Abstract
Buprenorphine, an opioid with mixed agonist-antagonist activity at classical opioid receptors, has been approved recently for the treatment of opioid dependency. Buprenorphine is also used as an analgesic. The buprenorphine dose-response curve is sometimes submaximal, or even bell-shaped, in nociceptive assays, depending upon the nature and intensity of the noxious stimulus. Moreover, buprenorphine, when administered with full agonists, such as morphine, antagonizes the action of these drugs. Partial agonism at the mu opioid receptor and, in some cases, antagonism at the kappa or delta opioid receptor have been considered as possible underlying mechanisms for the ceiling effect and bell-shaped dose-response curve of buprenorphine. While ceiling effects can be explained by partial agonist activity of buprenorphine, the bell-shaped dose-response curve cannot be a consequence of this property of the drug. Recently, buprenorphine has been shown to activate the opioid receptor-like (ORL-1; also known as NOP) receptor. Supraspinal activation of the ORL-1 receptor counteracts the antinociceptive and rewarding actions of morphine, raising the possibility that these actions of buprenorphine can also be altered by its ability to concomitantly activate the ORL-1 receptor. The use of molecular biological techniques has advanced our knowledge regarding the role of opioid receptors in modulation of pain and reward. In particular, generation of opioid receptor knockout mice has proven useful in this regard. Indeed, using knockout mice, we have recently shown that the antinociceptive effect of buprenorphine mediated primarily by the mu opioid receptor is attenuated by the ability of the drug to activate the ORL-1 receptor. Thus, the goal of this review is to provide evidence demonstrating that the ORL-1 receptor plays a functional role not only in the antinociceptive effect of buprenorphine but also in other actions of the drug as well.
Keywords: Buprenorphine, partial agonist, agonist-antagonist, antinociception, tolerance, dependence, ORL-1 receptors, knockout mice
INTRODUCTION
Buprenorphine, an oripavine derivative, is used clinically for pain management [21,74]. This opioid analgesic, which was the focus of many clinical trials for the treatment of opioid dependency [32,47,49,50,59], even in opiate addicts with a history of cocaine co-abuse [72,85,86], has recently been approved for the treatment of opioid dependency [30]. However, the mechanisms of action of buprenorphine are not fully understood. Martin and colleagues [56] originally described buprenorphine as a partial agonist at the mu opioid receptor. Subsequent studies showed that buprenorphine can also bind to kappa and delta opioid receptors (Tables 1 and 2). The existing data show that buprenorphine displays a 10-fold lower affinity for the delta as compared to the mu and kappa opioid receptors (Table 1) [see also, 31,71,83,84,98, 99]. Moreover, recent data demonstrate that buprenorphine, at low doses, blocks epsilon receptors as well [62]. Overall, the body of literature suggests that buprenorphine is an opioid with unique and complex pharmacology, i.e., it can act as an agonist and/or antagonist at different classes of opioid receptors [31,71,79,83,84,98,99].
Table 1.
Apparent Ki Values of Buprenorphine for the Various Members of the Opioid Receptor Family. Data are Mean ± s.e.m. (nM).
Table 2.
EC50 Values and Maximal Response of Buprenorphine in Stimulating [35S]GTPγS Binding to Membranes of CHO Cells Transfected with Various Members of the Opioid Receptor Family. Data are Mean ± s.e.m.
| Mu | Delta | Kappa | ORL-1 | |
|---|---|---|---|---|
| EC50 | 0.08 ± 0.01 nM | NS | 0.04 ± 0.01 nM | 35 ± 30 nM |
| Percent Stimulation | (38 ± 8%) | NS | (10 ± 4%) | (60 ± 10%) |
[31]; N.S., no stimulation
Soon after cloning of the delta opioid receptor [20,41], kappa and mu opioid receptors were cloned [7,106,108]. In 1994, several laboratories described a fourth receptor clone that showed approximately 65% homology, at the transmembrane domains, to the classical opioid receptors [5,6,22,66,102]. Despite the similarities, most opioid receptor ligands do not interact with this receptor which has distinct pharmacological characteristics [for reviews, see 60,65]. Therefore, the term opioid receptor-like (ORL-1) receptor was coined and the ORL-1 receptor was considered as the fourth member of the opioid receptor family.
Interestingly, this fourth member of the opioid receptor family is also coupled to the same second messenger systems [9,10,22,42,57,61,66,70,78,97]. Thus, activation of the ORL-1 receptor leads to inhibition of the enzyme adenylate cyclase [61,78] and calcium channel conductance [10,42,57]. Stimulation of the ORL-1 receptor also activates inwardly rectifying potassium channels [9,97].
In 1995, two independent laboratories isolated orphan in FQ (also known as nociceptin; OFQ/N) as the endogenous ligand of the ORL-1 receptor [61,78]. Interestingly, OFQ/N, a 17 amino acid peptide, is structurally similar to the endogenous opioid peptides, in particular to dynorphin A [33,61]. Paradoxically, supraspinal OFQ/N administration induces hyperalgesia [55,61,78] or, at least, blocks the antinociceptive effect of mu, delta and kappa opioid receptor agonists [64,67,92]. OFQ/N was accordingly proposed as an anti-opioid peptide in the brain [63]. However, OFQ/N can also exert modulatory actions similar to that of opioids on nociception depending on the behavioral state of the animal [73]. Furthermore, OFQ/N can exert actions similar to or opposite from mu and kappa opioid receptor agonists depending on which neurons are influenced by the peptide [73]. At the spinal level, OFQ/N produces effects ranging from allodynia to antinociception [for review, see 65].
Buprenorphine has recently been shown to interact with the ORL-1 receptor (Table 1) [2,26,31,53,105]. For example, we have shown that buprenorphine causes activation of the mitogen-activated protein kinase with similar efficacy but lower potency than OFQ/N in Chinese Hamster Ovary (CHO) cells expressing ORL-1 receptors [53]. Since activation of ORL-1 receptors leads to counter-opioid actions in the brain, the opioid receptor-mediated actions of buprenorphine could be altered by the ability of the drug to co-activate ORL-1 receptors. The aim of this review is therefore to provide evidence demonstrating the role of ORL-1 receptors in buprenorphine’s actions.
1. THE ROLE OF ORL-1 RECEPTORS IN THE ANTINOCICEPTIVE EFFECT OF BUPRENORPHINE
Agonistic actions of buprenorphine at mu and kappa opioid receptors have been proposed as the underlying mechanism for the antinociceptive effect of the drug [12,75,94]. However, these earlier studies did not use selective kappa opioid receptor ligands to identify the contribution of the kappa opioid receptor in buprenorphine-induced antinociception [46,75,94]. Given the fact that buprenorphine fails to produce antinociception in the presence of selective mu opioid receptor antagonists [36,62] or in mu opioid receptor deficient CXBK mice [37], the role of the kappa opioid receptor in the antinociceptive effect of buprenorphine remains controversial. The use of molecular biological techniques has advanced our knowledge regarding the role of opioid receptors in modulating pain and reward. In particular, generation of opioid receptor knockout mice has proven useful in this regard. Indeed, we have recently shown that buprenorphine produces no antinociceptive effect in mu opioid receptor knockout mice [53], providing further support for the notion that the action of buprenorphine is primarily mediated by the mu opioid receptor. Although one could still argue that the presence of mu opioid receptors may be necessary for the antinociceptive effect of buprenorphine through activation of kappa opioid receptors, it is of interest to note that the antinociceptive effect of U50,488H, a kappa opioid receptor agonist, is not altered in mice lacking the mu opioid receptor [58].
Despite the fact that buprenorphine is a potent analgesic, at higher doses the antinociceptive effect of the drug sometimes reaches a plateau without producing a maximal response, i.e., it produces a submaximal antinociceptive effect [12,18,51]. Partial agonism at the mu opioid receptor is thought to be the underlying mechanism for this action of buprenorphine [27,46]. In addition, the term “auto-inhibition” is used to describe the submaximal antinociceptive effect of buprenorphine, i.e., the mu and kappa opioid receptor-mediated actions of buprenorphine are inhibited by the ability of the drug to interact with the delta opioid receptor [83,84]. The majority of available data shows that buprenorphine acts as a delta opioid receptor antagonist. Since delta opioid receptor antagonists do not decrease the antinociceptive effect of mu opioid receptor agonists, other mechanism(s) may be involved. As stated above, the ORL-1 and classical opioid receptor systems represent two opposing mechanisms of pain modulation [55]; thus, it was proposed that the auto-inhibitory mechanism occurs through activation of the ORL-1 receptor, i.e., the mu opioid receptor-mediated antinociceptive effect of buprenorphine is attenuated by its ability to concomitantly activate the ORL-1 receptor. Consistent with this hypothesis, the antinociceptive effect of buprenorphine is greater in mice lacking the ORL-1 receptor [53], whereas the antinociceptive effect of morphine, which does not interact with the ORL-1 receptor [53], is not altered in these mice [53,96]. Thus, it is possible that the antinociceptive efficacy of buprenorphine is modified by concomitant activation of ORL-1 receptors. Given the problems associated with the use of knockout mice, the increased efficacy of buprenorphine in ORL-1 receptor knockout mice might be due to compensatory changes that could occur during development in these animals. However, we have recently shown that the antinociceptive effect of buprenorphine is also enhanced in the presence of J-113397, an ORL-1 receptor antagonist [38], in wild type mice, but not in mice lacking the ORL-1 receptor, which further supports the notion that the antinociceptive efficacy of buprenorphine is altered by its ability to co-activate the ORL-1 receptor and not because of compensatory developmental changes. Thus, activation of the ORL-1 receptor contributes to the ceiling effect of buprenorphine [53].
Previous studies have shown that buprenorphine not only produces a submaximal antinociceptive effect but, in some cases, is associated with a bell-shaped dose-response curve. It is of interest to note that the curvilinear antinociceptive dose-response relation for buprenorphine is only obtained in tests utilizing high intensity noxious stimuli [53]. For example, the dose-response relation for buprenorphine in rats resembles an inverted U in the 50°C tail withdrawal test or in the early phase of the formalin test. In contrast, buprenorphine gives a sigmoidal dose-response curve against the lower stimulus associated with the late phase of the formalin test [104]. Although one may relate the ceiling effect of buprenorphine to the partial agonist activity of the drug at the mu opioid receptor, the bell-shaped dose-response curve cannot be explained by this property of the drug. Indeed, we have recently shown that the bell-shaped dose-response curve of buprenorphine can also be blocked by inhibition of the ORL-1 receptor [53]. Thus, activation of the ORL-1 receptor may also contribute to the bell-shaped dose-response relationship of buprenorphine.
2. THE ROLE OF ORL-1 RECEPTORS IN THE ABILITY OF BUPRENORPHINE TO BLOCK THE ANTINOCICEPTIVE EFFECT OF FULL AGONISTS
Buprenorphine produces not only a submaximal antinociceptive effect [12,18,51], but also attenuates the action of morphine and other full agonists. In some cases, buprenorphine totally abolishes the action of full agonists [12,45]. The underlying mechanism for this antagonism remains elusive. Previous studies have used the term “partial agonism” at mu opioid receptors [12,18] or antagonism at the kappa opioid receptor [45] to describe the antagonistic action of buprenorphine. Since intracerebroventricular OFQ/N administration blocks the antinociceptive effect of morphine and other opioid analgesics [for review, see 65], we hypothesized that buprenorphine also inhibits the action of morphine via activation of the ORL-1 receptor. Indeed, our preliminary data demonstrate that buprenorphine attenuates the antinociceptive effect of morphine in wild type mice to a significantly (p<0.05) greater degree than in ORL-1 receptor knockout mice (Fig. 1).
Fig. (1).
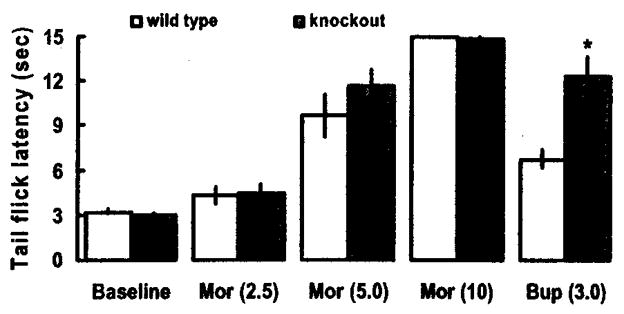
Buprenorphine attenuates morphine-induced antinociception in wild type, but not ORL-1 receptor knockout, mice in the radiant heat tail flick assay. Mice were tested for baseline tail flick latency, injected with morphine (2.5 mg/kg, s.c.) and tested 30 min later. The same mice were injected with the next dose of morphine (5 mg/kg,) and tested 30 min later. After the tail flick latency was measured 30 min following the last dose of morphine (10 mg/kg, s.c.), mice were injected with buprenorphine (3 mg/kg, s.c.) and tested after a further 15-min delay. Data are means (± s.e.m.) of 5–6 mice/genotype.
*indicates a significant difference between wild type (WT) and knockout (KO) mice (t = 4.07; p<0.05).
3. THE ROLE OF ORL-1 RECEPTORS IN TOLERANCE TO BUPRENORPHINE
Opioid analgesics are used widely for the treatment of moderate to severe pain. The clinical usefulness of these drugs is often hampered by the development of tolerance after chronic treatment [13,103]. Among others, a proposed mechanism of tolerance is increased activity of the so-called anti-opiate peptides in the brain [23,25]. Regarding the role of OFQ/N in opioid tolerance, a recent study showed increased levels of OFQ/N in cerebroventricular perfusate, periaqueductal gray and amygdala from morphine-tolerant rats [107]. On this basis, it has been proposed that continuous administration of morphine accelerates the biosynthesis and/or release of OFQ/N to antagonize the effect of morphine, thereby contributing to the phenomenon of tolerance. In support of this notion, intracerebroventricular injection of an antibody raised against OFQ/N partially reversed the expression of morphine tolerance [93]. Moreover, morphine tolerance is partially inhibited in mice lacking the ORL-1 receptor [96].
Although tolerance to the antinociceptive effect of buprenorphine has been demonstrated, the onset is slower than tolerance to morphine [12]. Indeed, an early clinical report suggested that there was no need to escalate the dose of buprenorphine for patients with chronic pain for several weeks to months of treatment [27]. Recently, concomitant OFQ/N treatment with morphine was shown to attenuate the development of morphine tolerance in rats [54], raising the possibility that tolerance to buprenorphine can be altered by its ability to concomitantly activate the ORL-1 receptor. Thus, we determined the development of tolerance to the antinociceptive action of buprenorphine in mice lacking the ORL-1 receptor. Mice were injected with saline or buprenorphine (1 mg/kg, s.c.) once daily for 4 days and tested 24 h later. On day 5, mice were tested for baseline tail flick latency, injected with buprenorphine (1 mg/kg, s.c.), and re-tested 15 min later. Two-factor analysis of variance revealed a main effect of genotype (F1,19 = 3.82; p<0.05), and a main effect of treatment (F1,19 = 12.33; p<0.05). Post-hoc analysis of the data showed that buprenorphine elicited a significantly (p<0.05) greater antinociceptive effect in mice lacking the ORL-1 receptor (Fig. 2; compare WT/SAL vs. KO/SAL). Whereas limited and non-significant (p>0.05) tolerance occurred in wild type mice after repeated buprenorphine treatment (compare WT/SAL vs. WT/BUP), the antinociceptive effect of the drug was significantly (p<0.05) reduced in mice lacking the ORL-1 receptor (Fig. 2; compare KO/SAL vs. KO/BUP). Thus, activation of the ORL-1 receptor may also contribute to the limited tolerance associated with buprenorphine. It is unclear, however, whether activation of the ORL-1 receptor modulates the development and/or expression of tolerance. One plausible explanation would be that tolerance develops to the mu opioid receptor-mediated action of buprenorphine; however, it is confounded by a reduced antinociceptive effect of the drug (floor effect), due to activation of the ORL-1 receptor, in wild type mice.
Fig. (2).
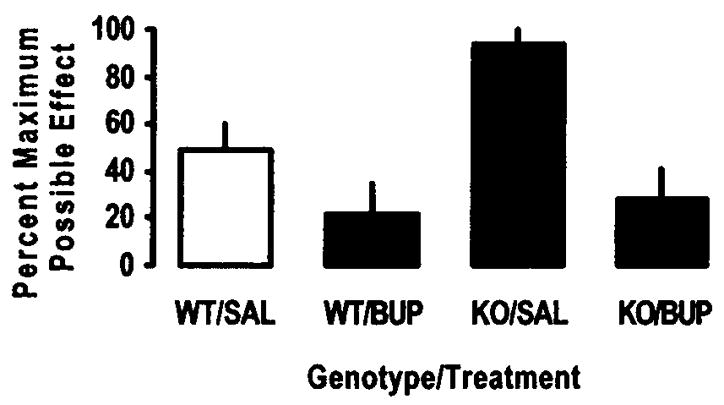
Tolerance develops to the antinociceptive effect of buprenorphine in mice lacking the ORL-1 receptor in the radiant heat tail flick assay. Mice were injected with either saline or buprenorphine (1 mg/kg, s.c.) once daily for 4 days. On day 5, mice were tested for baseline tail flick latency, injected with buprenorphine (1 mg/kg, s.c.) and re-tested after 15 min. Percent maximum possible effect (%MPE) was obtained using the formula: %MPE = [(Test latency − Baseline) / (Cut-off (15 sec) − Baseline)] × 100. Data are means (+ s.e.m.) of 5–7 mice/treatment/group.
4. THE ROLE OF ORL-1 RECEPTORS IN LIMITED DEPENDENCE LIABILITY OF BUPRENORPHINE
Buprenorphine has been under extensive investigation for the treatment of opioid dependency [32,48,59,72,85]. Chronic use of opioid analgesics can lead to a state of drug dependency, manifested by abstinence or antagonist-precipitated withdrawal [13]. Buprenorphine is associated with a limited physical dependence capacity in humans and laboratory animals [56,100]. Buprenorphine also has the ability to either block naloxone-precipitated withdrawal (jumping) in morphine-dependent mice [46,76] or to elicit jumping in mice implanted with morphine pellets [11].
Buprenorphine dissociates extremely slowly from its receptor [76]. The partial agonist activity at the mu opioid receptor [56] along with its slow dissociation rate may be rate-limiting factors in dependence aspects involving buprenorphine. Recent evidence, however, suggests that the ORL-1 receptor may also be involved in drug dependency [39,44]. Intracerebroventricular OFQ/N administration, for example, blocks wet-dog shakes induced by naloxone in morphine-dependent rats [44]. Furthermore, naloxone-precipitated withdrawal jumping is significantly increased in OFQ/N transgenic knockout mice [39]. Since buprenorphine interacts with the ORL-1 receptor, it is also possible that the dependence liability of the drug would be altered in mice lacking the ORL-1 receptor or if the drug is administered in the presence of an ORL-1 receptor antagonist. Thus, further studies are needed to determine whether activation of the ORL-1 receptor contributes to the limited dependence liability of buprenorphine and in the ability of the drug to block signs of naloxone-precipitated withdrawal in opioid-dependent rodents.
5. THE ROLE OF ORL-1 RECEPTORS IN THE REWARDING ACTION OF BUPRENORPHINE
Opioids exert their motor stimulatory and rewarding effects by facilitating neurotransmission along the mesolimbic dopaminergic reward circuitry [for review, see 43]. For example, it has been demonstrated that mu and delta opioid receptor agonists increase, while kappa opioid receptor agonists decrease, extracellular dopamine in the nucleus accumbens [14,91]. The action of OFQ/N is somewhat similar to that of kappa opioid receptor agonists. Thus, intracerebroventricular OFQ/N administration decreases basal [14,69] and cocaine-induced elevation of extracellular dopamine in the nucleus accumbens in anesthetized rats [52]. Moreover, OFQ/N attenuates morphine-induced increases in extracellular dopamine [15,16] and blocks acquisition of conditioned place preference induced by morphine [8,68]. Importantly, buprenorphine increases extracellular dopamine and elicits rewarding actions in a bell-shaped manner [3], suggesting that the mu opioid receptor-mediated actions of buprenorphine can be compromised by its agonist activity at the ORL-1 receptor. Further studies are required to determine the role of ORL-1 receptors in the rewarding actions of buprenorphine.
Opioids and other drugs of abuse, under certain conditions, produce behavioral sensitization, a phenomenon that is thought to be the basis for drug craving [for reviews, see 81,82]. The phenomenon of behavioral sensitization or “reverse tolerance” refers to an increase in motor stimulatory and stereotypic behaviors of stimulant drugs [35,80,87]. Psychomotor stimulant drugs, such as cocaine and amphetamine, as well as mu opioid receptor agonists can elicit behavioral sensitization [1,34,90]. Kappa opioid receptor agonists, on the other hand, due to their inhibitory action on dopamine release and motor activity, block behavioral sensitization induced by psychostimulants [28,29,88]. Moreover, norbinaltorphimine, a kappa opioid receptor antagonist, has been shown to act synergistically with morphine to induce a greater degree of sensitization [90]. Our preliminary data show that buprenorphine produces its motor stimulatory action primarily through the mu opioid receptor (Lutfy and Kieffer, unpublished data). Repeated treatment with buprenorphine produces a sensitized response to a subsequent challenge dose of the drug given on day 11 in wild type mice. However, buprenorphine fails to produce sensitization in mice lacking the mu opioid receptor (Lutfy and Kieffer, unpublished data). As stared above, the action of OFQ/N on the dopamine system is opposite to that of mu and delta opioid receptor agonists but somewhat similar to kappa opioid receptor agonists. Thus, it is possible to observe an increase in motor activity and a greater behavioral sensitization after repeated buprenorphine treatment in ORL-1 receptor knockout mice. Similarly since the rewarding action of morphine is also mediated through the mu opioid receptor [for review, see 40], it is possible that the morphine-like rewarding action of buprenorphine win be enhanced in ORL-1 receptor knockout mice.
PRECLINICAL PHARMACOLOGY AND CLINICAL EXPERIENCE
Much has been written about the shape and nature of the buprenorphine dose-response curve, obtained in certain preclinical nociceptive assays [19,104], The many confirmatory reports of inverted U-shaped curves with buprenorphine have heightened interest in this unusual compound over the past 30 years. Theories on the receptor pharmacology of buprenorphine have appeared in parallel with technological advances and have accompanied the passage of this analgesic from a Laboratory mouse to human addict and patient in pain. In retrospect, the buprenorphine curvilinear dose-response relation has turned out to be a rodent phenomenon. From a practical point of view, concern over possible submaximal analgesic effects with buprenorphine in humans with moderate to severe pain has not been realized. However, a ceiling effect for respiratory depression has been described for increasingly higher doses of sublingual buprenorphine in humans [101]. This frequently quoted result, which was attributed to pharmacodynamic rather than to pharmacokinetic factors, underscores the encouraging safety profile of buprenorphine. Additionally, this result is in line with buprenorphine data on blood gases (pCO2 values) obtained from conscious rats [17].
Little attention has been paid to the possible role of buprenorphine metabolites in explanations of the biphasic dose-response curve. Norbuprenorphine, the major N-dealkylated metabolite of buprenorphine, has its own distinct profile at opioid receptors, including full agonism but low potency at the ORL-1 receptor (EC50 > 1 μM in both stimulation of [35S]GTPγS binding and inhibition of forskolin-stimulated adenylate cyclase) [31]. In the mouse writhing assay, sigmoidal dose-response curves were associated with buprenorphine; and with norbuprenorphine. Both compounds had comparable antinociceptive efficacy after subcutaneous administration, with buprenorphine being about 3 times more potent than the metabolite. That the accumulation of norbuprenorphine might influence the receptor kinetics of buprenorphine in certain laboratory test procedures [95] is currently being revisited, prompted by the assumption that norbuprenorphine contributes to the overall in vivo pharmacology of buprenorphine.
The unique receptor profile of buprenorphine, together with high potency and such physicochemical characteristics as high lipophilicity and low molecular weight have recently triggered a new way in which this analgesic may be used in pain management. Thus, transdermal formulations of buprenorphine are now available [4,89] or under development [24.77]. These patches provide consistent buprenorphine delivery for 3 or 7 days and hold promise for improved ease of use and patient compliance in a variety of severe and chronic pain-related indications.
CONCLUSION
Buprenorphine is used clinically for pain management and has recently been approved for the treatment of opioid dependency. However, the mechanisms of action of buprenorphine are not fully understood. Recent data suggest that the action of buprenorphine at the ORL-1 receptor could be responsible for the ceiling effect and for the bell-shaped dose-response curves observed after administration of the drug in the radiant heat tail flick assay. Since activation of the ORL-1 receptor blocks the rewarding actions of morphine, the ability of buprenorphine to curb drug dependency may also stem from its interaction with ORL-1 receptors.
References
- 1.Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization) to the excitatory effects of morphine. A motility study in post-depentent rats. Neuropharmacology. 1983;22:1193–1196. doi: 10.1016/0028-3908(83)90080-1. [DOI] [PubMed] [Google Scholar]
- 2.Bloms-Funke P, Gillen C, Schuettler AJ, Wnendt S. Agonistic effects of the Opioid buprenorphine on the nociceptin/OFQ receptor. Peptides. 2000;21:1141–1146. doi: 10.1016/s0196-9781(00)00252-7. [DOI] [PubMed] [Google Scholar]
- 3.Brown EE, Finlay JM, Wong JT, Damsma G, Fibiger HC. Behavioral and neurochemical interactions between cocaine and buprenorphine: implications for the pharmacotheraphy of cocaine abuse. J Pharmacol Exp Ther. 1991;256:119–126. [PubMed] [Google Scholar]
- 4.Budd K, Collett BJ. Old dog--new (ma)trix. Br J Anaesth. 2003;90:722–724. doi: 10.1093/bja/aeg133. [DOI] [PubMed] [Google Scholar]
- 5.Bunzow JR, Snez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a mu, delta or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-Opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12. [PubMed] [Google Scholar]
- 8.Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- 9.Connor M, Vaughan CW, Chieng B, Christie MJ. Nociceptin receptor coupling to a potassium conductance in rat locus coeruleus neurones in vitro. Br J Pharmacol. 1996;119:1614–1618. doi: 10.1111/j.1476-5381.1996.tb16080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor M, Yeo A, Henderson G. The effect of nociceptin on Ca2+ channel current and intracellular Ca2+ in the SH-SY5Y human neuroblastoma cell line. Br J Pharmacol. 1996;118:205–207. doi: 10.1111/j.1476-5381.1996.tb15387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan A. Use of the mouse jumping test for estimating antagonistic potencies of morphine antagonists. J Pharm Pharmacol. 1976;28:177–182. doi: 10.1111/j.2042-7158.1976.tb04126.x. [DOI] [PubMed] [Google Scholar]
- 12.Cowan A, Lewis JW, Macfarlane IR. Antagonistic and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cox BM. Drug tolerance and physical dependence. In: Pratt WB, Taylor P, editors. Principals of drug action: the basis of pharmacology. Churchill Livingstone, Inc; New York, NY: 1990. pp. 639–690. [Google Scholar]
- 14.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- 15.Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- 16.Di Giannuario A, Pieretti S, Catalani A, Loizzo A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- 17.Doxey JC, Everitt JE, Frank LW, MacKenzie JE. A comparison of the effects of buprenorphine and morphine on the blood gases of conscious rats. Br J Pharmacol. 1982;75:118. [Google Scholar]
- 18.Dum J, Blasig J, Herz A. Buprenorphine: demonstration of physical dependence liability. Eur J Pharmacol. 1981;70:293–300. doi: 10.1016/0014-2999(81)90163-1. [DOI] [PubMed] [Google Scholar]
- 19.Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 21.Finco G, Polati E, Gottin L, Bartoloni A, Milan B, Zanoni L, Valle L. Intravenous patient-controlled analgesia (PCA) in the treatment of postoperative pain: rationale and clinical application. Chir Ital. 1995;47:20–25. [PubMed] [Google Scholar]
- 22.Fukuda K, Kato S, Mori K, Nishi M, Takeshima H, Iwabe N, Miyata T, Houtani T, Sugimoto T. cDNA cloning and regional distribution of a novel member of the opioid receptor family. FEBS Lett. 1994;343:42–46. doi: 10.1016/0014-5793(94)80603-9. [DOI] [PubMed] [Google Scholar]
- 23.Goodman CB, Heyliger S, Emilien B, Partilla JS, Yang HY, Lee CH, Cadet JL, Rothman RB. Regulation of mu binding sites after chronic administration of antibodies directed against specific anti-opiate peptides. Peptides. 1998;19:1703–1709. doi: 10.1016/s0196-9781(98)00121-1. [DOI] [PubMed] [Google Scholar]
- 24.Hale ME, Karpow SA, Miller J, Zalman MA, Spyker DA. Dose proportionality and the dose response of buprenorphine transdermal system in patients with chronic pain. J Clin Pharmacol. 2001;41:027. [Google Scholar]
- 25.Harrison LM, Kastin AJ, Zadina JE. Opiate tolerance and dependence: receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–1630. doi: 10.1016/s0196-9781(98)00126-0. [DOI] [PubMed] [Google Scholar]
- 26.Hawkinson JE, Acosta-Burruel M, Espitia SA. Opioid activity profiles indicate similarities between the nociceptin/orphanin FQ and opioid receptors. Eur J Pharmacol. 2000;389:107–114. doi: 10.1016/s0014-2999(99)00904-8. [DOI] [PubMed] [Google Scholar]
- 27.Heel RC, Brogden RN, Speight TM, Avery GS. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1979;17:81–110. doi: 10.2165/00003495-197917020-00001. [DOI] [PubMed] [Google Scholar]
- 28.Heidbreder CA, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: influence of kappa opioid receptor agonists. J Pharmacol Exp Ther. 1995;275:150–163. [PubMed] [Google Scholar]
- 29.Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- 30.Heit HA, Gourlay DL. Office-based treatment of opiate addiction. N Engl J Med. 2003;349:2567–2568. doi: 10.1056/NEJM200312253492619. [DOI] [PubMed] [Google Scholar]
- 31.Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- 32.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–516. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 33.Julius D. Pharmacology. Home for an orphan endorphin. Nature. 1995;377:476. doi: 10.1038/377476a0. [DOI] [PubMed] [Google Scholar]
- 34.Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J Neurochem. 1988;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 35.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 36.Kamei J, Saitoh A, Suzuki T, Misawa M, Nagase H, Kasuya Y. Buprenorphine exerts its antinociceptive activity via mu I-opioid receptors. Life Sci. 1995;56:PL285–290. doi: 10.1016/0024-3205(95)00078-x. [DOI] [PubMed] [Google Scholar]
- 37.Kamei J, Sodeyama M, Tsuda M, Suzuki T, Nagase H. Antinociceptive effect of buprenorphine in muI-opioid receptor deficient CXBK mice. Life Sci. 1997;60:PL333–337. doi: 10.1016/s0024-3205(97)00170-7. [DOI] [PubMed] [Google Scholar]
- 38.Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: I-[(3R, 4R)-1 - cyclooctylmethyl - 3- hydroxymethyl-4-piperidyl]-3-ethyl-l, 3 - dihydro - 2H - benzimidazol - 2-one (J-113397) J Med Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- 39.Kest B, Hopkins E, Palmese CA, Chen ZP, Mogil JS, Pintar JE. Morphine tolerance and dependence in nociceptin/orphanin FQ transgenic knock-out mice. Neuroscience. 2001;104:217–222. doi: 10.1016/s0306-4522(01)00037-9. [DOI] [PubMed] [Google Scholar]
- 40.Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 41.Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. The delta-opioid receptor: isolation of a cDNA by expression cloning and pharmacological characterization. Proc Natl Acad Sci USA. 1992;89:12048–12052. doi: 10.1073/pnas.89.24.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knoflach F, Reinscheid RK, Civelli O, Kemp JA. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J Neurosci. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 44.Kotlinska J, Suder P, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin inhibits morphine withdrawal. Life Sci. 2000;66:PL119–123. doi: 10.1016/s0024-3205(99)00648-7. [DOI] [PubMed] [Google Scholar]
- 45.Leander JD. Buprenorphine is a potent kappa-opioid receptor antagonist in pigeons and mice. Eur J Pharmacol. 1988;151:457–461. doi: 10.1016/0014-2999(88)90543-2. [DOI] [PubMed] [Google Scholar]
- 46.Lewis JW. Buprenorphine. Drug Alcohol Depend. 1985;14:363–372. doi: 10.1016/0376-8716(85)90067-5. [DOI] [PubMed] [Google Scholar]
- 47.Ling W, Charuvastra C, Collins JF, Batki S, Brown LS, Jr, Kintaudi P. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 48.Ling W, Rawson RA, Compton MA. Substitution pharmacotherapies for opioid addiction: from methadone to LAAM and buprenorphine. J Psychoactive Drugs. 1994;26:119–128. doi: 10.1080/02791072.1994.10472259. [DOI] [PubMed] [Google Scholar]
- 49.Ling W, Wesson DR, Charuvastra C, Klett CJ. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53:401–407. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- 50.Litten RZ, Allen JP. Medications for alcohol, illicit drug, and tobacco dependence. An update of research findings. J Subst Abuse Treat. 1999;16:105–112. doi: 10.1016/s0740-5472(98)00028-2. [DOI] [PubMed] [Google Scholar]
- 51.Lizasoain I, Leza JC, Lorenzo P. Buprenorphine: bell-shaped dose-response curve for its antagonist effects. Gen Pharmacol. 1991;22:297–300. doi: 10.1016/0306-3623(91)90452-c. [DOI] [PubMed] [Google Scholar]
- 52.Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lutfy K, Eitan S, Bryant CD, Yang YC, Saliminejad N, Walwyn W, Kieffer BL, Takeshima H, Carroll FI, Maidment NT, Evans CJ. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J Neurosci. 2003;23:10331–10337. doi: 10.1523/JNEUROSCI.23-32-10331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lutfy K, Hossain SM, Khaliq I, Maidment NT. Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br J Pharmacol. 2001;134:529–534. doi: 10.1038/sj.bjp.0704279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutfy K, Maidment NT. Blockade of mu-opioid receptors reveals the hyperalgesic effect of orphanin FQ/nociceptin in the rat hot plate test. Br J Pharmacol. 2000;131:1684–1688. doi: 10.1038/sj.bjp.0703746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- 57.Matthes H, Seward EP, Kieffer B, North RA. Functional selectivity of orphanin FQ for its receptor coexpressed with potassium channel subunits in Xenopus laevis oocytes. Mol Pharmacol. 1996;50:447–450. [PubMed] [Google Scholar]
- 58.Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, Denavit-Saubie M, Severini C, Negri L, Roques BP, Maldonado R, Kieffer BL. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mello NK, Mendelson JH. Buprenorphine treatment of cocaine and heroin abuse. In: Cowan A, Lewis JW, editors. Buprenorphine: combating drug abuse with a unique opioid. Wiley-Liss; New York: 1995. pp. 241–287. [Google Scholar]
- 60.Meunier JC. Nociceptin/orphanin FQ and the opioid receptor-like ORL1 receptor. Eur J Pharmacol. 1997;340:1–15. doi: 10.1016/s0014-2999(97)01411-8. [DOI] [PubMed] [Google Scholar]
- 61.Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 62.Mizoguchi H, Spaulding A, Leitermann R, Wu HE, Nagase H, Tseng LF. Buprenorphine blocks epsilon- and micro-opioid receptor-mediated antinociception in the mouse. J Pharmacol Exp Ther. 2003;306:394–400. doi: 10.1124/jpet.103.048835. [DOI] [PubMed] [Google Scholar]
- 63.Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- 64.Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci Lett. 1996;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- 65.Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- 66.Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 67.Morgan MM, Grisel JE, Robbins CS, Grandy DK. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport. 1997;8:3431–3434. doi: 10.1097/00001756-199711100-00003. [DOI] [PubMed] [Google Scholar]
- 68.Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- 69.Murphy NP, Ly HT, Maidment NT. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- 70.Narita M, Mizoguchi H, Oji DE, Dun NJ, Hwang BH, Nagase H, Tseng LF. Identification of the G-protein-coupled ORL1 receptor in the mouse spinal cord by [35S]-GTPgammaS binding and immunohistochemistry. Br J Pharmacol. 1999;128:1300–1306. doi: 10.1038/sj.bjp.0702907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Negus SS, Bidlack JM, Mello NK, Furness MS, Rice KC, Brandt MR. Delta opioid antagonist effects of buprenorphine in rhesus monkeys. Behav Pharmacol. 2002;13:557–570. doi: 10.1097/00008877-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Oliveto AH, Feingold A, Schottenfeld R, Jatlow P, Kosten TR. Desipramine in opioid-dependent cocaine abusers maintained on buprenorphine vs methadone. Arch Gen Psychiatry. 1999;56:812–820. doi: 10.1001/archpsyc.56.9.812. [DOI] [PubMed] [Google Scholar]
- 73.Pan Z, Hirakawa N, Fields HL. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron. 2000;26:515–522. doi: 10.1016/s0896-6273(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 74.Picard PR, Tramer MR, McQuay HJ, Moore RA. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain. 1997;72:309–318. doi: 10.1016/s0304-3959(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 75.Pick CG, Peter Y, Schreiber S, Weizman R. Pharmacological characterization of buprenorphine, a mixed agonist-antagonist with kappa 3 analgesia. Brain Res. 1997;744:41–46. doi: 10.1016/s0006-8993(96)01069-4. [DOI] [PubMed] [Google Scholar]
- 76.Rance MJ. Animal and molecular pharmacology of mixed agonist-antagonist analgesic drugs. Br J Clin Pharmacol. 1979;7:281S–286S. doi: 10.1111/j.1365-2125.1979.tb04701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reidenberg BA, El-Tahtawy A, Munera C, Cipriano A, Tonelli A, Reder R. Absolute bioavailability of a novel buprenorphine transdermal system (BTDS) applied for 7 days. J Clin Pharmacol. 2001;41:1026. [Google Scholar]
- 78.Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 79.Robinson SE. Buprenorphine: an analgesic with an expanding role in the treatment of opioid addiction. CNS Drug Rev. 2002;8:377–390. doi: 10.1111/j.1527-3458.2002.tb00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- 81.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 82.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95:S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 83.Sadee W, Richards ML, Grevel J, Rosenbaum JS. In vivo characterization of four types of opioid binding sites in rat brain. Life Sci. 1983;33:187–189. doi: 10.1016/0024-3205(83)90474-5. [DOI] [PubMed] [Google Scholar]
- 84.Sadee W, Rosenbaum JS, Herz A. Buprenorphine: differential interaction with opiate receptor subtypes in vivo. J Pharmacol Exp Ther. 1982;223:157–162. [PubMed] [Google Scholar]
- 85.Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993;34:66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- 86.Schottenfeld RS, Pakes JR, Oliveto A, Ziedonis D, Kosten TR. Buprenorphine vs methadone maintenance treatment for concurrent opioid dependence and cocaine abuse. Arch Gen Psychiatry. 1997;54:713–720. doi: 10.1001/archpsyc.1997.01830200041006. [DOI] [PubMed] [Google Scholar]
- 87.Segal DS, Schuckitt MA. Animal models of stimulant-induced psychosis. In: Creese I, editor. Stimulants: Neurochemical, Behavioral and Clinical Perspectives. Raven Press; New York: 1983. pp. 131–167. [Google Scholar]
- 88.Shippenberg TS, Lefevour A, Heidbreder C. Kappa-opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 89.Sittl R, Griessinger N, Likar R. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther. 2003;25:150–168. doi: 10.1016/s0149-2918(03)90019-1. [DOI] [PubMed] [Google Scholar]
- 90.Spanagel R. Modulation of drug-induced sensitization processes by endogenous opioid systems. Behav Brain Res. 1995;70:37–49. doi: 10.1016/0166-4328(94)00176-g. [DOI] [PubMed] [Google Scholar]
- 91.Spanagel R, Herz A, Shippenberg TS. Opposing tonic-ally active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc, Natl Acad Sci USA. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, Han JS. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian JH, Zhang W, Fang Y, Xu W, Grandy DK, Han JS. Endogenous orphanin FQ: evidence for a role in the modulation of electroacupuncture analgesia and the development of tolerance to analgesia produced by morphine and electroacupuncture. Br J Pharmacol. 1998;124:21–26. doi: 10.1038/sj.bjp.0701788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol. 1980;69:503–512. doi: 10.1111/j.1476-5381.1980.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tzeng T-B, Gopal S, Kung L-P, Cowan A. Mechanism behind the bell-shaped dose-response curve of buprenorphine in rats: antagonism by norbuprenorphine. NIDA Res Monogr. 2000;180:66. [Google Scholar]
- 96.Ueda H, Yamaguchi T, Tokuyama S, Inoue M, Nishi M, Takeshima H. Partial loss of tolerance liability to morphine analgesia in mice lacking the nociceptin receptor gene. Neurosci Lett. 1997;237:136–138. doi: 10.1016/s0304-3940(97)00832-x. [DOI] [PubMed] [Google Scholar]
- 97.Vaughan CW, Christie MJ. Increase by the ORL1 receptor (opioid receptor-like 1) ligand, nociceptin, of inwardly rectifying K conductance in dorsal raphe nucleus neurones. Br J Pharmacol. 1996;117:1609–1611. doi: 10.1111/j.1476-5381.1996.tb15329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villiger JW. Binding of buprenorphine to opiate receptors. Regulation by guanyl nucleotides and metal ions. Neuropharmacology. 1984;23:373–375. doi: 10.1016/0028-3908(84)90201-6. [DOI] [PubMed] [Google Scholar]
- 99.Villiger JW, Taylor KM. Buprenorphine: characteristics of binding sites in the rat central nervous system. Life Sci. 1981;29:2699–2708. doi: 10.1016/0024-3205(81)90529-4. [DOI] [PubMed] [Google Scholar]
- 100.Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–S27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 101.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 102.Wang JB, Johnson PS, Imai Y, Persico AM, Ozenberger BA, Eppler CM, Uhl GR. cDNA cloning of an orphan opiate receptor gene family member and its splice variant. FEBS Lett. 1994;348:75–79. doi: 10.1016/0014-5793(94)00557-5. [DOI] [PubMed] [Google Scholar]
- 103.Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- 104.Wheeler-Aceto H, Cowan A. Buprenorphine and morphine cause antinociccption by different transduction mechanisms. Eur J Pharmacol. 1991;195:411–413. doi: 10.1016/0014-2999(91)90485-9. [DOI] [PubMed] [Google Scholar]
- 105.Wnendt S, Kruger T, Janocha E, Hildebrandt D, Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- 106.Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yuan L, Han Z, Chang JK, Han JS. Accelerated release and production of orphanin FQ in brain of chronic morphine tolerant rats. Brain Res. 1999;826:330–334. doi: 10.1016/s0006-8993(99)01337-2. [DOI] [PubMed] [Google Scholar]
- 108.Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen LY. Cloning of a human kappa opioid receptor from the brain. Life Sci. 1995;56:PL201–207. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]


