Abstract
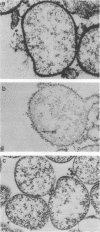
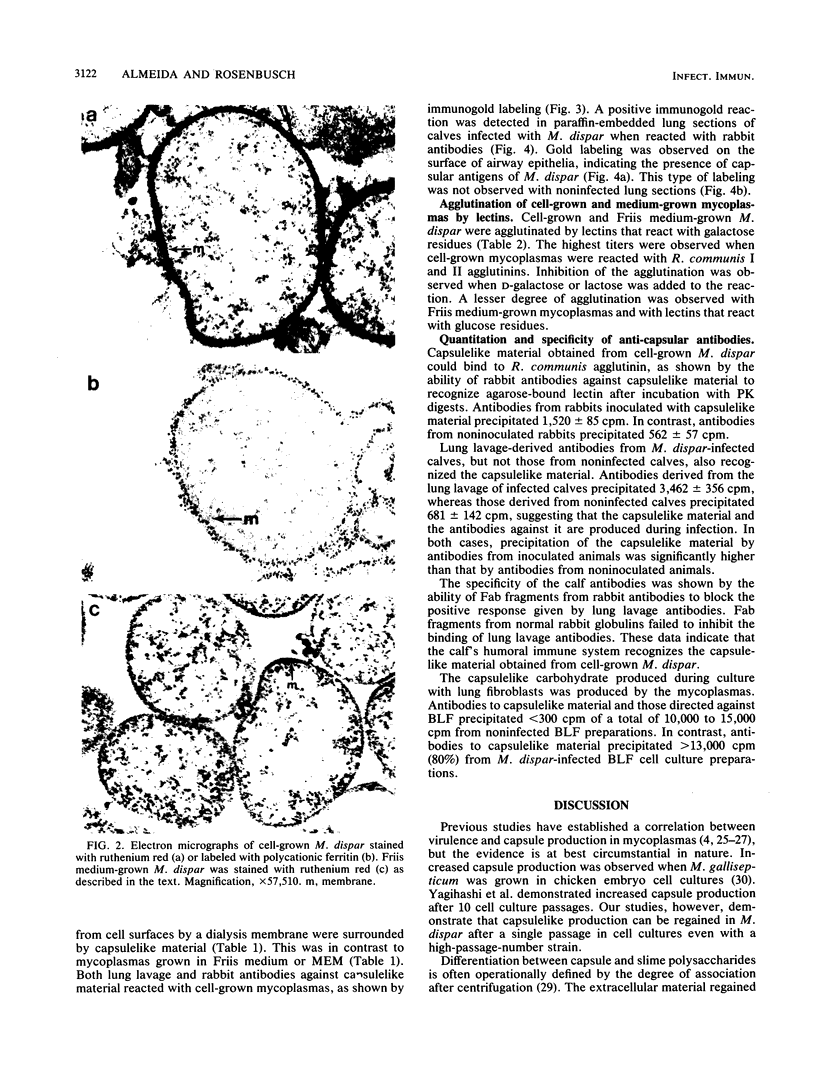
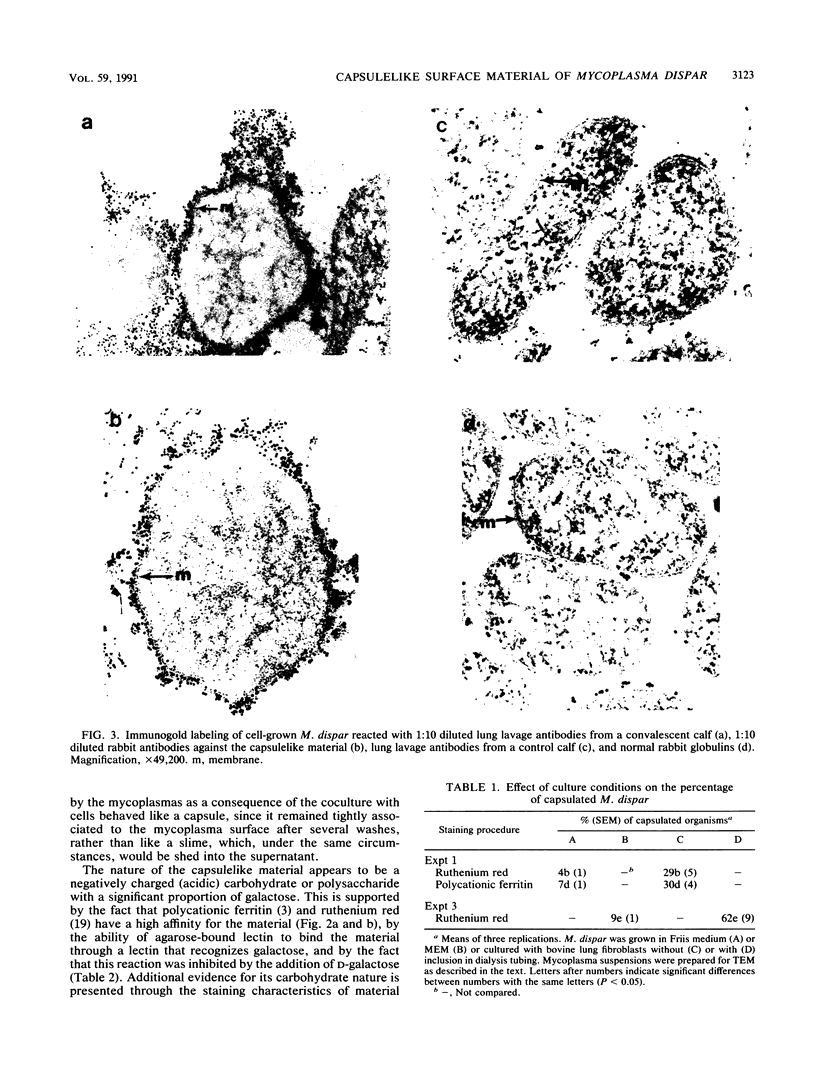
Electron microscopy has been used to show that Mycoplasma dispar produces an external capsulelike material in vivo that has an affinity for both ruthenium red and polycationic ferritin. This extracellular material is lost upon passage in culture medium but can be regained with a single passage on bovine lung fibroblast (BLF) cells. To confirm that the extracellular material associated with cell-grown mycoplasmas was the same as that observed in infected calves, rabbit antibodies were produced to purified capsulelike material isolated by protease digestion of cell-grown organisms. These antibodies bound to capsulelike material on the surface of M. dispar cells colonizing the bronchial epithelium of infected calves and to capsulelike material from cell-grown mycoplasmas. Calves infected with M. dispar produced antibodies in lung secretions that were capable of binding to the purified capsulelike material. The Fab fragments of rabbit antibodies to in vitro-produced capsulelike material could block this binding, indicating that the capsulelike material was similar in both in vivo-grown and cell-grown organisms. The carbohydrate nature of the capsular material suggested by the ruthenium red and polycationic staining characteristics was confirmed by its binding to Ricinus communis agglutinin, a galactose-specific lectin. These studies confirm that capsule material produced during infections with M. dispar share antigenic determinants with the material produced under in vitro conditions and that association with mammalian cells induces production of this material.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W. Determination of protein in preparations of microsomes. Anal Biochem. 1975 Apr;64(2):485–493. doi: 10.1016/0003-2697(75)90458-3. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Visualization of the bacterial polysaccharide capsule. Curr Top Microbiol Immunol. 1990;150:129–157. doi: 10.1007/978-3-642-74694-9_7. [DOI] [PubMed] [Google Scholar]
- Gilmour N. J., Menzies J. D., Donachie W., Fraser J. Electronmicroscopy of the surface of Pasteurella haemolytica. J Med Microbiol. 1985 Feb;19(1):25–34. doi: 10.1099/00222615-19-1-25. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Phagocytosis of microorganisms. Rev Infect Dis. 1982 Jan-Feb;4(1):104–123. doi: 10.1093/clinids/4.1.104. [DOI] [PubMed] [Google Scholar]
- Howard C. J., Gourley R. N. An electron-microscopic examination of certain bovine mycoplasmas stained with ruthenium red and the demonstration of a capsule on Mycoplasma dispar. J Gen Microbiol. 1974 Aug;83(2):393–398. doi: 10.1099/00221287-83-2-393. [DOI] [PubMed] [Google Scholar]
- Howard C. J. Variation in the susceptibility of bovine mycoplasmas to killing by the alternative complement pathway in bovine serum. Immunology. 1980 Nov;41(3):561–568. [PMC free article] [PubMed] [Google Scholar]
- Hudson J. R., Buttery S., Cottew G. S. Investigations into the influence of the galactan of Mycoplasma mycoides on experimental infection with that organism. J Pathol Bacteriol. 1967 Oct;94(2):257–273. doi: 10.1002/path.1700940204. [DOI] [PubMed] [Google Scholar]
- Jacques M., Foiry B. Electron microscopic visualization of capsular material of Pasteurella multocida types A and D labeled with polycationic ferritin. J Bacteriol. 1987 Aug;169(8):3470–3472. doi: 10.1128/jb.169.8.3470-3472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitany R. A., Zebrowski E. J. A high resolution PAS stain for polyacrylamide gel electrophoresis. Anal Biochem. 1973 Dec;56(2):361–369. doi: 10.1016/0003-2697(73)90202-9. [DOI] [PubMed] [Google Scholar]
- Knudtson W. U., Reed D. E., Daniels G. Identification of Mycoplasmatales in pneumonic calf lungs. Vet Microbiol. 1986 Feb;11(1-2):79–91. doi: 10.1016/0378-1135(86)90009-x. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Hermerath C. A. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984 Mar;43(3):879–886. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd L. C., Buttery S. H., Hudson J. R. The effect of the galactan and other antigens of Mycoplasma mycoides var. Mycoides on experimental infection with that organism in cattle. J Med Microbiol. 1971 Nov;4(4):425–439. doi: 10.1099/00222615-4-4-425. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurangirwa F. R., McGuire T. C., Magnuson N. S., Kibor A., Chema S. Composition of a polysaccharide from mycoplasma (F-38) recognised by antibodies from goats with contagious pleuropneumonia. Res Vet Sci. 1987 Mar;42(2):175–178. [PubMed] [Google Scholar]
- Russell R. R., Johnson K. G. SDS-polyacrylamide gel electrophoresis of lipopolysaccharides. Can J Microbiol. 1975 Dec;21(12):2013–2018. doi: 10.1139/m75-289. [DOI] [PubMed] [Google Scholar]
- Schiefer H. G., Gerhardt U., Brunner H., Krüpe M. Studies with lectins on the surface carbohydrate structures of mycoplasma membranes. J Bacteriol. 1974 Oct;120(1):81–88. doi: 10.1128/jb.120.1.81-88.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F. Lipoglycans from mycoplasmas. Crit Rev Microbiol. 1984;11(2):157–186. doi: 10.3109/10408418409105476. [DOI] [PubMed] [Google Scholar]
- Tajima M., Yagihashi T. Interaction of Mycoplasma hyopneumoniae with the porcine respiratory epithelium as observed by electron microscopy. Infect Immun. 1982 Sep;37(3):1162–1169. doi: 10.1128/iai.37.3.1162-1169.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima M., Yagihashi T., Miki Y. Capsular material of Mycoplasma gallisepticum and its possible relevance to the pathogenic process. Infect Immun. 1982 May;36(2):830–833. doi: 10.1128/iai.36.2.830-833.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Furr P. M., Davies H. A., Manchee R. J., Mouches C., Bove J. M. Mycoplasmal adherence with particular reference to the pathogenicity of Mycoplasma pulmonis. Isr J Med Sci. 1981 Jul;17(7):599–603. [PubMed] [Google Scholar]
- Tinant M. K., Bergeland M. E., Knudtson W. U. Calf pneumonia associated with Mycoplasma dispar infection. J Am Vet Med Assoc. 1979 Oct 15;175(8):812–813. [PubMed] [Google Scholar]
- Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988 Apr;34(4):415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]