Abstract
Patients with Smith–Lemli–Opitz syndrome (SLOS) are born with multiple congenital abnormalities. Postnatal cholesterol supplementation is provided; however, it cannot correct developmental malformations due to in utero cholesterol deficit. Increased transport of cholesterol from maternal to fetal circulation might attenuate congenital malformations. The cholesterol transporters Abca1, Abcg1, and Sr-b1 are present in placenta; however, their potential role in placental transport remains undetermined. In mice, expression analyses showed that Abca1 and Abcg1 transcripts increased 2–3-fold between embryonic days 13.5 and 18.5 in placental tissue; whereas, Sr-b1 expression decreased. To examine the functional role of Abca1, Abcg1 and Sr-b1 we measured the maternal–fetal transfer of 14C-cholesterol in corresponding mutant embryos. Disruption of either Abca1 or Sr-b1 decreased cholesterol transfer by ∼30%. In contrast, disruption of the Abcg1 had no effect. Treatment of pregnant C57Bl/6 female mice with TO901317, an LXR-agonist, increased both Abca1 expression and maternal–fetal cholesterol transfer to the fetus. In an SLOS mouse model (Dhcr7−/−), which is incapable of de novo synthesis of cholesterol, in utero treatment with TO901317 resulted in increased cholesterol content in Dhcr7−/− embryos. Our data support the hypothesis that Abca1, and possibly Sr-b1, contributes to transport maternal cholesterol to the developing fetus. Furthermore, we show, as a proof of principle, that modulating maternal–fetal cholesterol transport has potential for in utero therapy of SLOS.
INTRODUCTION
Cholesterol is essential for proper fetal development. It is clear that fetal development depends on fetal synthesis of cholesterol, because fetuses with defects in the de novo cholesterol synthesis are born with congenital abnormalities. The most common inborn error of cholesterol synthesis (7-dehydrocholesterol reductase deficiency) is Smith–Lemli–Opitz syndrome (SLOS). SLOS is characterized by various congenital malformations, impaired growth, behavioral abnormalities and mental retardation. The incidence of SLOS is approximately 1:10 000 to 1:60 000, and SLOS is more common in individuals of Northern European descent (1).
Currently, only postnatal or late fetal cholesterol supplementation of SLOS patients is possible (1,2). The human blood–brain barrier closes to cholesterol transport during early embryonic gestation (3). Thus, these therapeutic interventions occur after closure of the blood–brain barrier and therefore cannot prevent congenital neurological deficits. If, however, maternal–fetal cholesterol transport could be induced prior to closure of the fetal blood–brain barrier, neurological and behavioral abnormalities might be ameliorated. Recently, data were published suggesting that high-density lipoprotein (HDL) particles from SLOS fetuses are better acceptors of cholesterol than HDL particles from normal fetuses (4), raising the possibility that if more cholesterol could be transferred to the fetal circulation the fetus would in fact be capable of accepting it and circulating it in HDL particles.
The role of maternal–fetal cholesterol transport across the placenta has been controversial (5); however, evidence supporting this pathway has been accumulating. Although some of the earliest experimental studies using radioactively labeled cholesterol to trace the transport from maternal to fetal circulation (6–8) were carried out in the 1970s, the molecular mechanisms involved in the transport of cholesterol from mother to fetus have not been sufficiently elucidated. Improving our knowledge of the molecular mechanisms involved in maternal cholesterol transport may provide new insights into the processes that are essential for normal fetal development, may explain some of the phenotypic variability observed in SLOS and may provide a means for prenatal therapy.
Cholesterol transfer between lipoproteins and cells depends on cholesterol transporters and receptors. ATP-binding cassette transporter A1 (Abca1) is a cellular transmembrane protein that mediates efflux of cholesterol from cells to lipid poor apo-A1 and thus contributes to the formation of HDL particles (9). The ATP-binding cassette transporter G1 (Abcg1) belongs to the same family of transporters. Although less extensively studied, Abcg1 appears also to be involved in efflux of cholesterol from cells; however, in this case, the acceptors of the cholesterol are HDL2 and HDL3 particles (10). Scavenger receptor class B type 1 (Scarb1, hereafter Sr-b1) is a multi-ligand receptor that is capable of mediating cellular uptake of cholesterol from HDL-particles (11,12), as well as cholesterol efflux from cells (13,14).
Abca1, Abcg1 and Sr-b1 mRNAs are all expressed in mouse placenta (15–17). Immunohistological studies have localized ABCA1 to syncytiotrophoblasts, and fetal endothelial cells in human term placenta; thus, ABCA1 faces both the maternal and the fetal circulation (18). SR-B1 mRNA was identified in human first trimester and term trophoblast preparations (19), and the Sr-b1 protein was localized to the maternal side of the labyrinth in embryonic day (E)12.5 murine placenta (16). The cellular localization of Abcg1 in placenta has not been described.
Expression of both Abca1 and Abcg1 is regulated by the liver-X-receptor transcription factors (LXRs) (20,21). The LXRs belong to the nuclear receptor family, and regulate cholesterol metabolism (22). LXRs control gene expression by forming hetero-dimers with retinoid-X-receptors that bind to the LXR-response-elements of their target genes (23,24). Both LXR subtypes, LXR-α and LXR-β, are expressed in placenta (25,26). Plosch et al. (27) have previously proposed that placental cholesterol transport might be modulated by LXRs; however, this hypothesis is currently based on limited in vitro data (26,28).
In the present study we seek to gain insight into novel ways to alleviate the cholesterol deficiency in developing fetuses with SLOS, by studying the role of Abca1, Abcg1 and Sr-b1 in maternal–fetal cholesterol transport. We have characterized the temporal expression of Abca1, Abcg1 and Sr-b1 in murine placentas, and using Abca1, Abcg1 and Sr-b1 deficient mice we tested the functional role of these proteins in maternal–fetal cholesterol transport. Finally, we have increased placental expression of Abca1 and Abcg1 by treatment of pregnant females with an LXR-agonist, and we have investigated the therapeutic potential of these agonists in a SLOS mouse model.
RESULTS
Phenotype
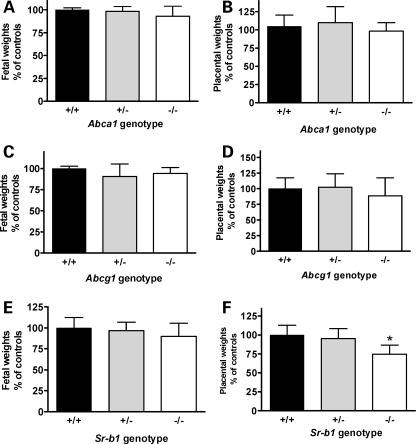
We observed no evidence of decreased viability for either Abca1−/− or Abcg1−/− embryos. Genotypic distributions in offspring from crosses of heterozygous males and females (+/+: +/−: −/−) were 6:11:8 and 8:17:8 for Abca1 and Abcg1, respectively. In addition, both fetal and placental weights did not differ between control, heterozygote and mutant Abca1 (Fig. 1A and B) or Abcg1 (Fig. 1C and D) embryos. Although Sr-b1−/− embryos were of normal weight (Fig. 1E), placental weight was decreased by 25% compared with control embryos (Fig. 1F). In addition, a lower than expected number of Sr-b1 mutant embryos were found (genotypic ratio of 13:17:5).
Figure 1.
Fetal and placental weights. Fetal (A) and placental (B) weights from Abca1+/− crosses, n = 6, 11 and 8 for controls, heterozygote and mutants, respectively. Fetal (C) and placental (D) weights from Abcg1+/− crosses, n = 9, 11 and 7 for controls, heterozygote and mutants, respectively. Fetal (E) and placental (F) weights from Sr-b1 +/− crosses, n = 6, 11 and 5 for controls, heterozygote and mutants, respectively. ANOVA *P < 0.01, Dunnets multiple comparison test, P < 0.01 when comparing Sr-b1+/+ with Sr-b1−/−. Values percent of mean of +/+ within each litter.
Expression
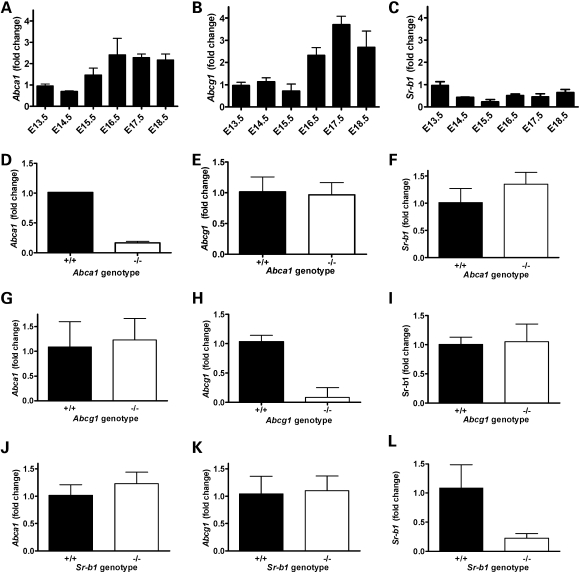
Expression of Abca1, Abcg1 and Sr-b1 was studied in placental tissue between E13.5 and E18.5. Abca1 expression increased during gestation, starting at E15.5 and increasing by over 2-fold between E16.5 and 18.5 (Fig. 2A). A similar increase in Abcg1 expression was observed during gestation (Fig. 2B). In contrast, Sr-b1 expression decreased after E13.5 (Fig. 2C). In placentas derived from Abca1 (Fig. 2 D–F), Abcg1 (Fig. 2 G– I) and Sr-b1 (Fig. 2 J–L) mice the expression of the disrupted gene was, as expected, decreased significantly in the mutant compared with control placentas. However, no compensatory increases were observed.
Figure 2.
Abca1, Abcg1 and Sr-b1 expression in placental tissue. Fold change of mRNA expression in mouse placentas. Expression of Abca1 (A), Abcg1 (B) and Sr-b1 (C) during gestation from E13.5 to E18.5. n = 4 from same litter for each time point. Data are normalized to E13.5 expression levels. The average CT- values for E13.5 placenta were 25.7, 24.1 and 24.2 for Abca1, Abcg1 and Sr-b1, respectively. Expression of Abca1, Abcg1 and Sr-b1 in placentas from Abca1+/+ and Abca1−/− fetuses, n = 2 and 4 (D–F); from Abcg1+/+ and Abcg1−/− fetuses, n = 7 and 6 (G–I) and from Sr-b1+/+ and Sr-b1−/− mutants, n = 7 and 5 (J–L). Values are fold change compared with mean of +/+.
Cholesterol transfer studies
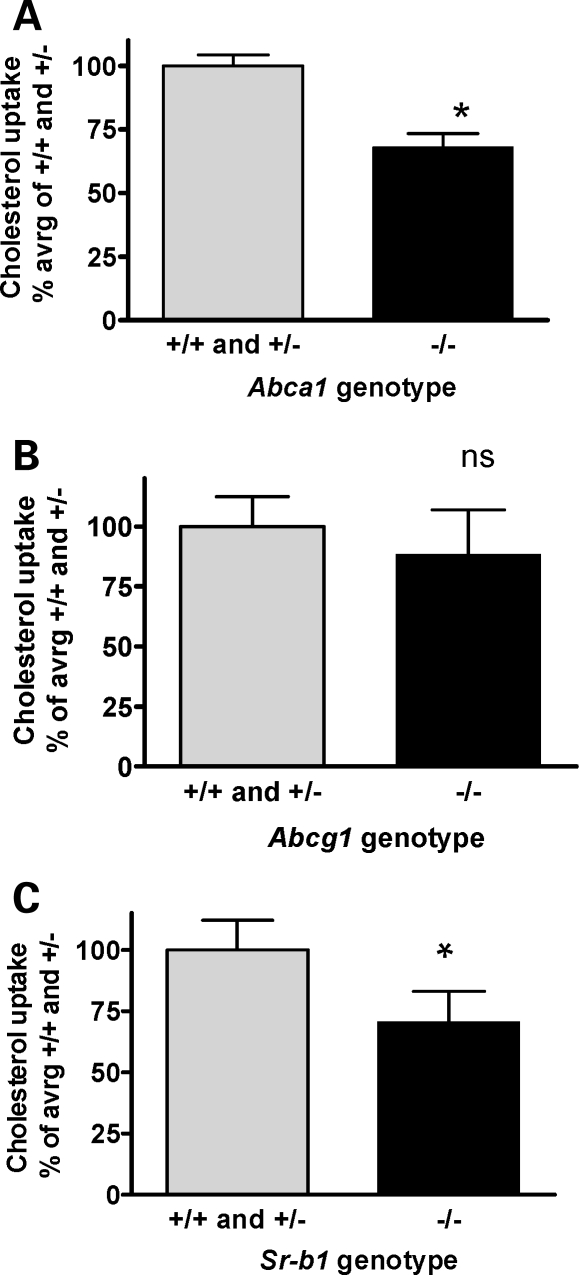
Mouse lipoproteins were labeled by equilibration of 14C-labeled cholesterol into mouse serum. Under the conditions used, rapid labeling of all lipoprotein fractions is expected (29). On E13.5, pregnant female mice were injected with labeled serum, and on E16.5 placental and fetal tissues were harvested. Maternal–fetal transfer of 14C-cholesterol was reduced by 31% (P < 0.0001) in Abca1−/− embryos compared with either Abca1+/− or Abca1+/+ embryos (Fig. 3A). Similarly, we observed a 29% (P < 0.003) reduction in maternal–fetal 14C-cholesterol transport in Sr-b1−/− embryos compared with Sr-b1+/− or Sr-b1+/+ embryos (Fig. 3C). Abcg1 deficiency did not affect the transport of 14C-cholesterol from mother to fetus (Fig. 3B).
Figure 3.
Maternal–fetal cholesterol transfer. (A) 14C-cholesterol transfer from mother to fetus in Abca1 pregnancies. Abca1+/+, n = 6, Abca1+/−, n = 11, and Abca1−/−, n = 10, from four litters; *P < 0.0001, unpaired t-test. (B) 14C-cholesterol transfer from mother to fetus in Abcg1 pregnancies. Abcg1+/+, n = 8, Abga1+/−, n = 11 and Abcg1−/−, n = 7; from three litters; P = 0.08, unpaired t-test. (C) 14C-cholesterol transfer from mother to fetus in Sr-b1 pregnancies. Sr-b1+/+, n = 6, Sr-b1+/−, n = 11 and Sr-b1−/−, n = 5, from four litters (Sr-b1−/− in two litters); *P < 0.003, unpaired t-test. Values are percent of mean of +/+ and +/− within each litter. +/+ and +/− did not differ significantly for any of the genotypes.
LXR-agonist treatment
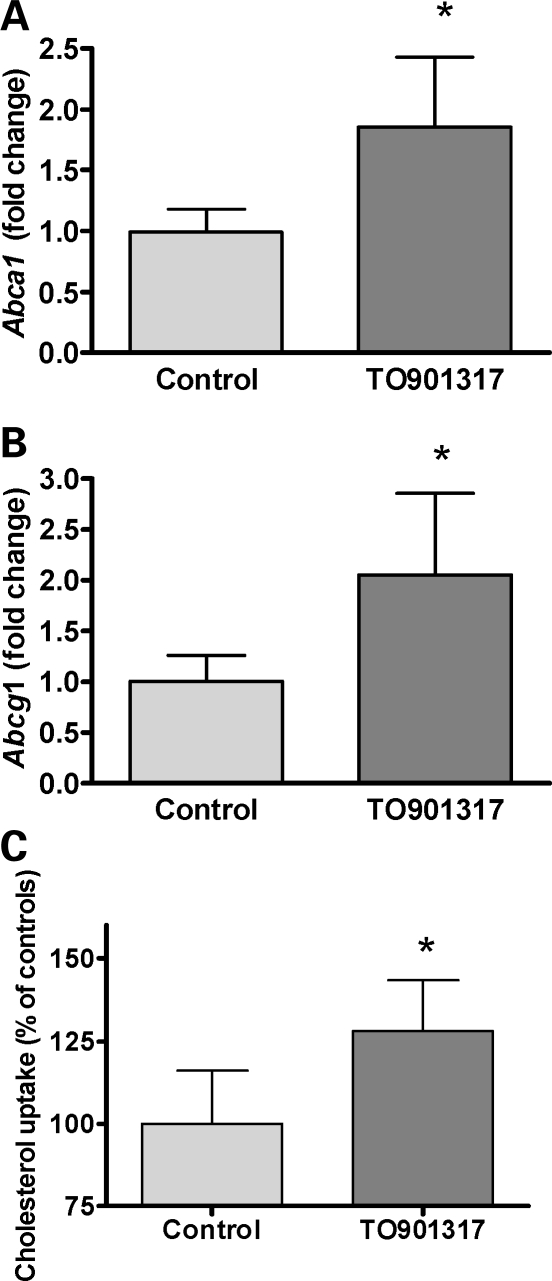
Pregnant C57Bl/6 females were treated daily from E11.5 to E15.5 with 40 mg TO901317/kg bodyweight or with vehicle. On E13.5, the females were injected with 14C-labeled cholesterol equilibrated in control mouse serum. Maternal blood, placenta and fetal tissues were collected at E16.5. Placental expression of Abca1 and Abcg1 increased approximately 2-fold (P < 0.0001 for both) in the TO90131-treated animals compared with vehicle-treated controls (Fig. 4A and B), and the transport of 14C-cholesterol from mother to fetus was increased 25% (P < 0.005) in TO90131-treated animals (Fig. 4C).
Figure 4.
Effect of LXR-agonist treatment on maternal–fetal cholesterol transfer. Placental gene expression in C57Bl/6-mice treated with LXR-agonist. Fold change in mRNA expression compared with the mean control value. Controls: n = 18, six pups from three litters; LXR-treated: n = 24, six pups from four litters. (A) *Abca1, P < 0.0001; (B) *Abcg1, P < 0.0001, both unpaired t-test. Values are percent of mean for the controls. (C) The effect of LXR-agonist treatment on 14C-cholesterol transfer from mother to fetus. Values are 14C-cholesterol in fetuses in relation to 14C-cholesterol in maternal blood on E 16.5 and standardized to mean of controls. Controls: n = 6 from two litters; TO901317 treated: n = 9 from three litters, *P = 0.005, unpaired t-test.
To investigate whether an LXR agonist, such as TO901317, could have potential therapeutic utility in SLOS, we studied the efficiency of TO901317 to promote maternal–fetal cholesterol transport in Dhcr7 mutant embryos. For this experiment pregnant females were treated with 20 mg/kg/d of TO901317 from E10.5 to E15.5. Control mice were treated with a similar volume of vehicle without drug. For these experiments we reduced the dose, but increased the period of administration of TO901317 in attempt to minimize maternal hepatosteatosis observed in the previous experiments using 40 mg/kg/d and thereby keeping the physiology of the female as normal as possible.
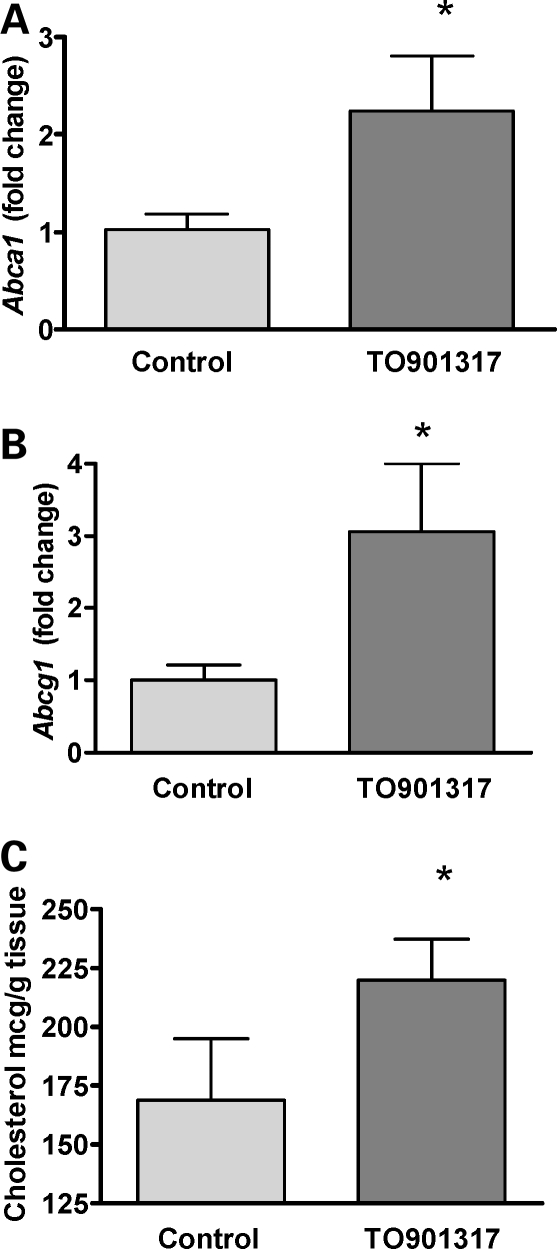
Although the amount of TO901317 was reduced, a similar induction of Abca1 and Abcg1 expression was observed (Fig. 5A and B). Cholesterol mass was assessed by gas chromatography/mass spectrophotometry. Consistent with the increased maternal–fetal 14C-cholesterol transfer observed in TO901317-treated mice described earlier, we observed a 30% increase (P = 0.001) in fetal cholesterol content in Dhcr7 mutant embryos from females treated with LXR agonist (Fig. 5C).
Figure 5.
Increased cholesterol content in Dhcr7−/− fetuses by maternal LXR-agonist treatment. Placental gene expression in Dhcr7-mice treated with LXR-agonist. Fold change in mRNA expression compared with the mean control value. Mutant controls: n = 11; mutant TO901317 treated: n = 11. (A) *Abca1, P = 0.0003. (B) *Abcg1, P < 0.0001, both unpaired t-test. (C) The effect of LXR-agonist treatment on cholesterol content of Dhcr7−/− fetuses. Mutant controls: n = 7; mutant TO901317 treated: n = 7; *P = 0.001, unpaired t-test.
DISCUSSION
The present study suggests that Abca1 is involved with cholesterol transport from the maternal to the fetal circulation. This may possibly also be true for Sr-b1. Both genes are expressed in placental tissue, and disruption of either gene reduces maternal–fetal cholesterol transport by about a third. In addition, both placental Abca1 expression and maternal–fetal cholesterol transport were increased in mice treated with an LXR agonist. Although previous studies have reported placental malformation, increased in utero mortality and decreased fetal weight in Abca1−/− placenta (15,30–32), we did not observe this. In our mouse line fetal weight and viability were normal, thus it is unlikely that the decreased maternal–fetal cholesterol transport observed in Abca1 mutant embryos was due to placental insufficiency. These observed differences might be a function of genetic background. Because increased fetal loss and decreased fetal weight were observed in Sr-b1 mutants, we cannot exclude that the decrease in maternal–fetal cholesterol transport was due to placental insufficiency. Another potential variable was that we used probucol, as previously reported (33,34), to maintain Sr-b1 pregnancies. There are two potential confounding issues in using mouse models for placental studies. First, unlike the mouse, where the yolk sac persists during gestation (35), the yolk sac probably only plays a role in transfer of nutrients during the first 2–3 weeks in humans (36). Sr-b1 is expressed in mouse yolk sac on the membranes facing the maternal blood at E10.5 and E12.5 (16), but no data are available with respect to Abca1 and Abcg1 expression. Secondly, structural differences in placental anatomy exist between mice and humans. The functional unit where fetal and maternal blood circulate to exchange gases and nutrients is in humans shaped as a villous tree, whereas the analogous structure in the mouse has the shape of a labyrinth. Furthermore, the trophoblasts facing the maternal blood consists of one layer in humans, but three layers in mice (37). Thus, one must keep in mind that the present studies were done in mice, and future work will be necessary to confirm applicability to human maternal–fetal cholesterol transport.
In contrast to the results obtained for Abca1; although Abcg1 expression increased during gestation similar to what was observed for Abca1, maternal–fetal cholesterol transport was not impaired in Abcg1−/− embryos. This result, in addition to the lack of compensatory gene expression reported in other studies (32,38), suggest that Abcg1 does not play a major role in placental cholesterol transport between E13.5 and E18.5. However, Abca1 and AbcG1 often act synergistically in cholesterol transport (32,38,39) and Abcg1 expression is increased in LXR-treated placenta. Thus, we cannot conclude that Abcg1 does not act synergistically with Abca1 in the LXR-treated mice.
Patients with SLOS have a deficiency of 7-dehydrocholesterol reductase activity. This results in impaired cholesterol synthesis. The deficiency of cholesterol contributes to various congenital malformations, growth retardation, learning disabilities and autistic traits (1). Current therapeutic interventions are limited, because they do not address the in utero cholesterol deficit. Increasing the supply of exogenous cholesterol to the fetus might attenuate or even prevent developmental malformations in these fetuses. To test this hypothesis we investigated the potential of LXR agonists to increase the expression of Abca1. Previous data show that LXR-agonists increases Abca1 and Abcg1 transcription in various other cell types (40–42), but their effect in placenta has not been studied. LXRs regulate transcription of Abca1 and Abcg1 in macrophages resulting in increased cholesterol efflux and reduced atherosclerosis (43). Current LXR agonists, including TO901317, affects various target genes and various tissues; among these the liver. Via activation of sterol response element binding protein 1-c and fatty acid synthetase hepatic triglyceride synthesis is enhanced resulting in hypertriglyceridemia and hepatic steatosis (44). Thus, the data we present in this paper represent a proof-of-principle, and an actual therapeutic trial in humans would depend on the development of more selective LXR agonists, with a more restricted pattern of gene induction or development of drugs that specifically modulate ABCA1 expression. Because of the beneficial effects on the cholesterol content of macrophages, there is great interest in development of LXR drug agonists for the prevention and treatment of atherosclerosis. Thus, future LXR agonists may be developed that would have potential for in utero therapy of SLOS.
Treating pregnant mice with TO901317, an LXR agonist, upregulated expression of both Abca1 and Abcg1 in placental tissue, and functionally increased maternal–fetal cholesterol transport. LXR agonists modulate the expression of multiple genes involved in cholesterol transport, synthesis and homeostasis. Among these LXR agonists modulate the expression of ApoE in mice (45). ApoE is expressed in both mouse and human placenta (46,47), thus modulation of ApoE expression or isotype could play a role in fetal cholesterol transport. In SLOS, the fetal phenotype appears to be influenced by the maternal ApoE isotype (48). Future work should focus on the potential role that ApoE may be playing in maternal–fetal cholesterol transport.
Based on these results from LXR treatment in control mice, we explored the possibility that LXR agonists could increase cholesterol transfer to Dhcr7−/− embryos, and we found that TO901317 treatment could significantly increase cholesterol content in Dhcr7 mutant embryos. Although the treated embryos still have a cholesterol deficit, these experiments provide a proof-of-principle that upregulation of maternal–fetal cholesterol transport may provide a means of in utero therapy. The SLOS mouse model used in this study has no residual Dhcr7 activity. This is in contrast to the majority of SLOS patients, who have hypomorphic mutant alleles with residual enzymatic function (49). It is plausible that increasing maternal–fetal cholesterol transport, even to a relatively small degree, in this situation could have a significant clinical impact.
In summary, the data from the present study suggest that Abca1, and possibly Sr-b1, are involved in cholesterol transport from mother to fetus. In addition, they suggest that it may be possible to increase the expression of Abca1 in placenta and thereby the transfer of cholesterol to Smith–Lemli–Opitz fetuses. Whether or not such treatment will be effective in humans and can mitigate the SLOS phenotype awaits further studies.
METHODS AND MATERIALS
Animals, husbandry and sample collection
Mice were housed under controlled condition with a 12/12 h light/dark cycle. All experiments were approved by the Animal Care and Use Committee of the NHLBI (#H-0022), or NICHD (# 06–021), NIH. Abca1+/– mice (DBA/1-Abca1tm1Jdm/J) (30) and Sr-b1+/– mice (B6;129S2-Scarb1tm1Kri/J) (50) were obtained from Jackson Laboratories, Bar Harbor, ME, USA. Abcg1+/− mice were obtained from Deltagen Inc., San Carlos, CA (51). C57Bl/6 were obtained from Jackson Laboratories. For genotyping of Abca1 mice, the primer set: 5′-CCTCCAGCCTATTCCTTTCTC-3′ and 5′-GTGCAATCCATCTTGTTCAATC -3′ were amplifying the mutant allele, whereas the primer set: 5′-TGGGATCATCTCTGTCTCCTTT-3′ and 5′-TCCTGAGGTAGATCTTGGGAGA-3′ were amplifying the wild-type allele. For genotyping of the Sr-b1 mice the primer set: 5′-CTTGGGTGGAGAGGCTATTC-3′ and 5′-AGGTGAGATGACAGGAGATC-3′ were amplifying the mutant allele, whereas the primer set: 5′-CGTCTCCTTCAGGTCCTGAG-3′ and 5′-CATGAGGATCATGACAACGC-3′ were amplifying the wild-type allele. For genotyping of Abcg1 mice, the primer set: 5′-GGG CCA GCT CAT TCC TCC CAC TCA T-3′ and 5′-GTG AGC AGA GCT TCT GGT AGC AAA C-3′ were amplifying the mutant allele, whereas the primer set: 5′-GGG ATC TCT GGG AAA TTC AAC AGT G-3′ and 5′-GTG AGC AGA GCT TCT GGT AGC AAA-3′ were amplifying the wild-type allele. Dhcr7 mice were genotyped as previously described (52).
Heterozygous Abca1, Abcg1, Sr-b1 and Dhcr7 mice were intercrossed to obtain mutant fetuses and placentas. The date of the vaginal plug was designated E0.5. Placentas from C57Bl/6 mice were harvested on day E13.5, E14.5, E15.5, E16.5, E17.5 and E18.5 for expression analysis and rapidly frozen on dry ice. Sr-b1+/− females were maintained on mouse chow supplemented with probucol (33).
Expression analysis
RNA was extracted from tissues, using an RNAeasy Mini Kit (Qiagen, cat.no. 74106). RNA (100 ng) was reverse transcribed, using a High-Capacity cDNA Archive kit (Applied Biosystems, cat.no. 4368813), as per manufacturer’s protocol. Quantitative PCR assays were performed, using Abca1, Abcg1 and Srb1 Assays on Demand from Applied Biosystems. Analysis was performed on an ABI Prism 7000 or ABI 7300. All assays were validated, performed in triplicate and normalized to Gapdh. Fold-change relative to control levels was determined, using the ΔΔCt method.
Fetal cholesterol uptake
Ten microcuries of 14C-cholesterol in 100 µl toluene (GE-healthcare, cat.no. CFA128-250UCI) was evaporated under nitrogen and redissolved in 200 µl mouse plasma with 1% ethanol at 37°C for 1 h. Pregnant mice were anaesthetized with Avertin [2, 2, 2-tribromoethanol (Sigma-Aldrich, cat.no.T4840-2); 2.5% solution, 0.0125 ml/g of bodyweight] on E13.5, an incision was made over the saphenous vein and the 14C-cholesterol containing plasma was injected. At E16.5, the females were euthanized and both fetal and placental tissues were harvested.
Cholesterol from the whole fetus was extracted using a modified version of a Folch extraction. Briefly, fetal tissue was homogenized in chloroform/methanol (2:1) (19 µl/mg) and extracted for 4 h at 25°C. The solvent was washed with 0.2 volume of 0.9% NaCl solution; the tubes were shaken and maintained at 4°C overnight to allow the phases to separate. The organic phase was isolated and evaporated under nitrogen. The extract was resuspended in Cholesterol Assay buffer (Cholesterol Quantitation Kit, Biovision, cat.no: K603-100) with 1% Triton-X 100. Scintillation counting was performed to quantify the amount of 14C-labeled cholesterol in the fetus. Total cholesterol mass was measured as per manufacturer’s protocol, using a cholesterol/cholesteryl ester quantitation kit (Cholesterol Quantitation Kit, Biovision) in order to validate the cholesterol extractions. Cholesterol uptake was calculated as CPM/mg tissue and standardized to the mean of control values.
LXR treatment studies
C57 Bl/6 females were treated daily from E11.5 to E15.5 with 40 mg of TO901317 (Sigma-Aldrich, cat. No. T2320)/kg body weight by gavage. The drug was dissolved in DMSO at a concentration of 25 mg/ml and mixed with 1× PBS with 0.75% hydroxypropylmethyl-cellulose (Sigma-Aldrich, cat.no.H-8384), 1:2 vol/vol. The controls were treated with a similar volume of vehicle only. On E13.5, pregnant mice were injected with 10 µCi of 14C-cholesterol/30 g of bodyweight as described earlier. On E16.5, the females were anaesthetized, maternal blood and placental and fetal tissues were collected and rapidly frozen on dry ice. Dhcr7+/− mice were intercrossed and pregnant mice were treated daily from E10.5 to E15.5 with 20 mg of TO901317/kg. Placental and fetal tissues were harvested on E16.5. For sterol analyses, sample preparation was carried out as previously described by Kelley with slight modifications (53). Briefly the samples were weighed then homogenized in PBS. Twenty micrograms of coprostan-3-ol (Sigma-Aldrich, cat.no: C7578-10MG) was added to each sample as a surrogate internal standard. The samples were saponified in 4% KOH in 100% ethanol for 1 h at 60°C, extracted with an equal volume of ethyl acetate and centrifuged for 5 min at 640g. The organic phase was removed and blown to dryness under nitrogen. The samples were derivatized with Bis-trimethylsilytrifluoroacetamide (BSTFA) plus 1% Trimethylchlorosilane (TMCS) (Pierce, cat.no: 38831) for 1 h at 60°C. The derivatized samples were injected onto a gas chromatogram/mass spectrometer (Finnigan Trace DSQ) utilizing a ZB-1701, 30 m×0.25 mm×0.25 µm column (Phenomenex). On injection the oven temperature was 170°C and ramped at 21°C per min to 250°C then ramped at 3°C per min to 290°C. Total amounts of cholesterol and 7DHC were determined based on comparison to the surrogate internal standard coprostan-3-ol.
Statistical analysis
Data are reported as mean±standard deviation. Unless otherwise specified, P < 0.05 was considered significant. ANOVA with appropriate post-test or unpaired t-test was used as indicated.
FUNDING
This work was supported by the intramural programs of Eunice Kennedy Schriver National Institute of Child Health and Human Development; National Heart, Lung and Blood Institute, National Institutes of Health; The Lundbeck Foundation and the Danish Medical Research Council (#271-06-0722).
ACKNOWLEDGEMENTS
The technical assistance of Cornelio Duarte, Steve Demosky, Erin Merkel and Katharine Porter is greatly appreciated.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Porter F.D. Smith-Lemli-Opitz syndrome: pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008;16:535–541. doi: 10.1038/ejhg.2008.10. [DOI] [PubMed] [Google Scholar]
- 2.Irons M.B., Nores J., Stewart T.L., Craigo S.D., Bianchi D.W., D’Alton M.E., Tint G.S., Salen G., Bradley L.A. Antenatal therapy of Smith-Lemli-Opitz syndrome. Fetal Diagn. Ther. 1999;14:133–137. doi: 10.1159/000020906. [DOI] [PubMed] [Google Scholar]
- 3.Plotz E.J., Kabara J.J., Davis M.E., LeRoy G.V., Gould R.G. Studies on the synthesis of cholesterol in the brain of the human fetus. Am. J. Obstet. Gynecol. 1968;101:534–538. doi: 10.1016/0002-9378(68)90565-6. [DOI] [PubMed] [Google Scholar]
- 4.Jenkins K.T., Merkens L.S., Tubb M.R., Myatt L., Davidson W.S., Steiner R.D., Woollett L.A. Enhanced placental cholesterol efflux by fetal HDL in Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. 2008;94:240–247. doi: 10.1016/j.ymgme.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woollett L.A. Maternal cholesterol in fetal development: transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 2005;82:1155–1161. doi: 10.1093/ajcn/82.6.1155. [DOI] [PubMed] [Google Scholar]
- 6.Connor W.E., Lin D.S. Placental transfer of cholesterol-4-14C into rabbit and guinea pig fetus. J. Lipid Res. 1967;8:558–564. [PubMed] [Google Scholar]
- 7.Lin D.S., Pitkin R.M., Connor W.E. Placental transfer of cholesterol into the human fetus. Am. J. Obstet. Gynecol. 1977;128:735–739. doi: 10.1016/0002-9378(77)90713-x. [DOI] [PubMed] [Google Scholar]
- 8.Pitkin R.M., Connor W.E., Lin D.S. Cholesterol metabolism and placental transfer in the pregnant Rhesus monkey. J. Clin. Invest. 1972;51:2584–2592. doi: 10.1172/JCI107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oram J.F., Vaughan A.M. ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins. Curr. Opin. Lipidol. 2000;11:253–260. doi: 10.1097/00041433-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Wang N., Lan D., Chen W., Matsuura F., Tall A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl Acad. Sci. USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Acton S., Rigotti A., Landschulz K.T., Xu S., Hobbs H.H., Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 12.Xu S., Laccotripe M., Huang X., Rigotti A., Zannis V.I., Krieger M. Apolipoproteins of HDL can directly mediate binding to the scavenger receptor SR-BI, an HDL receptor that mediates selective lipid uptake. J. Lipid Res. 1997;38:1289–1298. [PubMed] [Google Scholar]
- 13.de l.L.-M., Rothblat G.H., Connelly M.A., Kellner-Weibel G., Sakr S.W., Phillips M.C., Williams D.L. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J. Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- 14.Thuahnai S.T., Lund-Katz S., Dhanasekaran P., de l.L.-M., Connelly M.A., Williams D.L., Rothblat G.H., Phillips M.C. Scavenger receptor class B type I-mediated cholesteryl ester-selective uptake and efflux of unesterified cholesterol. Influence of high density lipoprotein size and structure. J. Biol. Chem. 2004;279:12448–12455. doi: 10.1074/jbc.M311718200. [DOI] [PubMed] [Google Scholar]
- 15.Christiansen-Weber T.A., Voland J.R., Wu Y., Ngo K., Roland B.L., Nguyen S., Peterson P.A., Fung-Leung W.P. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 2000;157:1017–1029. doi: 10.1016/S0002-9440(10)64614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatzopoulos A.K., Rigotti A., Rosenberg R.D., Krieger M. Temporal and spatial pattern of expression of the HDL receptor SR-BI during murine embryogenesis. J. Lipid Res. 1998;39:495–508. [PubMed] [Google Scholar]
- 17.Langmann T., Mauerer R., Zahn A., Moehle C., Probst M., Stremmel W., Schmitz G. Real-time reverse transcription-PCR expression profiling of the complete human ATP-binding cassette transporter superfamily in various tissues. Clin. Chem. 2003;49:230–238. doi: 10.1373/49.2.230. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht C., Soumian S., Tetlow N., Patel P., Sullivan M.H., Lakasing L., Nicolaides K., Williamson C. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta. 2007;28:701–708. doi: 10.1016/j.placenta.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Wadsack C., Hammer A., Levak-Frank S., Desoye G., Kozarsky K.F., Hirschmugl B., Sattler W., Malle E. Selective cholesteryl ester uptake from high density lipoprotein by human first trimester and term villous trophoblast cells. Placenta. 2003;24:131–143. doi: 10.1053/plac.2002.0912. [DOI] [PubMed] [Google Scholar]
- 20.Costet P., Luo Y., Wang N., Tall A.R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000;275:28240–28245. doi: 10.1074/jbc.M003337200. [DOI] [PubMed] [Google Scholar]
- 21.Venkateswaran A., Repa J.J., Lobaccaro J.M., Bronson A., Mangelsdorf D.J., Edwards P.A. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 22.Zelcer N., Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawla A., Repa J.J., Evans R.M., Mangelsdorf D.J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 24.Edwards P.A., Kennedy M.A., Mak P.A. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul. Pharmacol. 2002;38:249–256. doi: 10.1016/s1537-1891(02)00175-1. [DOI] [PubMed] [Google Scholar]
- 25.Marceau G., Volle D.H., Gallot D., Mangelsdorf D.J., Sapin V., Lobaccaro J.M. Placental expression of the nuclear receptors for oxysterols LXRalpha and LXRbeta during mouse and human development. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005;283:175–181. doi: 10.1002/ar.a.20157. [DOI] [PubMed] [Google Scholar]
- 26.Weedon-Fekjaer M.S., Duttaroy A.K., Nebb H.I. Liver X receptors mediate inhibition of hCG secretion in a human placental trophoblast cell line. Placenta. 2005;26:721–728. doi: 10.1016/j.placenta.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Plosch T., van Straten E.M., Kuipers F. Cholesterol transport by the placenta: placental liver X receptor activity as a modulator of fetal cholesterol metabolism? Placenta. 2007;28:604–610. doi: 10.1016/j.placenta.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Schmid K.E., Davidson W.S., Myatt L., Woollett L.A. Transport of cholesterol across a BeWo cell monolayer: implications for net transport of sterol from maternal to fetal circulation. J. Lipid Res. 2003;44:1909–1918. doi: 10.1194/jlr.M300126-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz C.C., Zech L.A., VandenBroek J.M., Cooper P.S. Cholesterol kinetics in subjects with bile fistula. Positive relationship between size of the bile acid precursor pool and bile acid synthetic rate. J. Clin. Invest. 1993;91:923–938. doi: 10.1172/JCI116314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeish J., Aiello R.J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K.L., Roach M.L., Royer L.J., de W.J., et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl Acad. Sci. USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orso E., Broccardo C., Kaminski W.E., Bottcher A., Liebisch G., Drobnik W., Gotz A., Chambenoit O., Diederich W., Langmann T., et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and Abc1-deficient mice. Nat. Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 32.Yvan-Charvet L., Ranalletta M., Wang N., Han S., Terasaka N., Li R., Welch C., Tall A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miettinen H.E., Rayburn H., Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J. Clin. Invest. 2001;108:1717–1722. doi: 10.1172/JCI13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S.C., Zhao S.P., Wu Z.H. Effect of probucol on HDL metabolism and class B type I scavenger receptor (SR-BI) expression in the liver of hypercholesterolemic rabbits. Int. J. Cardiol. 2007;115:29–35. doi: 10.1016/j.ijcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Jollie W.P. Development, morphology, and function of the yolk-sac placenta of laboratory rodents. Teratology. 1990;41:361–381. doi: 10.1002/tera.1420410403. [DOI] [PubMed] [Google Scholar]
- 36.Moore K.L., Persaud T.V.N. Before We are Born. Essentials of Embryology and Birth Defects. Philadelphia, PA: Saunders/Elsevier; 2008. [Google Scholar]
- 37.Georgiades P., Ferguson-Smith A.C., Burton G.J. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 38.Ranalletta M., Wang N., Han S., Yvan-Charvet L., Welch C., Tall A.R. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1−/− bone marrow. Arterioscler. Thromb. Vasc. Biol. 2006;26:2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan A.M., Oram J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006;47:2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Drouineaud V., Sagot P., Garrido C., Logette E., Deckert V., Gambert P., Jimenez C., Staels B., Lagrost L., Masson D. Inhibition of progesterone production in human luteinized granulosa cells treated with LXR agonists. Mol. Hum. Reprod. 2007;13:373–379. doi: 10.1093/molehr/gam019. [DOI] [PubMed] [Google Scholar]
- 41.Li T., Chen W., Chiang J.Y. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J. Lipid Res. 2007;48:373–384. doi: 10.1194/jlr.M600282-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Panzenboeck U., Kratzer I., Sovic A., Wintersperger A., Bernhart E., Hammer A., Malle E., Sattler W. Regulatory effects of synthetic liver X receptor- and peroxisome-proliferator activated receptor agonists on sterol transport pathways in polarized cerebrovascular endothelial cells. Int. J. Biochem. Cell Biol. 2006;38:1314–1329. doi: 10.1016/j.biocel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Tall A.R. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J. Intern. Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 44.Grefhorst A., Elzinga B.M., Voshol P.J., Plosch T., Kok T., Bloks V.W., van der Sluijs F.H., Havekes L.M., Romijn J.A., Verkade H.J., et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 45.Ulven S.M., Dalen K.T., Gustafsson J.A., Nebb H.I. Tissue-specific autoregulation of the LXRalpha gene facilitates induction of apoE in mouse adipose tissue. J. Lipid Res. 2004;45:2052–2062. doi: 10.1194/jlr.M400119-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Overbergh L., Lorent K., Torrekens S., Van Leuven F., Van den B.H. Expression of mouse alpha-macroglobulins, lipoprotein receptor-related protein, LDL receptor, apolipoprotein E, and lipoprotein lipase in pregnancy. J. Lipid Res. 1995;36:1774–1786. [PubMed] [Google Scholar]
- 47.Rindler M.J., Traber M.G., Esterman A.L., Bersinger N.A., Dancis J. Synthesis and secretion of apolipoprotein E by human placenta and choriocarcinoma cell lines. Placenta. 1991;12:615–624. doi: 10.1016/0143-4004(91)90496-3. [DOI] [PubMed] [Google Scholar]
- 48.Witsch-Baumgartner M., Gruber M., Kraft H.G., Rossi M., Clayton P., Giros M., Haas D., Kelley R.I., Krajewska-Walasek M., Utermann G. Maternal apo E genotype is a modifier of the Smith-Lemli-Opitz syndrome. J Med Genet. 2004;41:577–584. doi: 10.1136/jmg.2004.018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassif C.A., Krakowiak P.A., Wright B.S., Gewandter J.S., Sterner A.L., Javitt N., Yergey A.L., Porter F.D. Residual cholesterol synthesis and simvastatin induction of cholesterol synthesis in Smith-Lemli-Opitz syndrome fibroblasts. Mol. Genet. Metab. 2005;85:96–107. doi: 10.1016/j.ymgme.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Rigotti A., Trigatti B.L., Penman M., Rayburn H., Herz J., Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc. Natl Acad. Sci. USA. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy M.A., Barrera G.C., Nakamura K., Baldan A., Tarr P., Fishbein M.C., Frank J., Francone O.L., Edwards P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Wassif C.A., Zhu P., Kratz L., Krakowiak P.A., Battaile K.P., Weight F.F., Grinberg A., Steiner R.D., Nwokoro N.A., Kelley R.I., et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith–Lemli–Opitz syndrome. Hum. Mol. Genet. 2001;10:555–564. doi: 10.1093/hmg/10.6.555. [DOI] [PubMed] [Google Scholar]
- 53.Kelley R.I. Diagnosis of Smith-Lemli-Opitz syndrome by gas chromatography/mass spectrometry of 7-dehydrocholesterol in plasma, amniotic fluid and cultured skin fibroblasts. Clin. Chim. Acta. 1995;236:45–58. doi: 10.1016/0009-8981(95)06038-4. [DOI] [PubMed] [Google Scholar]