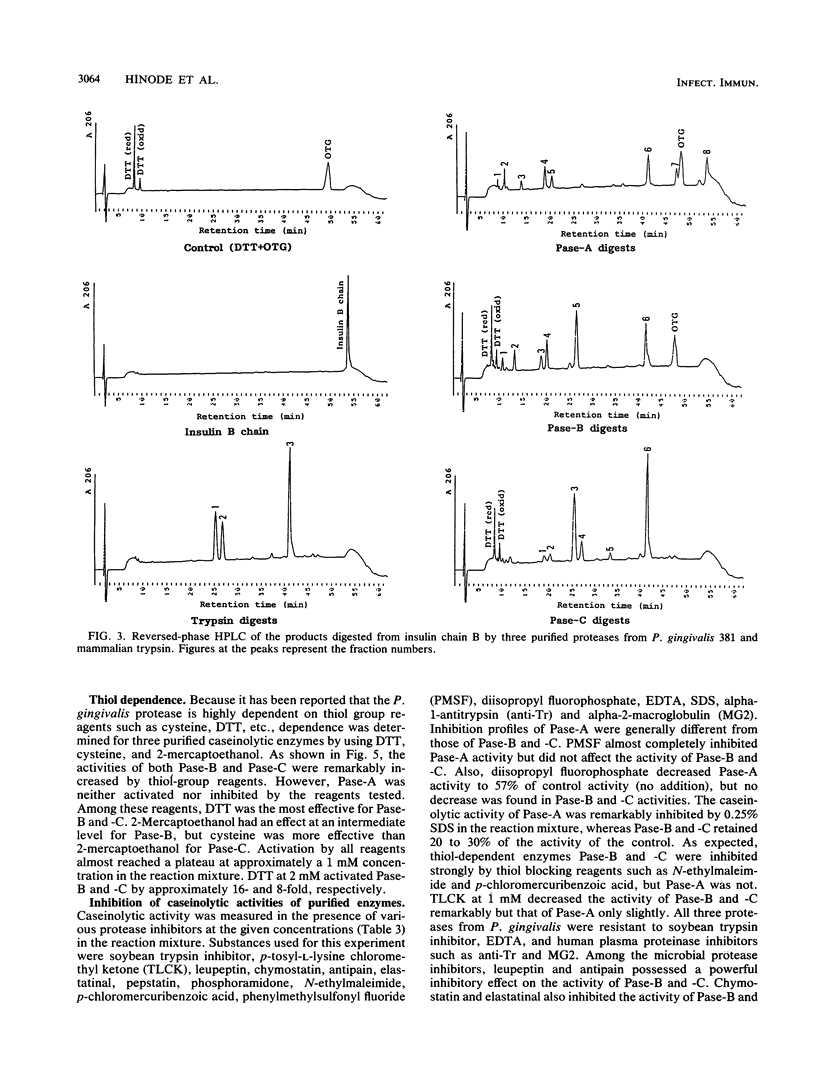
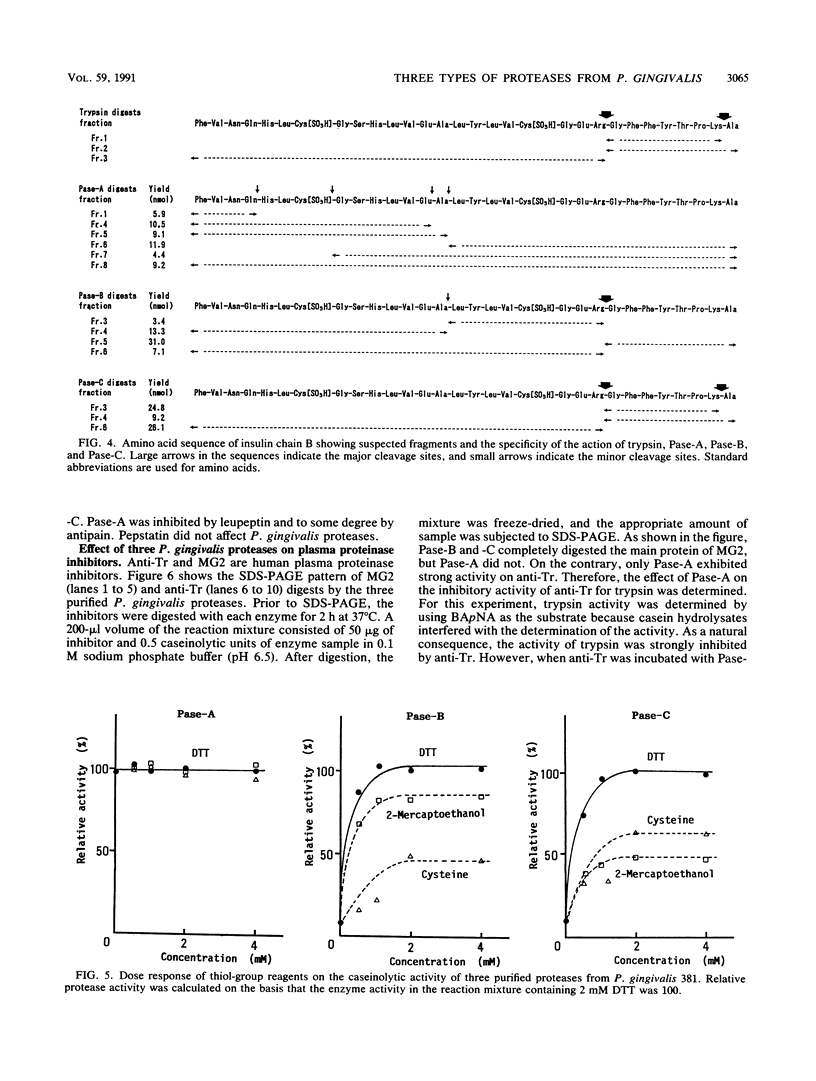
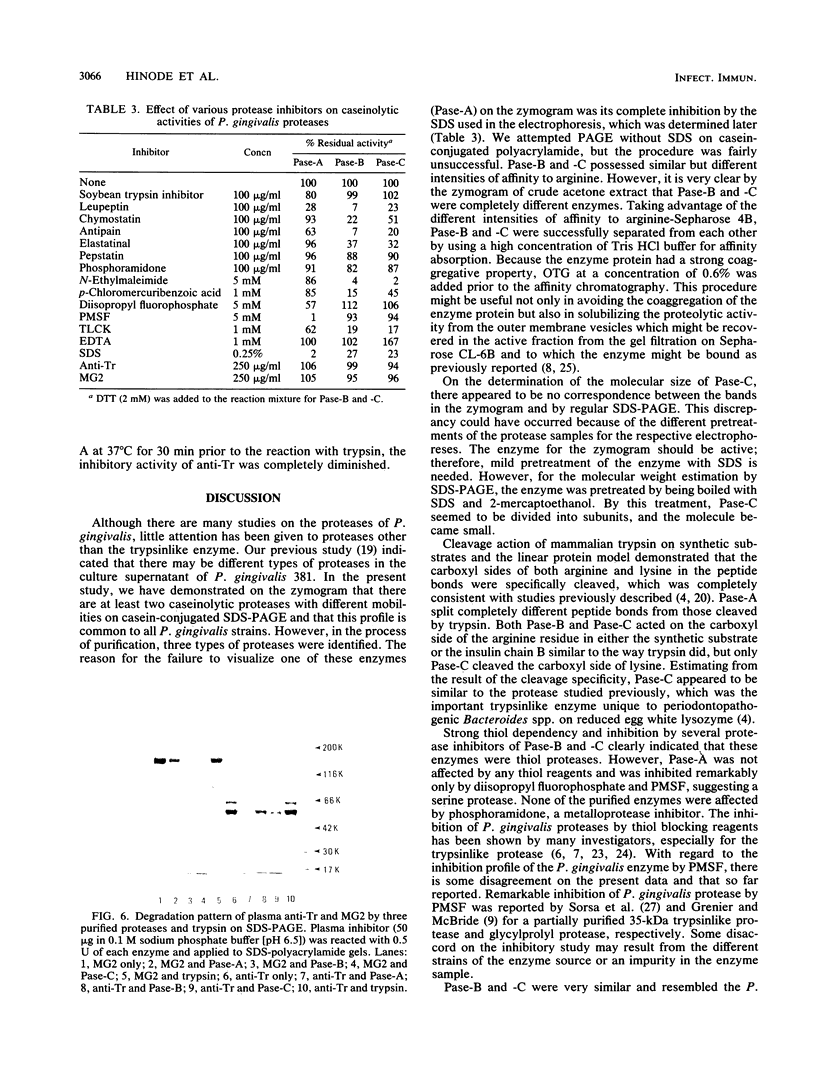
Three types of caseinolytic proteases (Pase-A, Pase-B, and Pase-C) were isolated and purified from culture supernatants of Porphyromonas gingivalis 381 by the combined procedures of acetone precipitation, gel filtration, solubilization with octylthioglucoside followed by affinity chromatography on arginine-Sepharose 4B, high-performance liquid chromatography (HPLC) on Biofine IEC-DEAE, and HPLC on TSK-G4000SW. By sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Pase-A and -B showed diffuse protein bands of 105 to 110 and 72 to 80 kDa, respectively, and Pase-C showed a clear band of about 44 kDa. Pase-B and -C hydrolyzed some synthetic substrates for trypsin, but Pase-B did not act on the carboxyl side of lysine in insulin chain B or on a synthetic substrate which trypsin and Pase-C acted on. Pase-A did not act on the synthetic substrates but cleaved the peptide bonds Glu-Ala and Ala-Leu of insulin. Leupeptin inhibition of the caseinolytic activity of both Pase-A and -B was similar to its inhibition of Pase-C. Phenylmethylsulfonyl fluoride and diisopropyl fluorophosphate strongly inhibited Pase-A, but no significant effect on the other enzymes was observed, suggesting that only Pase-A is a serine protease. The inhibitory characteristics of Pase-B and -C were very similar. Pase-A was not thiol dependent for enzyme activity, but Pase-B was strongly dependent, i.e., even more so than Pase-C. Pase-A inactivated the inhibitory activity of plasma alpha-1-antitrypsin, but the other two did not. These results show that P. gingivalis produces different types of proteases other than the trypsinlike protease generally reported.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Starkey P. M. The interaction of alpha 2-macroglobulin with proteinases. Characteristics and specificity of the reaction, and a hypothesis concerning its molecular mechanism. Biochem J. 1973 Aug;133(4):709–724. doi: 10.1042/bj1330709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E., Zambon J. J., Barwa P. K., Neiders M. E. Characterization of collagenolytic activity from strains of Bacteroides gingivalis. J Periodontal Res. 1988 Jul;23(4):258–264. doi: 10.1111/j.1600-0765.1988.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Herrmann B. F., Höfling J. F., Sundqvist G. K. Degradation of the human proteinase inhibitors alpha-1-antitrypsin and alpha-2-macroglobulin by Bacteroides gingivalis. Infect Immun. 1984 Feb;43(2):644–648. doi: 10.1128/iai.43.2.644-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Endo J., Otsuka M., Ohara E., Sato M., Nakamura R. Cleavage action of a trypsin-like protease from Bacteroides gingivalis 381 on reduced egg-white lysozyme. Arch Oral Biol. 1989;34(11):911–916. doi: 10.1016/0003-9969(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Isolation and characterization of a protease from Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):716–720. doi: 10.1128/iai.55.3.716-720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura S., Nakamura T. Purification and characterization of a 43-kDa protease of Bacteroides gingivalis. Oral Microbiol Immunol. 1990 Dec;5(6):360–362. doi: 10.1111/j.1399-302x.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., McBride B. C. Isolation of a membrane-associated Bacteroides gingivalis glycylprolyl protease. Infect Immun. 1987 Dec;55(12):3131–3136. doi: 10.1128/iai.55.12.3131-3136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kelleher P. J., Juliano R. L. Detection of proteases in polyacrylamide gels containing covalently bound substrates. Anal Biochem. 1984 Feb;136(2):470–475. doi: 10.1016/0003-2697(84)90246-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. Rapid identification of Bacteroides gingivalis. J Clin Microbiol. 1982 Feb;15(2):345–346. doi: 10.1128/jcm.15.2.345-346.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C., Edwards T., Jensen S. Characterization of Bacteroides asaccharolyticus and B. melaninogenicus oral isolates. Can J Microbiol. 1980 Oct;26(10):1178–1183. doi: 10.1139/m80-197. [DOI] [PubMed] [Google Scholar]
- Mitchell W. M., Harrington W. F. Purification and properties of clostridiopeptidase B (Clostripain). J Biol Chem. 1968 Sep 25;243(18):4683–4692. [PubMed] [Google Scholar]
- Otsuka M., Endo J., Hinode D., Nagata A., Maehara R., Sato M., Nakamura R. Isolation and characterization of protease from culture supernatant of Bacteroides gingivalis. J Periodontal Res. 1987 Nov;22(6):491–498. doi: 10.1111/j.1600-0765.1987.tb02060.x. [DOI] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. 2. The investigation of peptides from enzymic hydrolysates. Biochem J. 1951 Sep;49(4):481–490. doi: 10.1042/bj0490481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Otsuka M., Maehara R., Endo J., Nakamura R. Degradation of human secretory immunoglobulin A by protease isolated from the anaerobic periodontopathogenic bacterium, Bacteroides gingivalis. Arch Oral Biol. 1987;32(4):235–238. doi: 10.1016/0003-9969(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Schwabe C. A fluorescent assay for proteolytic enzymes. Anal Biochem. 1973 Jun;53(2):484–490. doi: 10.1016/0003-2697(73)90098-5. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Gharbia S. E., Kowlessur D., Wilkie E., Brocklehurst K. Isolation and characterization of gingivain, a cysteine proteinase from Porphyromonas gingivalis strain W83. Biochem Soc Trans. 1990 Aug;18(4):578–579. doi: 10.1042/bst0180578. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Gharbia S. E. Lysis of erythrocytes by the secreted cysteine proteinase of Porphyromonas gingivalis W83. FEMS Microbiol Lett. 1989 Oct 1;52(1-2):213–217. doi: 10.1016/0378-1097(89)90199-7. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J., Shuttleworth C. A. The degradation of type I collagen and human plasma fibronectin by the trypsin-like enzyme and extracellular membrane vesicles of Bacteroides gingivalis W50. Arch Oral Biol. 1988;33(5):323–329. doi: 10.1016/0003-9969(88)90065-9. [DOI] [PubMed] [Google Scholar]
- Smalley J. W., Birss A. J. Trypsin-like enzyme activity of the extracellular membrane vesicles of Bacteroides gingivalis W50. J Gen Microbiol. 1987 Oct;133(10):2883–2894. doi: 10.1099/00221287-133-10-2883. [DOI] [PubMed] [Google Scholar]
- Sorsa T., Uitto V. J., Suomalainen K., Turto H., Lindy S. A trypsin-like protease from Bacteroides gingivalis: partial purification and characterization. J Periodontal Res. 1987 Sep;22(5):375–380. doi: 10.1111/j.1600-0765.1987.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Suido H., Neiders M. E., Barua P. K., Nakamura M., Mashimo P. A., Genco R. J. Characterization of N-CBz-glycyl-glycyl-arginyl peptidase and glycyl-prolyl peptidase of Bacteroides gingivalis. J Periodontal Res. 1987 Sep;22(5):412–418. doi: 10.1111/j.1600-0765.1987.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Saito S. Use of n-octyl-beta-D-thioglucoside, a new nonionic detergent, for solubilization and reconstitution of membrane proteins. J Biochem. 1984 Nov;96(5):1593–1597. doi: 10.1093/oxfordjournals.jbchem.a134989. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Kinouchi T., Wakano Y., Ohnishi Y. Purification and characterization of a protease from Bacteroides gingivalis 381. Infect Immun. 1987 Feb;55(2):420–427. doi: 10.1128/iai.55.2.420-427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]