Abstract
Cl− transport is essential for lung development. Because gamma-aminobutyric acid (GABA) receptors allow the flow of negatively-charged Cl− ions across the cell membrane, we hypothesized that the expression of ionotropic GABA receptors are regulated in the lungs during development. We identified 17 GABA receptor subunits in the lungs by real-time PCR. These subunits were categorized into 4 groups: Group 1 had high mRNA expression during fetal stages and low in adults; Group 2 had steady expression to adult stages with a slight up-regulation at birth; Group 3 showed an increasing expression from fetal to adult lungs; and Group 4 displayed irregular mRNA fluctuations. The protein levels of selected subunits were also determined by Western blots and some subunits had protein levels that corresponded to mRNA levels. Further studied subunits were primarily localized in epithelial cells in the developing lung with differential mRNA expression between isolated cells and whole lung tissues. Our results add to the knowledge of GABA receptor expression in the lung during development.
Keywords: lungs, ionotropic GABA receptor, fetal lung development, chloride homeostasis, lung fluid transport, real time PCR, gene expression, alveolar epithelial cells
1. RESULTS AND DISCUSSION
1.1 Significance
Cl− channels play an important role in the lung throughout an animal’s life. During the fetal stages, the lung is a fluid-secreting organ. The main driving force for fluid secretion is the active secretion of Cl− into the developing lungs. Therefore, Cl− channels are indispensable for fetal lung development and morphogenesis (Folkesson et al., 1998; Olver et al., 2004; Bland and Nielson, 1992). On the other hand, the adult lung is air-filled and maintains a “dry” state. The apical surface is covered by a thin layer of alveolar surface fluid. This layer of fluid is required for the proper air exchange and airway defenses. Cl− channels are also essential for maintaining the composition and volume of the alveolar surface fluid (Brochiero et al., 2004).
Several Cl− channels have previously been studied in the lung. The cystic fibrosis transmembrane conductance regulator (CFTR) (Harris et al., 1991), the voltage-gated Cl− channels, ClC2 (Thiemann et al., 1992), ClC3 (Lamb et al., 2001), and ClC5 (Edmonds et al., 2002), and Na+-K+-Cl− cotransporter (NKCC) (Rochelle et al., 2000) have been detected in fetal lungs, distal airway epithelial cells and alveolar epithelial cells. Some of the channels have been shown to be involved in fluid homeostasis in fetal and adult lungs (Brochiero et al., 2004; Blaisdell et al., 2000; Fang et al., 2002). These Cl− channels appear in early gestation and are expressed relatively high throughout the fetal stage, but decrease at late gestation and remain low during adult stages (Harris et al., 1991; Murray et al., 1996; Lamb et al., 2001; Murray et al., 1995). The Cl− channels may participate in fluid secretion in fetal lungs since the lung undergoes a change from fluid secretion to absorption at birth. However, CFTR and NKCC knock-out mice were not fatal (Gillie et al., 2001; Snouwaert et al., 1992). In these mice, other Cl− channels may compensate for those channels.
Ionotropic γ-aminobutyric acid (GABA) receptors are the most important Cl− channels in the central nervous system (CNS), and their expression has also been found in peripheral organs. These receptors mediate a fast inhibitory neurotransmission in the CNS but their functions in peripheral organs have not been extensively studied (Akinci and Schofield, 1999). Ionotropic GABA receptors can be categorized into GABAA and GABAC receptors based on their subunit compositions and pharmacological properties (Bormann, 2000). Nineteen GABA receptor subunits have been cloned from rats, which include α1–6, β1–3, γ1–3, ρ1–3, δ, θ, ε, and π (Whiting et al., 1999). We have recently identified the GABAA receptor π subunit as a novel alveolar epithelial type II cell marker (Chen et al., 2004b). We have also detected the α1, α3, β2, γ2, and γ3 subunits in adult type II cells (Jin et al., 2005). Furthermore, we have provided evidence that GABA receptors contribute to fluid balance in adult lungs (Jin et al., 2006).
A functional GABAA or GABAC receptor requires five subunits (Whiting et al., 1999). While GABAA receptors are assembled from at least 3 different subfamilies of subunits, GABAC receptors can be formed from only the ρ subunit family. The most common combination of a GABAA receptor is αβγ, in which α and β subunits appear to be essential and the γ subunit can be replaced by other subunits (Sieghart et al., 1999). In addition to those common subunits, “rare” subunits such as θ, ε, δ and π can assemble with αβγ (Sieghart and Sperk, 2002; Sieghart et al., 1999). For example, the π subunit was found to assemble with α1β3γ2 or α5β3γ3 in the co-transfected cell lines (Hedblom and Kirkness, 1997; Neelands and Macdonald, 1999).
In this study, we investigated the developmental regulation of all the ionotropic GABA receptor subunits in the lung and pulmonary epithelial cells. This is the first study on the dynamic changes of GABA receptor subunits in developing lungs.
1.2 Quantification of the mRNA levels of GABA receptor subunits in fetal rat lungs and pulmonary epithelial cells
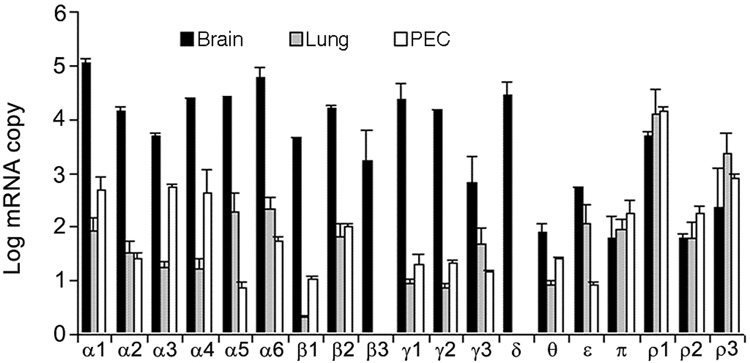
By using real-time PCR, we determined the mRNA abundance of 19 GABA receptor subunits (α1–6, β1–3, γ1–3, δ, θ, ε, π, and ρ1–3) in fetal lungs and fetal pulmonary epithelial cells on gestational day 18 (D18), which was selected to represent the late pseudoglandular stage of fetal lung development. Brain tissue from adult rats was used as a positive control and contained more than 50 copies per 108 copies of 18S rRNA for all the subunits (Fig. 1). The α1 subunit had the highest expression in brain compared with other subunits (>105 copies per 108 copies of 18S rRNA). Brain contained 104~105 copies per 108 copies of 18S rRNA of α2–6, β1–3, γ1–3, δ, ε, ρ1 and ρ3 subunits. In the lung, α5, α6, ε, π, ρ1 and ρ3 subunits had >100 copies per 108 copies of 18S rRNA. The copy numbers for α1, α2, α3, α4, β2, γ3, and ρ2 subunits in the lung were 10–100 copies per 108 copies of 18S rRNA. In pulmonary epithelial cells, α1, α3, α4, β2, π, and ρ1–3 subunits had >100 copies per 108 copies of 18S rRNA. Some of those subunits (α1, α3, α4, β2, and π) appear to be enriched in pulmonary epithelial cells in comparison with lung tissue. As a whole, the mRNA levels of GABA receptor subunits in the lung and pulmonary epithelial cells were much lower than that in the brain, especially for α, β, and γ subunit families. However, π, ρ1, ρ2 and ρ3 subunits had a similar or higher abundance of mRNA in lungs in comparison with that in brains (Fig. 1).
Figure 1. Expression of GABA receptor subunits in fetal rat lungs and pulmonary epithelial cells.
Total RNA was isolated from rat fetal lungs and pulmonary epithelial cells (PEC) at gestational day 18 and reverse-transcribed into cDNA. The mRNA abundance of 19 ionotropic GABA receptors was quantified by real-time PCR, normalized to 18S rRNA and expressed as log copy number per 108 copies of 18S rRNA. The adult brain was used as a positive control. Data shown are means ± SE (n ≥3 independent cell preparations).
1.3 Developmental alteration of GABA receptor mRNA in rat lungs
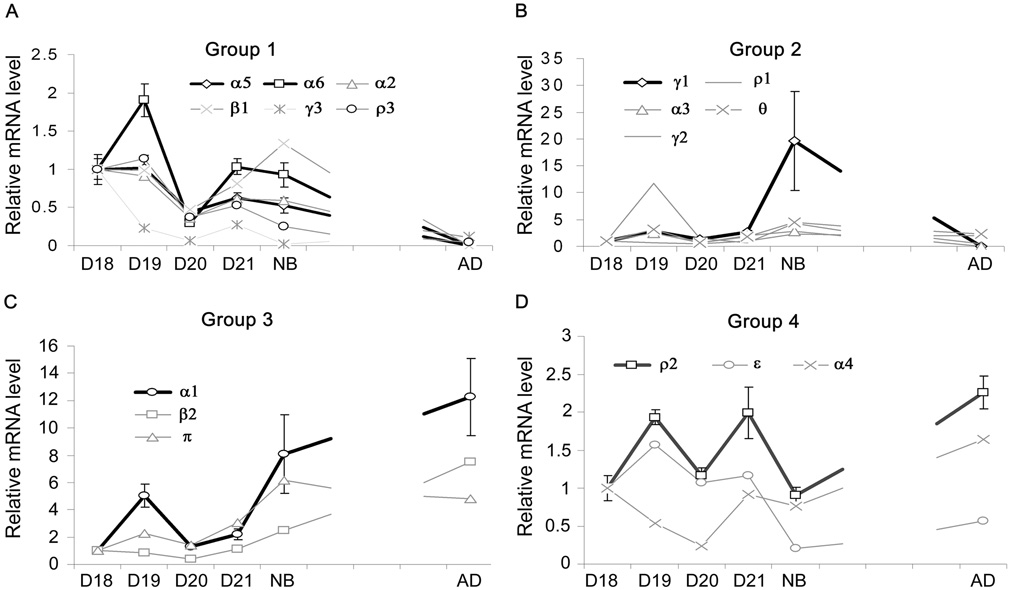
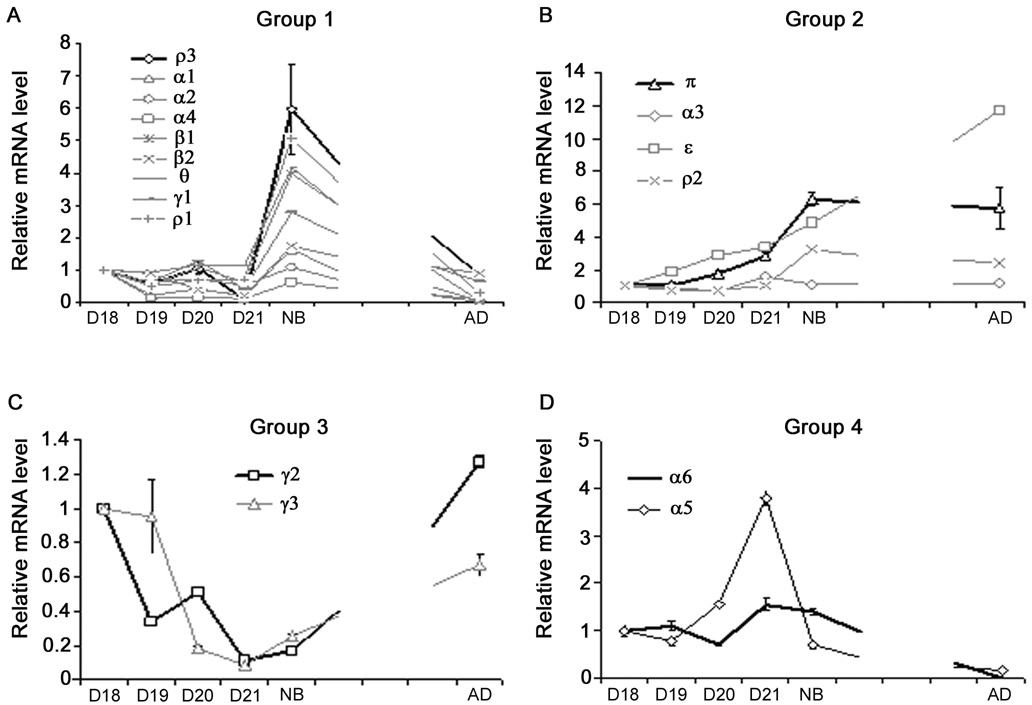
The 17 subunits expressed in the lung can be classified into 4 groups based on the similarity of changes in expression during lung development (Fig. 2A–D). Group 1 was composed of 6 subunits (α2, α5, α6, γ3, β1, and ρ3). The genes in this group exhibited a decreasing trend from D18 to adult. These subunits had significantly lower mRNA expression in adult lungs in comparison with the fetal or newborn lungs (Fig. 2A). Group 2 had 5 subunits (α3, γ1, γ2, θ, and ρ1). The mRNA expression in this group was relatively steady from fetal to adult lungs, but was slightly up-regulated at birth (Fig. 2B). Group 3 included 3 subunits (α1, β2, and π). The genes in this group showed an increasing trend from fetal D18 to adult (Fig. 2C). Group 4 was composed of 3 subunits (α4, ε, and ρ2). The genes in this group exhibited irregular fluctuation and had no significant difference among all the time points (Fig. 2D).
Figure 2. Developmental alteration of GABA receptor mRNA in rat lungs.
Total RNA was isolated from fetal lungs at gestational day 18 (D18), day 19 (D19), day 20 (D20) and day 21 (D21), or from newborn rat lungs (NB) and adult rat lungs (AD). Total RNA was reverse-transcribed into cDNA, mRNA levels of GABA receptor subunits were determined with real-time PCR and the results expressed as log copy number per 108 copies of 18S rRNA. Those subunits were classified into 4 groups according to their expression patterns. Group 1, α2, α5, α6, β1, γ3 and ρ3 (A); Group 2, α3, γ1, γ2, θ and ρ1 (B); Group 3, α1, β2 and π (C); Group 4, α4, ε and ρ2 (D). Data shown are means ± SE (n ≥ 3 independent preparations).
1.4 Developmental changes of GABA receptor protein during lung development
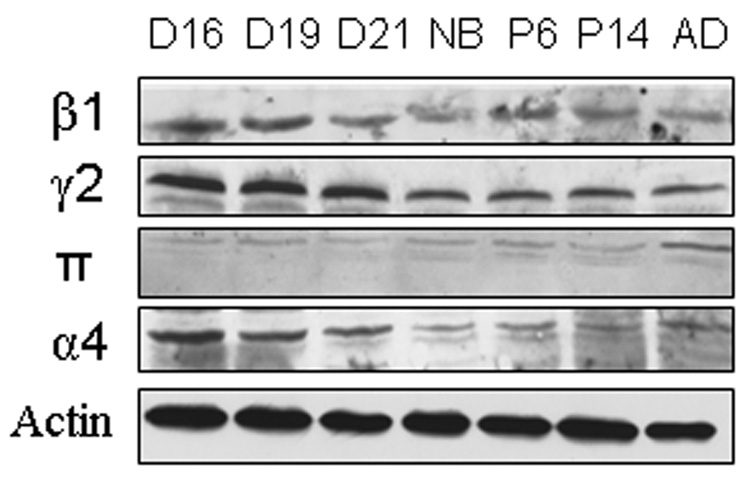
We further determined the changes in protein levels of GABA receptor subunits during lung development. We chose one subunit in each group as a representative. Whole lung tissue was run on SDS-PAGE, and Western blot was performed using specific antibodies. As shown in Fig. 3, the protein level of the β1 subunit in Group 1 was high at D16 and D19 and declined after D21. The γ2 subunit (Group 2) protein levels were the same at D16, D19 and D21, and then dropped after birth. On the other hand, the π subunit (Group 3) protein level was the highest in the adult lungs. Finally, the α4 subunit (Group 4) expressed the greatest amount of protein at D16, followed by a gradual decline in expression. The equal protein loading was confirmed by using anti-β-actin antibodies (Fig. 3).
Figure 3. Protein expression levels of GABA receptors during lung development.
Rat fetal lung tissues at gestational day 16 (D16), day 19 (D19), day 21 (D21) and lung tissues from newborn (NB), postnatal day 6 (P6), day 14 (P14) and adult (AD) rats were separated on SDS-PAGE. Each subunit (β1, γ2, π and α4) was detected with specific antibodies by Western blot. β-actin was used as a loading control. The blots shown are one representative of two independent preparations.
1.5 Cellular localization of GABA receptors in fetal lung tissue
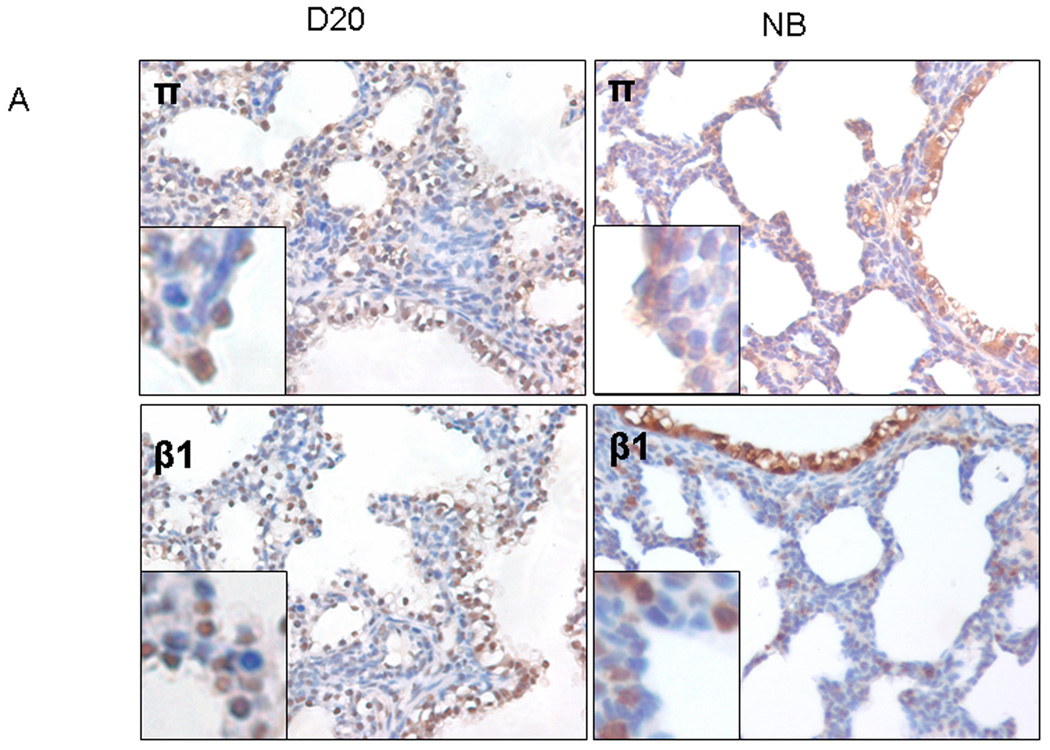
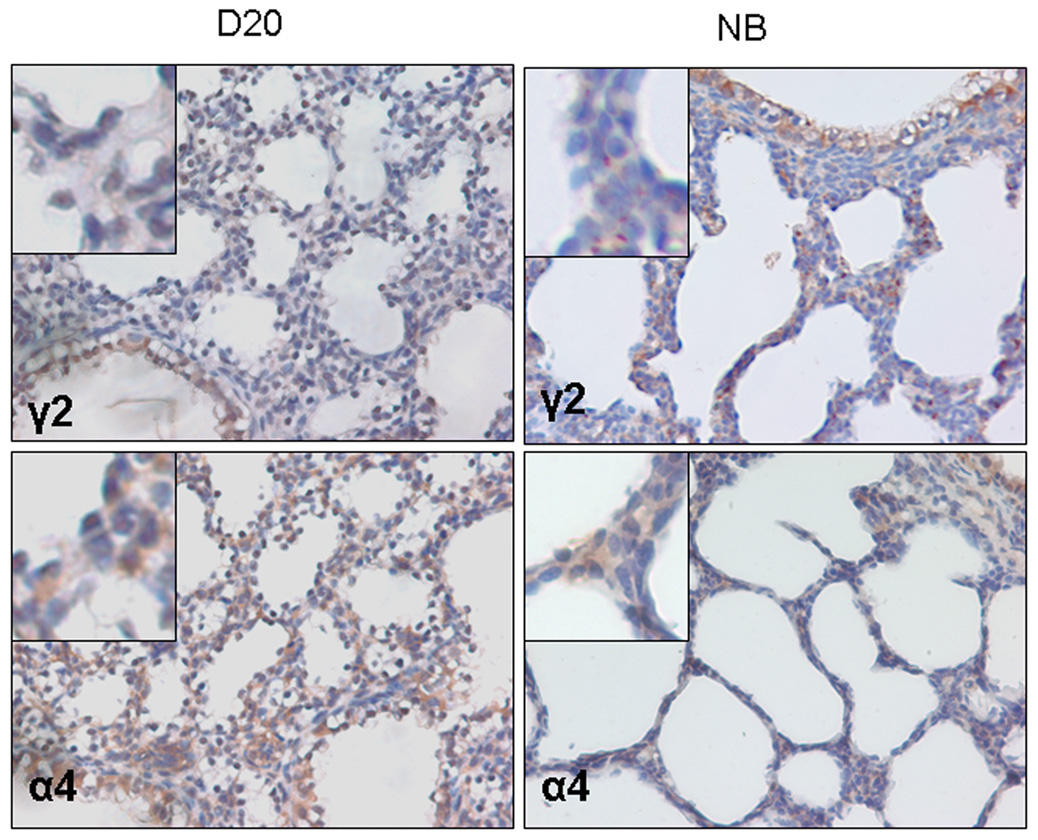
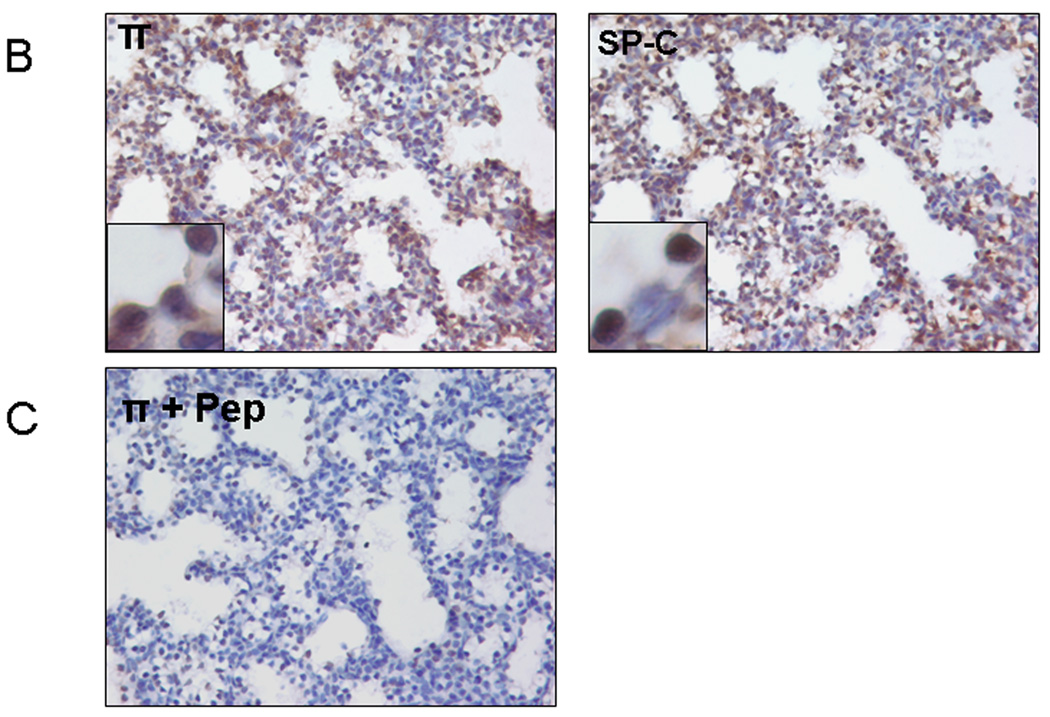
Using immunostaining, we analyzed the location of GABA receptors in D20 and new born (NB) lung tissues. The π subunit showed positive staining primarily in the airway epithelial cells in both D20 and NB lung tissues in particular at D20 (Fig. 4A insert). The π subunit also co-localized partially with SP-C, an alveolar type II cell marker (Fig. 4B). The β1 subunit had a similar staining pattern as that of the π subunit. The γ2 subunit appeared to be mainly present in the large airway epithelial cells. However, the α4 subunit exhibited a diffuse staining pattern, showing signals in epithelial cells and mesenchymal cells. The negative controls with omitted primary antibodies (data not shown) or with π subunit blocking peptide did not show positive signals (Fig. 4C).
Figure 4. Localization of GABA receptors in fetal lungs.
(A) Fetal lung tissues at gestational day 20 (D20) or newborn lung (NB) tissues were stained with anti-GABA receptor β1, γ2, π and α4 subunits antibodies. (B) Co-localization of π subunit and SP-C (an alveolar type II cell marker). Two serial day 20 fetal lung sections were stained with anti-π subunit and anti-SP-C antibodies. (C) Negative control. Anti-π subunit antibodies were incubated with a blocking peptide (Pep) overnight before performing immunostaining on D20 lung tissue.
1.6 Developmental alteration of GABA receptor mRNA in pulmonary epithelial cells
With the realization of the potential importance of pulmonary epithelial cells in fluid transport of fetal lungs, we further determined the mRNA abundance of GABA receptor subunits in rat pulmonary epithelial cells during fetal lung development. Similar to the fetal lung tissue, we clustered the GABA receptor subunits based on their mRNA expression patterns (Fig. 5). They can also be grouped into 4 groups based on their relative mRNA expression patterns. Group 1 contained 9 subunits, α1, α2, α4, β1, β2, γ1, θ, ρ1 and ρ3. All of them had a peak expression at birth. The representative gene in Group 1 was subunit ρ3. Its mRNA level in newborn rat pulmonary epithelial cells was 6-fold higher than that in D18 and was significantly higher than all the other time points (Fig. 5A). Group 2 included α3, ε, π and ρ2 subunits (Fig. 5B). Those genes either kept a relative stable expression or showed an increasing trend during development. The representative gene in this cluster was the π subunit. Its mRNA levels in new born and adult were significantly higher than those of D18 and D19. Group 3 was composed from γ2 and γ3 subunits. Both genes showed a relative low expression on D20 and D21 in comparison with D18 and adult (Fig. 5C). The α5 and α6 subunits constituted Group 4, where both genes had a lower expression in adult pulmonary epithelial cells than in all other time periods (Fig. 5D).
Figure 5. Developmental alteration of GABA receptor mRNA in pulmonary epithelial cells.
Pulmonary epithelial cells were isolated from fetal rats at gestational day 18 (D18), day 19 (D19), day 20 (D20) and day 21 (D21), or from newborn rats (NB) and adult rats (AD). Total RNA was extracted and reverse-transcribed into cDNA. The mRNA levels of GABA receptor subunits were determined with real-time PCR and expressed as log copy number per 108 copies of 18S rRNA. These subunits were classified into 4 groups according to their expression patterns. Group 1, α1, α2, α4, β1, β2, γ1, θ, ρ1 and ρ3 (A); Group 2, α3, ε, π and ρ2 (B); Group 3, γ2 and γ3 (C); Group 4, α5 and α6 (D). Data shown are means ± SE (n ≥ 3 independent preparations).
1.7 Summary and further discussion
In the current study, we used real-time PCR, Western blot and immunohistochemtry to demonstrate that multiple ionotropic GABA receptor subunits are expressed in fetal rat lungs and pulmonary epithelial cells. The expression of those subunits is developmentally regulated in the lungs and pulmonary epithelial cells.
Essentially, all the GABA receptor subunits were expressed in the brain. The heterogeneous GABA receptors may reflect the complexity in controlling nervous network. Many of the receptors were also expressed in the fetal lung. Some of them were enriched in pulmonary epithelial cells, but others were expressed higher in the lung tissue than in pulmonary epithelial cells, indicating that GABA receptors may also be present in other lung cells rather than pulmonary epithelial cells. Subunits α3, α4, β1, γ1, γ2, θ and π in fetal pulmonary epithelial cells may also constitute functional receptors in these cells because their expression was higher in fetal pulmonary epithelial cells than in the fetal lung (Fig. 1). The GABA receptor ρ subunits in fetal pulmonary epithelial cells were more abundant than in brain. They could constitute the GABAC receptors, which could be homo-or hetero-pentameric. The ρ subunit family is also highly expressed in the retina (Enz et al., 1995). Functional GABAC receptors were also found in several hormone secretory organs, such as the pancreas and the pituitary glands (Gamel-Didelon et al., 2003; Jansen et al., 2000). Therefore, the necessary subunits needed to assemble a functional GABAA or GABAC receptor are present in fetal pulmonary epithelial cells.
The expression patterns of GABA receptors during fetal lung development are much more complicated than the channels encoded by a single gene because they are composed of multiple subunits and each subunit has multiple isoforms. Similar to CFTR and ClC in the developing lungs (Brochiero et al., 2004; Blaisdell et al., 2000; Harris et al., 1991; Murray et al., 1996; Lamb et al., 2001; Murray et al., 1995), the mRNA of some GABA receptor subunits (Group 1, Fig. 2A) were relatively high in mid-gestation, but decreased in late gestation and remained low in adult. Interestingly, some of those subunits, such as α2 and α5, were also less abundant in the adult brain than in the fetal brain (Poulter et al., 1992). Some subunits in the lung from Groups 2, 3 and 4 were constitutively expressed or only slightly fluctuated from mid-gestation to adult (Fig. 2B–D). This demonstrates that these subunits are ontogeny-“conserved” in the lung. Many of these subunits, including α3, α4, β2, and γ2, were also more developmentally “conserved” than other subunits in the brain (Huang and Dillon, 2002; Poulter et al., 1992). These subunits may be the basic components for forming functional GABA receptors in both embryonic and postnatal brains and lungs. The ρ1–3 subunits were only detected in the postnatal brain and their expression was up-regulated one week after birth. This is consistent with the results from the visual system (Alakuijala et al., 2005; Young and Cepko, 2004). In the lung, ρ1 subunit mRNA was up-regulated at gestational day 19 and at birth (Fig. 2B). The other two ρ subunits had a different expression pattern than that of the ρ1 subunit.
We also determined the protein levels of selected GABA receptor subunits during lung development. When the developmental trend in expression level was compared, π and β1 subunits had a better correlation between protein and mRNA than α4 and γ2 subunits. A large scale of comparison showed that mRNA and protein expression was not always correlated (Gygi et al., 1999).
The expression patterns of GABA receptor subunits in pulmonary epithelial cells displays a “clearer” pattern than those in whole lung tissue probably because of the heterogeneous nature of the lungs, which contains more than 40 types of cells. The most obvious expression characteristics for many of the subunits are that their mRNAs are relatively unchanged during fetal stages, but markedly up-regulated at birth (Group 1, Fig. 5A).
2. EXPERIMENTAL PROCEDURES
2.1 Materials
Pregnant Sprague-Dawley (S.D.) and adult male rats were purchased from Charles River Breeding Laboratories (Wilmington, MA). The Oklahoma State University Animal Use and Care Committee approved all the animal protocols used in this study. Nylon meshes (160-, 37-, and 15-µm) were purchased from Tetko (Elmsford, NY). Minimum essential medium (MEM), trypsin, collagenase V, DNase I, rat IgG were from Sigma-Aldrich (St Louis, MO). M-MLV reverse transcriptase was from Invitrogen (Calsbad, CA). Polyclonal goat anti-GABA receptor π subunit was from Santa Cruz Biotechnology (sc-17714, Santa Cruz, CA). Polyclonal rabbit antibodies against GABA receptor β1 and γ2 subunits were from Abcam (ab16703 and ab4073, Cambridge, MA), respectively. Polyclonal rabbit GABA receptor α4-subunit antibodies were from Abcam (ab4120) or Covance (PRB-549P, Berkeley, CA). Monoclonal anti-rat leukocyte common antigen antibodies (anti-LC) were from Accurate (Westbury, NY). Polyclonal anti-rat T1 α antibody was raised in rabbits by Custom Service of Affinity Bio-reagents (Golden, CO) and affinity-purified. HRP-conjugated goat anti-rabbit and donkey anti-goat IgG were from BioRad (Hercules, CA) and Jackson ImmunoResearch Lab (West Grove, PA). Goat anti-rabbit IgG BioMag® beads were from QIAGEN (Valencia, CA). Sheep anti-rat IgG magnetic beads and goat anti-mouse IgG Dynabeads were from Dynal Biotech (Lake Success, NY). Biotinylated horse anti-goat or anti-rabbit IgG and the ABC staining kit were from Vector Labs (Burlingame, CA). TRI reagent was from Molecular Research Center (Cincinnati, Ohio). DNA-free TM DNase was from Ambion (Austin, Texas). Random hexamer primers were from Promega (Madison, WI). Primers for real-time PCR were synthesized by MWG Biotech Inc. (High Point, NC).QuantiTech TM SYBR Green PCR master mix was from QIAGEN (Foster city, CA). GENECLEAN Turbo for PCR was obtained from Qbiogene (Carlsbad, CA).
2.2 Collection of fetal lung tissues and isolation of fetal pulmonary epithelial cells
Fetal lung tissue collection and pulmonary epithelial cell isolation were carried out as described (Fraslon-Vanhulle et al., 1991). Briefly, fetal lungs were removed from pregnant S.D. rats at gestational days 18, 19, 20 and 21. The fetal lungs of rats from the same mother were pooled as one group. After removing the trachea, bronchi, thymus and hearts, 3–4 fetal lungs were frozen in liquid nitrogen for RNA isolation; the other lungs were used for fetal pulmonary epithelial cell isolation. The fetal lungs were chopped and digested with an enzyme solution (1 mg/ml of collagenase V, 0.2 mg/ml of trypsin, and 20 µg/ml of DNAse I in MEM) for about 20 ~ 60 min until no tissue was left. The cell mixture was filtered sequentially through 160-and two 37-µm nylon meshes. Thereafter, the cells were further purified by panning on a bacterial culture plastic dish four times (40 min each time) at a density of 35 million cells per 100 mm dish for removing fibroblasts. Then a hypotonic solution (75 mM KCl) was applied to the cell suspension for 2 ~ 5 min to remove red blood cells. The protocol for pulmonary epithelial cells isolation from neonatal rats was the same as that for fetal lungs, except that 0.036 units/ml elastase was included in the digestion enzyme solution. The purity of the isolated fetal pulmonary epithelial cells cells was 90 ~ 95% as determined by alkaline phosphatase staining (Miller and Hook, 1990) and FITC-labeled Maclura pomifera agglutinin staining. The viability was >90% as determined by Trypan blue exclusion.
2.3 Isolation of highly pure adult type II cells
Highly pure type II cells were isolated from the lungs of adult male S.D. rats (180 ~ 200 g) according to our recently improved methods (Chen et al., 2004a). The perfused lungs were lavaged, digested with 3 units/ml elastase, chopped, and filtered through 160-, 37-, and two 15-µm nylon meshes. The cell mixture was panned twice on rat IgG-coated plates (40 min each). The cell suspension was further incubated with 40 µg/ml of rat IgG at room temperature for 15 min, and then sheep-anti-rat IgG magnetic beads (100 µl/rat) were applied to remove macrophages. The cells were again incubated with anti-LC (40 µg/ml) and anti-rat T1 α (40 µg/ml), followed by incubation with goat anti-mouse Dynabeads (100 µl/rat) and goat anti-rabbit IgG BioMag® beads (500 µl/rat) to remove leukocytes and type I cells. The purity of the resulting type II cell preparation was 95 ~ 98%. The viability was >98%.
2.4 Absolute quantitative real-time PCR
Total RNA was extracted from tissue or cells with TRI reagents. Absolute quantitative real-time PCR was performed as previously described (Jin et al., 2005). Briefly, the total RNA was digested with DNase I to remove genomic DNA. Absence of DNA contamination in the total RNA samples was verified by PCR, in which no PCR products were found from the amplification of the non-reverse transcribed RNA with 18S rRNA primers for 40 cycles. The integrity of the RNA was determined by running the RNA samples on a 1.2% agarose gel. The intensities of the 28S rRNA bands were twice of those of the 18S rRNA bands. The total RNA was reverse-transcribed into cDNA with M-MLV and random hexamer. The primers for real-time PCR were the same as previously described (Jin et al., 2006). The standards used for constructing the standard curves were amplified by traditional PCR and purified with GENECLEAN Turbo. Real-time PCR was performed on an ABI 7700 system (Applied Biosystems, Foster city, CA) using SYBR Green detection. The copy numbers of target genes in cDNA samples were normalized with 18S rRNA and expressed as log values.
2.5 Western Blot
The fetal lung tissues were collected and stored at −80°C until use. The tissues were lysed with the M-PER mammalian protein extraction reagent containing Halt Protease Inhibitor Cocktail (Pierce) and homogenized on ice. The lysates were centrifuged at 2,500 g for 10 min. The supernatants were then collected and the protein concentrations were determined using the BioRad Dc protein assay. The protein samples were aliquoted and stored at −80°C. The proteins were separated on SDS-PAGE and transferred to a nitrocellulose membrane. After being blocked, the blots were incubated with antibodies against GABA receptor α4-, β1-, γ2-and π-subunits (dilutions: 1:800, 1:800, 1:500 and 1:50, respectively) followed by incubation with goat-anti rabbit or donkey anti-goat secondary antibodies (dilutions: 1:2000, 1:2000, 1:1000 and 1:1000, respectively). The blots were developed with enhanced chemiluminescence reagents.
2.7 Immunohistochemistry
This was done as previously described (Weng et al., 2006). The D20 fetal lung tissue sections were treated with 3% H2O2 for 10 min. After being washed, the slides were boiled in 20 mM citrate buffer (pH 6.0) for 20 min and then permeabilized in 0.3% Trition X-100 for 10 min. The fetal lung tissues were blocked with 10% horse serum for 30 min. Slides were then incubated with antibodies against GABA receptor α4, β1, γ2 and π subunits (dilutions: 1:100, 1:100, 1:100 and 1:50, respectively) for 3 h at 37°C or overnight at 4°C inside a moisture chamber, followed by incubation with biotinylated horse anti-rabbit or anti-goat secondary antibodies (dilution: 1:100) for 45 min at 37°C, followed by incubation with the ABC reagents for 30 min at room temperature. The slides were stained with DAB peroxidase substrate, counterstained with hematoxylin QS solution and mounted with Permount solution.
2.8 Statistics
The relative expression levels were expressed as ratios to D18 lungs/cells. Data were means ± S.E. in all the graphs. Statistical analysis was performed using SigmaStat 3.2 by one-way ANOVA, followed with the Least Significance Difference (LSD). Significance was taken when P<0.05.
ACKNOWLEDGMENTS
We thank Dr. Melanie A. Breshears for her help in the evalution of lung tissue slides and Dr. Heidi M. Stricker, Ms. Candice Marsh and Ms. Tisha Posey for editorial assistance. This work was funded by NIH (R01 HL-052146, R01 HL-071628 and R01 HL-083188), March of Dimes (6FY05-76) and the American Heart Association (0315256Z, 0315260Z and 0610143Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akinci MK, Schofield PR. Widespread expression of GABA(A) receptor subunits in peripheral tissues. Neurosci Res. 1999;35:145–153. doi: 10.1016/s0168-0102(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternack M. GABA receptor rho subunit expression in the developing rat brain. Brain Res.Dev.Brain Res. 2005;154:15–23. doi: 10.1016/j.devbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Blaisdell CJ, Edmonds RD, Wang XT, Guggino S, Zeitlin PL. pH-regulated chloride secretion in fetal lung epithelia. Am.J.Physiol Lung Cell Mol.Physiol. 2000;278:L1248–L1255. doi: 10.1152/ajplung.2000.278.6.L1248. [DOI] [PubMed] [Google Scholar]
- Bland RD, Nielson DW. Developmental changes in lung epithelial ion transport and liquid movement. Annu.Rev.Physiol. 1992;54:373–394. doi: 10.1146/annurev.ph.54.030192.002105. [DOI] [PubMed] [Google Scholar]
- Bormann J. The 'ABC' of GABA receptors. Trends.Pharmacol.Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- Brochiero E, Dagenais A, Prive A, Berthiaume Y, Grygorczyk R. Evidence of a functional CFTR Cl(−) channel in adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L382–L392. doi: 10.1152/ajplung.00320.2002. [DOI] [PubMed] [Google Scholar]
- Carter EP, Wangensteen OD, O'Grady SM, Ingbar DH. Effects of hyperoxia on type II cell Na-K-ATPase function and expression. Am J Physiol. 1997;272:L542–L551. doi: 10.1152/ajplung.1997.272.3.L542. [DOI] [PubMed] [Google Scholar]
- Chen JW, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of hightly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest. 2004a;84:727–735. doi: 10.1038/labinvest.3700095. [DOI] [PubMed] [Google Scholar]
- Chen Z, Jin N, Narasaraju T, Chen J, McFarland LR, Scott M, Liu L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem.Biophys.Res.Commun. 2004b;319:774–780. doi: 10.1016/j.bbrc.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Edmonds RD, Silva IV, Guggino WB, Butler RB, Zeitlin PL, Blaisdell CJ. ClC-5: ontogeny of an alternative chloride channel in respiratory epithelia. Am.J.Physiol Lung Cell Mol.Physiol. 2002;282:L501–L507. doi: 10.1152/ajplung.00207.2001. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstatter JH, Hartveit E, Wassle H, Bormann J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur.J.Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS, Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen.Physiol. 2002;119:199–207. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Norlin A, Baines DL. Salt and water transport across the alveolar epithelium in the developing lung: Correlations between function and recent molecular biology advances (Review) Int.J.Mol.Med. 1998;2:515–531. doi: 10.3892/ijmm.2.5.515. [DOI] [PubMed] [Google Scholar]
- Fraslon-Vanhulle C, Bourbon JR, Batenburg JJ. Culture of fetal alveolar epithelial type II cells. Cell & Tissue Culture: Laboratory Procedures. 1991;2:2.1–2.12. [Google Scholar]
- Gamel-Didelon K, Kunz L, Fohr KJ, Gratzl M, Mayerhofer A. Molecular and physiological evidence for functional gamma-aminobutyric acid (GABA)-C receptors in growth hormone-secreting cells. J.Biol.Chem. 2003;278:20192–20195. doi: 10.1074/jbc.M301729200. [DOI] [PubMed] [Google Scholar]
- Gillie DJ, Pace AJ, Coakley RJ, Koller BH, Barker PM. Liquid and ion transport by fetal airway and lung epithelia of mice deficient in sodium-potassium-2-chloride transporter. Am J Respir Cell Mol Biol. 2001;25:14–20. doi: 10.1165/ajrcmb.25.1.4500. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol.Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A, Chalkley G, Goodman S, Coleman L. Expression of the cystic fibrosis gene in human development. Development. 1991;113:305–310. doi: 10.1242/dev.113.1.305. [DOI] [PubMed] [Google Scholar]
- Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J Biol Chem. 1997;272:15346–15350. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Functional characterization of GABA(A) receptors in neonatal hypothalamic brain slice. J.Neurophysiol. 2002;88:1655–1663. doi: 10.1152/jn.2002.88.4.1655. [DOI] [PubMed] [Google Scholar]
- Jansen A, Hoepfner M, Herzig KH, Riecken EO, Scherubl H. GABA(C) receptors in neuroendocrine gut cells: a new GABA-binding site in the gut. Pflugers Arch. 2000;441:294–300. doi: 10.1007/s004240000412. [DOI] [PubMed] [Google Scholar]
- Jin N, Kolliputi N, Gou D, Weng T, Liu L. A novel function of ionotropic gamma -aminobutyric acid receptors involving alveolar fluid homeostasis. J Biol.Chem. 2006;281:36012–36020. doi: 10.1074/jbc.M606895200. [DOI] [PubMed] [Google Scholar]
- Jin N, Narasaraju T, Kolliputi N, Chen J, Liu L. Differential expression of GABA(A) receptor pi subunit in cultured rat alveolar epithelial cells. Cell Tissue Res. 2005;321:173–183. doi: 10.1007/s00441-005-1130-8. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Guo Y, Helton ES, Matalon S, Jackson RM. Modulation of rat lung Na+,K(+)-ATPase gene expression by hyperoxia. Exp.Lung Res. 1998;24:173–188. doi: 10.3109/01902149809099581. [DOI] [PubMed] [Google Scholar]
- Lamb FS, Graeff RW, Clayton GH, Smith RL, Schutte BC, McCray PB., Jr Ontogeny of CLCN3 chloride channel gene expression in human pulmonary epithelium. Am.J.Respir.Cell Mol.Biol. 2001;24:376–381. doi: 10.1165/ajrcmb.24.4.4114. [DOI] [PubMed] [Google Scholar]
- Miller BE, Hook GE. Hypertrophy and hyperplasia of alveolar type II cells in response to silica and other pulmonary toxicants. Environ.Health Perspect. 1990;85:15–23. doi: 10.1289/ehp.85-1568321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CB, Chu S, Zeitlin PL. Gestational and tissue-specific regulation of C1C-2 chloride channel expression. Am.J.Physiol. 1996;271:L829–L837. doi: 10.1152/ajplung.1996.271.5.L829. [DOI] [PubMed] [Google Scholar]
- Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol. 1995;12:597–604. doi: 10.1165/ajrcmb.12.6.7766424. [DOI] [PubMed] [Google Scholar]
- Narasaraju TA, Jin N, Narendranath CR, Chen Z, Gou D, Liu L. Protein nitration in rat lungs during hyperoxia exposure: a possible role of myeloperoxidase. Am.J.Physiol Lung Cell Mol.Physiol. 2003;285:L1037–L1045. doi: 10.1152/ajplung.00008.2003. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Macdonald RL. Incorporation of the pi subunit into functional gamma-aminobutyric Acid(A) receptors. Mol Pharmacol. 1999;56:598–610. doi: 10.1124/mol.56.3.598. [DOI] [PubMed] [Google Scholar]
- O'Grady SM, Jiang X, Ingbar DH. Cl-channel activation is necessary for stimulation of Na transport in adult alveolar epithelial cells. Am.J.Physiol Lung Cell Mol.Physiol. 2000;278:L239–L244. doi: 10.1152/ajplung.2000.278.2.L239. [DOI] [PubMed] [Google Scholar]
- Olver RE, Walters DV, Wilson M. Developmental regulation of lung liquid transport. Annu.Rev.Physiol. 2004;66:77–101. doi: 10.1146/annurev.physiol.66.071702.145229. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Barker JL, O'Carroll AM, Lolait SJ, Mahan LC. Differential and transient expression of GABAA receptor alpha-subunit mRNAs in the developing rat CNS. J.Neurosci. 1992;12:2888–2900. doi: 10.1523/JNEUROSCI.12-08-02888.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle LG, Li DC, Ye H, Lee E, Talbot CR, Boucher RC. Distribution of ion transport mRNAs throughout murine nose and lung. Am.J.Physiol Lung Cell Mol.Physiol. 2000;279:L14–L24. doi: 10.1152/ajplung.2000.279.1.L14. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem.Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr.Top.Med.Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Weng T, Chen Z, Jin N, Gao L, Liu L. Gene Expression Profiling Identifies Regulatory Pathways Involved in the Late Stage of Rat Fetal Lung Development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1027–L1037. doi: 10.1152/ajplung.00435.2005. [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann.N.Y.Acad.Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41:867–879. doi: 10.1016/s0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matalon S. Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats. Proc Natl Acad Sci U S A. 1995;92:8418–8422. doi: 10.1073/pnas.92.18.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]