Abstract
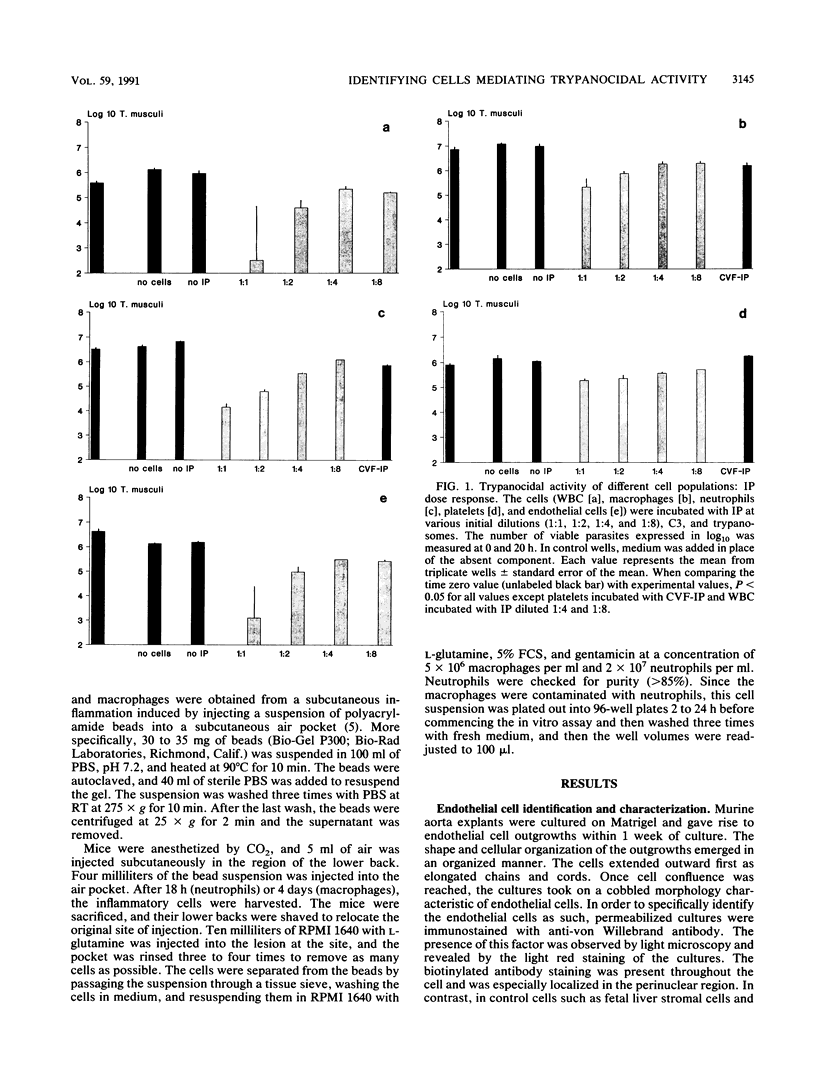
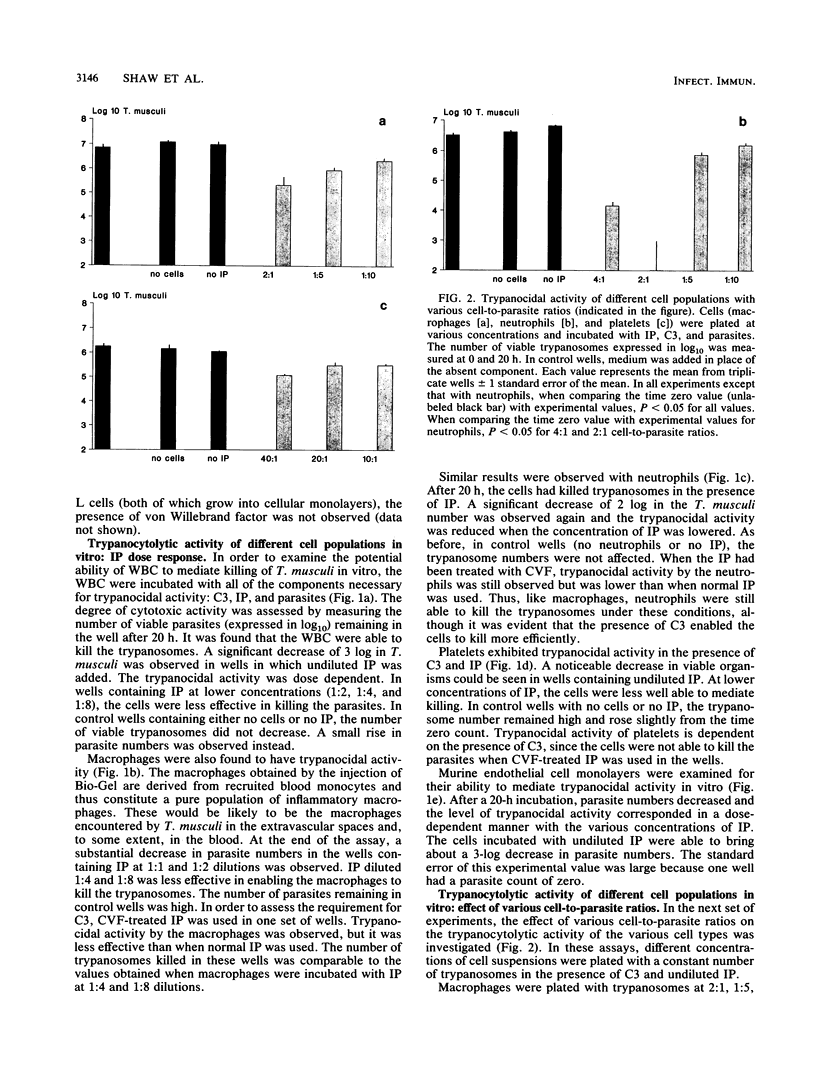
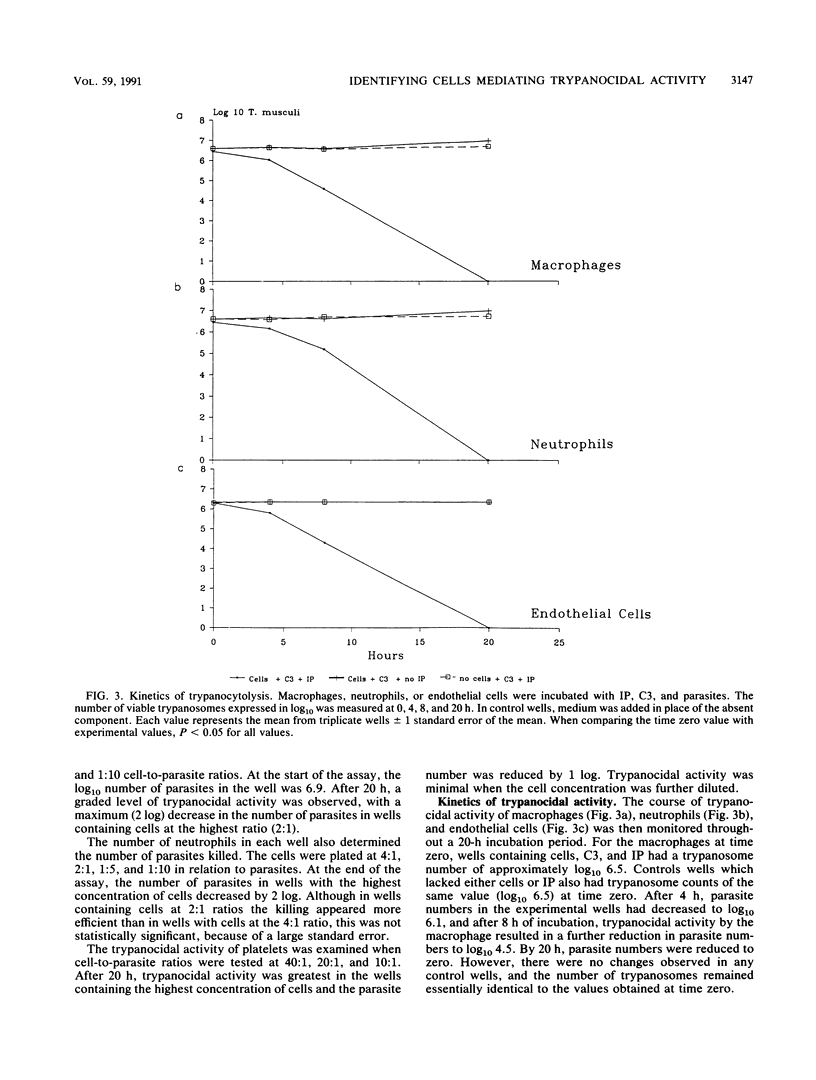
Cure of Trypanosoma musculi infection involves an effector mechanism mediated by immunoglobulin G2a antibody, C3, and an unidentified effector cell. In the present study, experiments were designed to identify the cell(s) within the vascular system that may be responsible for cure of trypanosomiasis. The ability of various cell populations to mediate killing of trypanosomes in the presence of C3 and immune plasma (IP) was tested in vitro. Blood-derived platelets or leukocytes or Bio-Gel-elicited macrophages or neutrophils were incubated at various concentrations with T. musculi, C3, and IP diluted up to 1 in 8. Trypanocidal activity was dependent upon the presence and concentration of IP and on the number of cells in the wells. Macrophages, neutrophils, and platelets were shown to kill with different potencies. With a 2:1 cell-to-parasite ratio, both macrophages and neutrophils reduced parasite numbers by 2 log, while platelets at a 40:1 ratio mediated a 1 log decrease. In addition, even in the absence of C3, the phagocytes were capable of killing trypanosomes while platelet trypanocidal activity was abrogated. The time course of trypanocidal activity was monitored for macrophages and neutrophils. The number of parasites decreased by 0.5 log by 4 h and 1 to 2 log by 8 h and by 20 h was reduced to zero. Cultured monolayers of endothelial cells were also tested for trypanocidal activity and shown to kill the parasites in the presence of IP and C3. The level of trypanocidal activity was dependent on the concentration of IP.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. In vitro growth of Trypanosoma musculi in cell-free medium conditioned by rodent macrophages and mercaptoethanol. Int J Parasitol. 1980 Apr;10(2):137–142. doi: 10.1016/0020-7519(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Büngener W. Verlauf con Trypanosoma musculi-Infektionen in NMIR-Mäusen. Tropenmed Parasitol. 1975 Sep;26(3):281–284. [PubMed] [Google Scholar]
- Büngener W. Verlauf von Trypanosoma musculi-Infektionen in mit Plasmodium berghei infizierten Mäusen. Tropenmed Parasitol. 1975 Sep;26(3):285–290. [PubMed] [Google Scholar]
- Dusanic D. G. Trypanosoma musculi infections in complement-deficient mice. Exp Parasitol. 1975 Apr;37(2):205–210. doi: 10.1016/0014-4894(75)90071-5. [DOI] [PubMed] [Google Scholar]
- Fauve R. M., Jusforgues H., Hevin B. Maintenance of granuloma macrophages in serum-free medium. J Immunol Methods. 1983 Nov 25;64(3):345–351. doi: 10.1016/0022-1759(83)90442-8. [DOI] [PubMed] [Google Scholar]
- Ferrante A. The role of the macrophage in immunity to Trypanosoma musculi. Parasite Immunol. 1986 Mar;8(2):117–127. doi: 10.1111/j.1365-3024.1986.tb00838.x. [DOI] [PubMed] [Google Scholar]
- James S. L., Hibbs J. B., Jr The role of nitrogen oxides as effector molecules of parasite killing. Parasitol Today. 1990 Sep;6(9):303–305. doi: 10.1016/0169-4758(90)90261-2. [DOI] [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi infections in normocomplementemic, C5-deficient, and C3-depleted mice. Infect Immun. 1977 May;16(2):557–563. doi: 10.1128/iai.16.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. M., Trenchev P., Faulk W. P. Immunological studies of human placentae. Binding of complexed immunoglobulin by stromal endothelial cells. Clin Exp Immunol. 1975 Oct;22(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Joseph M., Auriault C., Capron A., Vorng H., Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983 Jun 30;303(5920):810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- Joseph M., Capron A., Ameisen J. C., Capron M., Vorng H., Pancré V., Kusnierz J. P., Auriault C. The receptor for IgE on blood platelets. Eur J Immunol. 1986 Mar;16(3):306–312. doi: 10.1002/eji.1830160318. [DOI] [PubMed] [Google Scholar]
- Kongshavn P. A., Shaw K., Ghadirian E., Ulczak O. Failure to demonstrate a major role for Kupffer cells and radiosensitive leukocytes in immunoglobulin-mediated elimination of Trypanosoma musculi. Infect Immun. 1990 Jun;58(6):1971–1978. doi: 10.1128/iai.58.6.1971-1978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Cox F. E. Nonspecific defence mechanism: the role of nitric oxide. Immunol Today. 1991 Mar;12(3):A17–A21. doi: 10.1016/S0167-5699(05)80006-4. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Pancré V., Joseph M., Capron A., Delanoye A., Vorng H., Auriault C. Characterization of a suppressive factor of platelet cytotoxic functions in human and rat schistosomiasis mansoni. Clin Exp Immunol. 1989 Jun;76(3):417–421. [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Ristimäki A., Westerlund B. Binding of Escherichia coli S fimbriae to cultured human endothelial cells. Infect Immun. 1989 Jul;57(7):2256–2259. doi: 10.1128/iai.57.7.2256-2259.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Goodwin J. D., Vann J. M., Selvaraj M. P., Hart M. A. Role of C1q in phagocytosis of Salmonella minnesota by pulmonary endothelial cells. Infect Immun. 1989 May;57(5):1356–1362. doi: 10.1128/iai.57.5.1356-1362.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Ruan J. W. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981 Oct 30;214(4520):557–558. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- Ryan U. S. The endothelial surface and responses to injury. Fed Proc. 1986 Feb;45(2):101–108. [PubMed] [Google Scholar]
- Samarawickrema N. A., Howell M. J. Interactions between peritoneal cells and Trypanosoma musculi in mice. Int J Parasitol. 1988 Feb;18(1):69–73. doi: 10.1016/0020-7519(88)90038-0. [DOI] [PubMed] [Google Scholar]
- Slezak S., Symer D. E., Shin H. S. Platelet-mediated cytotoxicity. Role of antibody and C3, and localization of the cytotoxic system in membranes. J Exp Med. 1987 Aug 1;166(2):489–505. doi: 10.1084/jem.166.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper W. D., Bartlett S. P., Winn H. J. Lysis of antibody-coated cells by platelets. J Exp Med. 1982 Oct 1;156(4):1210–1221. doi: 10.1084/jem.156.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viens P., Dubois R., Kongshavn P. A. Platelet activity in immune lysis of Trypanosoma musculi. Int J Parasitol. 1983 Dec;13(6):527–530. doi: 10.1016/s0020-7519(83)80023-x. [DOI] [PubMed] [Google Scholar]
- Viens P., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to Trypanosoma musculi. I. Initial control of the infection and the effect of T-cell deprivation. Clin Exp Immunol. 1974 Feb;16(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Daëron M., Daulouede S. Identification of antibody classes and Fc receptors responsible for phagocytosis of Trypanosoma musculi by mouse macrophages. Infect Immun. 1986 Sep;53(3):600–605. doi: 10.1128/iai.53.3.600-605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Characterization of antibodies mediating protection and cure of Trypanosoma musculi infection in mice. Infect Immun. 1985 Jun;48(3):787–794. doi: 10.1128/iai.48.3.787-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Further characterization of the curative antibodies in Trypanosoma musculi infection. Infect Immun. 1988 Sep;56(9):2379–2384. doi: 10.1128/iai.56.9.2379-2384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Heat-labile IgG2a antibodies affect cure of Trypanosoma musculi infection in C57BL/6 mice. J Immunol. 1986 Nov 1;137(9):2968–2972. [PubMed] [Google Scholar]



