Abstract
The species composition and population dynamics of adult mosquitoes in a wetland near Iuka, MS, were analyzed over a 6-yr period (1997–2002) and reverse transcription-polymerase chain reaction (PCR) detection rates of arboviruses determined during five of those years. Blood meals of three likely vector species were identified using a PCR-based method that allows identification of the host to species. Culex erraticus (Dyar & Knab) composed 51.9% of the population during the 6-yr period with 295 females collected per trap night. Eastern equine encephalomyelitis (EEE) virus was detected in six genera of mosquitoes [Coquillettidia perturbans (Walker), Culex restuans Theobald, Culex salinarius Coquillett, Culex erraticus (Dyar & Knab), Anopheles crucians Wiedemann, Anopheles quadrimaculatus Say, Aedes vexans (Meigen), Ochlerotatus triseriatus Say, and Psorophora ferox Humboldt) with positive pools occurring in 1998, 1999, and 2002. Culiseta melanura Coquillett occurred at a low level (<1%) and was not infected. Saint Louis encephalitis virus was detected once in a single pool of Cx. erraticus in 1998. Neither West Nile virus nor LaCrosse virus was found. Minimum infection rates per 1000 females tested of competent vectors of EEE virus were variable and ranged from 0.14 for Cx. erraticus to 40.0 for Oc. triseriatus. Thirty-nine species of birds were identified in the focus with blood-engorged mosquitoes found to contain meals (n = 29) from eight avian species. The majority of meals was from the great blue heron, Ardea herodias L. (n = 55%), but when bird abundance data were adjusted for avian mass, the brown-headed cowbird, Molothrus ater (Boddaert); blue jay, Cyanocitta cristata (L.); and northern mockingbird, Mimus polyglottos (L.), were overrepresented as hosts.
Keywords: Saint Louis encephalitis, eastern equine encephalomyelitis virus, Culex erraticus, Culiseta melanura, blood meal identification
Long-term observations of mosquito populations in Mississippi are uncommon, particularly in relation to endemic arbovirus activity. Early reports dealt largely with information collected over short periods of time, primarily during outbreaks of Saint Louis encephalitis (SLE) virus (Powell and Blakey 1977) or sporadic cases of eastern equine encephalomyelitis (EEE) (Hugh-Jones and Samui 1993). In an attempt to extend information on the ecology of mosquitoes and arboviruses in this area, collections of mosquitoes were made systematically over a 6-yr period (1997–2002) in an isolated cypress/mixed hardwood habitat in northeastern Mississippi.
We describe here the composition and dynamics of mosquito populations in this wetland throughout the period of greatest insect activity, provide data on infection rates of virus during 5 of those 6 yr (1998–2002), and identify avian blood meals of several likely vector species by using a polymerase chain reaction (PCR)-based method that allows the identification of the blood meal host to the species level. These findings are discussed with regard to the biology of several vector species in relation to the local avifauna.
Materials and Methods
Site
The study habitat, located near Iuka, MS (34°49′41″N, 89°33′52″W), is a privately owned preserve of some 24–28 hectares (60–70 acres) in Tishomingo County that abuts the Bear Creek floodplain. The original old growth cypress trees, Taxodium distichum L., were logged some 65 yr ago, and substantial secondary growth of tupelo gum trees, Nyssa aquatica L., and new cypress has occurred since then. Water levels fluctuate seasonally based on rainfall, but the core area of the wetland remains permanently flooded. Monthly rainfall during the 6-mo mosquito season (April–September) was recorded each year at Iuka to correlate precipitation to mosquito abundance.
Mosquito Collections
Mosquitoes were collected from several locations in the core area of the swamp over a 6-yr period (1997–2002) by using portable CDC light-traps baited with CO2 (Sudia and Chamberlain 1967). Collections usually were made once a week during the same 5-mo period (May–October) each year when adult mosquitoes were most active. Specimens were transported live to the laboratory, placed on a chill table, and identified under a binocular microscope. Species were sorted into pools ranging from 1 to 50 mosquitoes and stored at −70°C. Mosquitoes containing blood were removed from collections and not processed for virus. Blood-engorged mosquitoes were collected in 2002 from black resting boxes (Edman et al. 1968) by using a vacuum suction device (Nasci 1981), returned to the laboratory, and identified as noted above.
Virus Identification
Mosquitoes collected during 1998–2002 were analyzed for EEE, SLE, and LaCrosse (LAC) viruses using a reverse transcription (RT)-PCR protocol (Lee et al. 2002). A separate assay for West Nile virus (WNV) was conducted on samples beginning in 2000. Pools of up to 50 individuals were homogenized in 1.5 ml of BA-1 tissue culture medium and then centrifuged at 13,000 × g for 5 min at room temperature. A total of 140 μl of the resulting supernatant was removed, and RNA was purified from the aliquot by using the QiaAMP viral RNA extraction kit (QIAGEN, Valencia, CA). RNA was prepared following the manufacturer’s instructions, with the exception that the number of washes with buffers AW1 and AW2 were increased from one to two.
RNA prepared from the mosquito pools was used as a template in two RT-PCR assays to detect the presence of viral RNA. EEE, SLE, and LAC RNA were detected in a multiplex RT-PCR as described previously (Lee et al. 2002). WNV RNA was detected using primers recommended by the Centers for Disease Control and Prevention (Lanciotti et al. 2000). In brief, 4 μl of RNA prepared as described above was used in a 50-μl total volume one-step RT-PCR amplification reaction by using reagents provided by QIAGEN (one-step RT-PCR) and the primers WNV 212 (5′ TTGTGTTGGCTCTCTTGGCGTTCTTT 3′) and WNV 619 (5′ CAGCCGACAGCACTGGACATTCATA 3′). Amplification reactions contained 1× QIAGEN one-step RT-PCR buffer, 400 μM each of dATP, dGTP, dCTP, and dTTP, 0.6 μM of each primer, and 2 μl of QIAGEN one-step RT-PCR enzyme mix. Reaction conditions consisted of 50°C for 30 min, 95°C for 15 min, followed by 40 cycles each consisting of 94°C for 30 s, 58°C for 30 s, and 68°C for 2 min. Reactions were completed with a final extension at 72°C for 10 min. Amplification products were detected by electrophoresis on a 1.5% agarose gel, followed by staining in 2 g/ml ethidium bromide. Samples were scored as putatively positive if an amplification product of the expected size was visible. Putative positive samples were confirmed in a second independent RT-PCR and by matching a DNA sequence of the resulting PCR product to the virus in question.
Calculation of Infection Rates
Minimum virus infection rates (MIRs) were calculated using a standard formula recently evaluated for reliable detection of alphaviruses (Nasci and Mitchell 1996). Infection rates were also analyzed using the Poolscreen2 program (http://main.uab.edu/show.asp? Durki = 46672; password bstpoolscreen2001) that calculates the prevalence of infection from the number of positive PCR pools (Katholi et al. 1995). This method is based upon the observation that the probability that a pool will have no positive mosquitoes can be represented in terms of the unknown probability that an individual insect is infected. The methodology can be applied when the pools are of equal size or when the pools have variable size. In either case, maximum likelihood estimates are computed and Klopper–Pearson confidence intervals are calculated.
Avian Census
In the spring and early summer, most species of birds vocalize frequently during the morning. By counting the number of individuals of each species that vocalize, a fast and accurate estimate of the abundance of species present within an area can be derived (Robbins et al. 1986). Using this approach, bird abundance at the study site during the breeding season was estimated by conducting point counts in June 2002. Except for the recruitment of young birds produced that year, the abundance of forest birds remains essentially stable throughout the summer, and estimates on 1 day during this period are normally representative for the entire period (Hamel et al. 1996).
Point counts were conducted by walking a jeep trail/foot path that skirts the southern edge of the wetland and counting birds at 20 stops positioned every 200 m. These counts began at first light and ended at 10:00 a.m. Point counts lasted 3 min, and all birds seen or heard within 100 m of the observer during the 3-min counts were recorded. This is a standard census technique used for rapid assessment of the density of breeding birds (Robbins et al. 1986, Bibby et al. 1992, Ralph et al. 1995). Although counts reveal displaying males during the day, these songbirds also roost within their territories.
Blood Meal Identification
Blood-engorged specimens were stored at −70°C until identification of avian host source to the species level was made using a combined PCR-heteroduplex method (Apperson et al. 2002). In brief, genomic DNA prepared from blood-fed mosquitoes was used as a template in a nested PCR amplification reaction by using primers that were designed to specifically amplify vertebrate cytochrome B sequences, as described previously (Lee et al. 2002). Two aliquots of 6 μl of the resulting PCR products then were mixed separately with 6 μl of PCR product driver derived from northern cardinal, Cardinalis cardinalis (L.), and Carolina chickadee, Poecile carolinensis (Audubon). The combined sample and driver PCR products were mixed with 8 μl of 10 mM Tris-HCl (pH 8.0), 1 mM EDTA and overlaid with 10 μl of mineral oil. The mixture was denatured at 99°C for 2.5 min and allowed to form heteroduplex products by slow cooling to room temperature. Heteroduplex products were separated as described previously (Lee et al. 2002), and samples were grouped according to their resulting pattern on the two heteroduplex gels. Representative samples from each group were then subjected to DNA sequencing and the identity of the group determined by comparison of the DNA sequences to data in the GenBank DNA sequence database.
Analysis of Relative Host-Feeding Preferences in Vector Mosquitoes
The blood meal data were compared with the avian abundance data derived from the point count by using the feeding index method of Kay et al. (1979). Initially, the feeding indices were calculated using the unadjusted point count data alone. The feeding indices then were recalculated using abundance data that was adjusted to take into account the overall biomass of each species at the site. Biomass corrected values were calculated by multiplying the number of individuals of each species observed by the average body mass of each species obtained from published sources (Dunning 1984).
Results
More than 102,000 mosquitoes representing eight genera and 24 species were collected in CO2-baited light traps during the 6-yr study (Table 1). As anticipated, the most abundant species were associated with permanent, standing water and composed >94% of the mosquitoes collected. The remainder was largely floodwater species that used temporary pools along the margins of the swamp and flood plain as larval habitats. On average during the 6-yr period, adult mosquito activity peaked in July and then declined gradually through September, at which time collections were usually terminated.
Table 1.
Mosquitoes collected at the Iuka, MS, study site (1997–2002)
| Species | No. of Adults | % of Collection |
|---|---|---|
| Cx. erraticus | 53,221 | 51.9 |
| Cq. perturbans | 16,963 | 16.6 |
| An. crucians | 16,891 | 16.5 |
| An. quadrimaculatus s.la | 9,287 | 9.0 |
| Ae. vexans | 1,941 | 1.9 |
| Ur. sapphirina | 1,453 | 1.4 |
| An. walkeri | 1,072 | 1.0 |
| Othersb | 1,651 | 1.6 |
An. quadrimaculatus occurs as a sibling species complex; no attempt was made to identify specimens to this level.
In descending order of abundance: Cx. spp., An. punctipennis, Ps. ferox, Ps. columbiae, Cx. salinarius, Cs. melanura, Oc. triseriatus, Oc. sticticus, Ae. albopictus, Ps. howardii, Ae. spp., Oc. fulvus pallens, Oc. atlanticus/tormentor, Oc. canadensis, Cx. territans, Oc. thibaulti, Ps. ciliata, and Ps. horrida.
Overall, an average of 396 mosquitoes per trap night was collected during 259 trap nights. Culex erraticus (Dyar & Knab) was the most abundant mosquito during the 6-yr period, with 295 females collected per trap night (fptn). Populations of Cx. erraticus fluctuated inversely in relation to seasonal rainfall, with the most intense activity occurring during 1999 and 2000 (312 and 471 females per trap night, respectively; Fig. 1), followed by a slow decline during 2001 and 2002 (Fig. 1). Other mosquitoes routinely collected during the 6-yr period included Coquillettidia perturbans (Walker) (an average of 65 fptn), Anopheles crucians Wiedemann (also 65 fptn), and Anopheles quadrimaculatus Say sensu lato (36 fptn). Aedesvexans(Meigen) (7 fptn), Uranotaenia sapphirina (Osten Sacken) (6 fptn), and Anopheles walkeri Theobald (4 fptn) were collected occasionally.
Fig. 1.
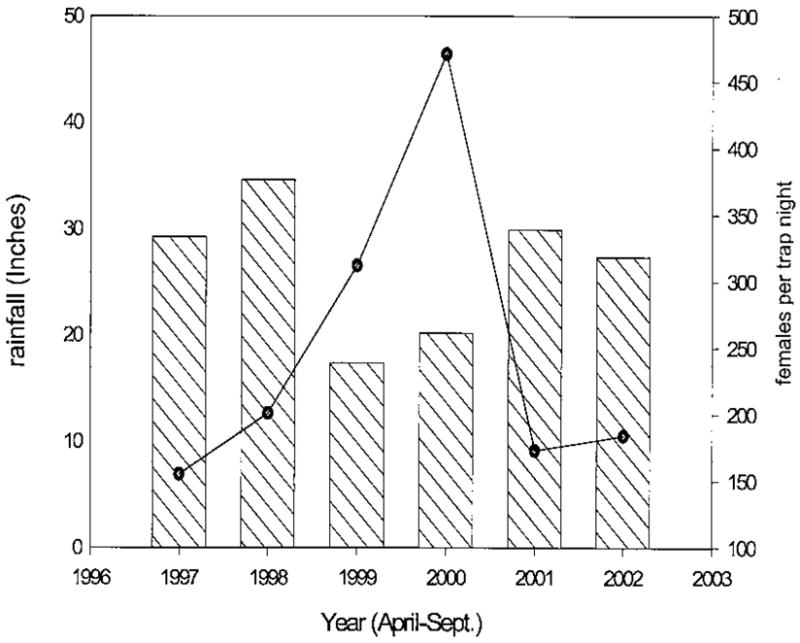
Fluctuation of Cx. erraticus populations in relation to seasonal rainfall. Bars represent the overall amount of rainfall reported in Iuka for the 5-mo collection season for 1997–2002. The line shows the average number of adult Cx. erraticus collected per trap night in each year.
Mosquitoes were tested for arboviral infection during 5 yr (Table 2). SLE virus only was detected during 1998, and no mosquito pools were positive for LAC or West Nile viruses when primers specific for those viruses were used in testing. EEE virus was detected during 1998, 1999, and 2002 in six genera of mosquitoes: Cq. perturbans, Culex restuans Theobald, Culex salinarius Coquillett, Cx. erraticus, An. crucians, and An. quadrimaculatus, Ae. vexans, Ochlerotatus triseriatus Say and Psorophora ferox Humboldt. Detection of virus occurred as early as the first week of April and as late as the last week of August.
Table 2.
Arboviral infection in mosquitoes at the Iuka, MS, study site (1998 –2002) with comparison of 2002 EEE infection rates by using MIR and Poolscreen methods
| Year | Species | Virus | Pools tested/+ | Total no. tested | MIR/1,000 | Poolscreen infection rate | Poolscreen 95% confidence intervala | Vector status |
|---|---|---|---|---|---|---|---|---|
| 1998 | Cq. perturbans | EEE | 20/1+ | 512 | 1.95 | nd | nd | (+) |
| 1998 | Cx. erraticus | SLE | 72/1+ | 2041 | 0.48 | nd | nd | Unknown |
| 1999 | Cx. restuans | EEE | 24/1+ | 27 | 37.03 | nd | nd | (+) |
| 2000 | 234 pools/10,493 mosquitoes tested - all negative | |||||||
| 2001 | 624 pools/11,359 mosquitoes tested - all negative | |||||||
| 2002 | An. crucians | EEE | 221/5+ | 8429 | 0.59 | 0.60 | 0.18–1.41 | (−) |
| 2002 | An. quadrimaculatis | EEE | 115/1+ | 3178 | 0.31 | 0.315 | 0.01–1.62 | (−) |
| 2002 | Ae. vexans | EEE | 42/1+ | 1108 | 0.90 | 0.92 | 0.03–4.74 | (+) |
| 2002 | Cx. salinarius | EEE | 24/2+ | 70 | 28.6 | 29.4 | 3.5–99.7 | (−) |
| 2002 | Oc. triseriatus | EEE | 11/1+ | 25 | 40.0 | 40.8 | 1.3–19.3 | (+) |
| 2002 | Cx. erraticus | EEE | 463/3+ | 20,485 | 0.14 | 0.147 | 0.03–0.43 | (+) |
| 2002 | Ps. ferox | EEE | 20/1+ | 75 | 13.3 | 13.3 | 0.41–66.8 | (+) |
| 2002 | Cq. perturbans | EEE | 279/0 | 11,365 | 0 | 0 | 0.00–0.168 | (+) |
nd, not determined.
Any two vectors in which the 95% confidence intervals do not overlap have significantly different infection rates at the P < 0.05 level.
MIRs for EEE virus were highly variable within the general mosquito population. Abundant species such as Cq. perturbans, Cx. erraticus, An. crucians, An. quadrimaculatus, and Ae. vexans had relatively low values compared with less common species tested (Table 2). Because the minimum infection rate assumes that any given positive pool contains a single infected mosquito, we used the Poolscreen2 program to recalculate the frequency of infection in the different mosquito species tested in 2002. The results from the two methods were comparable, although the infection rates estimated by the Poolscreen2 algorithm were generally higher than those estimated using the MIR method.
A total of 101 blooded mosquitoes representing three likely EEE vector species (Ae. vexans [n = 21], Cq. perturbans [n = 20], and Cx. erraticus [n = 60]) were collected during 2002 and analyzed to identify the blood meal hosts. Blood meals were identified to the species level of the host by using the PCR-heteroduplex assay methods described above. Of the 101 mosquitoes tested, 54 (54%) produced a positive result in the vertebrate specific PCR assay. Of these, 29 were avian in origin with eight species represented. The great blue heron, Ardea herodias L., was the major source (55%) of the avian blood meals identified
The avifauna at the site was estimated by point count resulting in the identification of 363 individuals, representing 39 species. Avian abundance and blood meal data then were compared using the feeding index method. In conducting these analyses, the abundance of each bird species was estimated based upon the unadjusted count data, and subsequently the count data were adjusted for total biomass. When analyzed on the basis of the unadjusted abundance data, the great blue heron, northern mockingbird, green-backed heron, wild turkey, brown-headed cowbird, and blue jay were all found to be highly overrepresented in the blood meals, i.e., a feeding index value of >10 (Table 3). However, when the data were adjusted for biomass, only the blue jay, brown-headed cowbird, and northern Mockingbird were highly overrepresented in the blood meals.
Table 3.
Feeding indices (FI) for avian blood meal hosts in mosquitoes collected from the Iuka study site in 2002
| Species | FI based on point count | FI based on body mass |
|---|---|---|
| Wild turkey | 13.4 | 0.1 |
| Carolina chickadee | 0.9 | 7.1 |
| Brown-headed cowbird | 27.8 | 47.9 |
| Northern cardinal | 0.6 | 1 |
| Blue jay | 14.4 | 12.5 |
| Great blue heron | 160 | 3.7 |
| Northern mockingbird | 13.4 | 20.85 |
| Green-backed heron | 13.4 | 4.74 |
Discussion
Based on these findings, the wetlands area seems to be a fairly stable focus for EEE virus. This virus was detected by RT-PCR during 3 of the 5 yr when mosquito pools were tested and fromat least six competent species (Chamberlain et al. 1954). These included species associated with permanent standing water (Cq. perturbans, Cx. restuans, and Cx. erraticus) as well as several floodwater/tree-hole taxa (Ae. vexans, Ps. ferox/Oc. triseriatus). This mixture of vectors indicates that transmission is likely occurring within the central zone of the swamp as well as along the periphery where Ae. vexans and Ps. ferox typically forage (Horsfall 1972). A number of bird species shown in previous studies to be EEE reservoirs were also at the site.
Two methods were used to calculate the prevalence of infection of EEE in the different mosquito species. The first, the MIR, is a standard method used to compare infection rates among potential vector species. However, the Poolscreen2 algorithm offers several advantages over the classical MIR calculation. First, because the MIR assumes that every positive pool contains only one infected insect, it can underestimate the actual prevalence of infection in the vector population, especially when a significant proportion of the pools are positive. In contrast, Poolscreen2 is based upon a probabilistic approach originally reported in 1962 (Chiang and Reeves 1962). This approach is based upon the probability that any given pool will contain no infected insects, given certain prevalence of infection in the population, and therefore considers the possibility that an infected pool may contain greater than one infected insect. Therefore, the infection estimates calculated by Poolscreen2 a real ways somewhat higher than those calculated using the MIR method. This difference is minor in the cases where the proportion of positive pools is small (as in this study), but increases dramatically when the proportion of positive pools is high.
Apart from providing a more accurate estimate of infection prevalence than does the MIR method, Poolscreen2 offers several other advantages over both the MIR and the original method proposed by Chiang and Reeves (1962). First, because Poolscreen2 was developed to estimate prevalences of infection in areas where infections are rare (≈ 1 per 1000 insects tested or less), the method permits accurate estimates of 95% confidence intervals surrounding the infection prevalence estimate, even when the number of positive pools is very low. This is not the case for either of the other methods, which require significant numbers of positive pools to obtain a 95% confidence interval (Chiang and Reeves 1962). Poolscreen2 also permits the use of data obtained from pools of unequal sizes. In contrast, the method proposed by Chiang and Reeves requires that the samples be divided into pools containing equal numbers of insects, or at most be divided into pools of two different sizes. The ability to analyze data from individual pools containing any given number of insects is advantageous, because it permits one to maintain important collection data (e.g., species, collection date or trap location) that might be lost when it is necessary to divide the collections into pools containing equal number of insects. Finally, Poolscreen2 permits one to estimate an upper bound on the probability of infection in a vector population when all insects tested are negative. This is valuable, for example, in determining whether transmission levels actually fall below a given threshold. Poolscreen2 has been incorporated into an easy to use computer package that requires little statistical background and may be obtained free of charge by contacting the corresponding author.
Cx. erraticus, a competent vector of EEE virus (Chamberlain et al. 1954), was highly abundant at the site and was repeatedly infected with this virus during 2002 (three positive pools). Data for 2003 indicate a similar pattern of infection with two positive pools of EEE virus (unpublished data). This species therefore seems to play a role in maintaining EEE virus, a role assumed in other foci along the Atlantic coast by Cs. melanura Coquillett (Scott and Weaver 1989). Populations of the latter were negligible at the Mississippi site during the 7-yr study period (<1%), and virus was not isolated from this species during the 5-yr testing period. During the first four decades of the 20th century, Cs. melanura was considered a rare species in the southeastern United States (King et al. 1939, 1942) and was not reported from Mississippi until the mid-1940s (Michener 1947). Based on a 1961 historical account of species distribution in the Tennessee Valley (Breeland et al. 1961), we postulate that Cs. melanura populations were probably established in the study site before the 1938 cypress deforestation but largely were replaced by Cx. erraticus after the drastic change in the habitat. A similar pattern was reported at an EEE focus in central Alabama where small numbers of Cs. melanura occur with a highly abundant Cx. erraticus population in an area that had been clear-cut for farming >100 yr ago but is returning slowly to a hardwood swamp habitat (Cupp et al. 2003). In that situation, Cs. melanura populations exist at a low level and have relatively high MIRs for EEE virus, whereas broad temporal infection of Cx. erraticus occurs annually.
Adult Cx. erraticus populations fluctuated inversely with the amount of rainfall occurring during the 6-mo mosquito season. This variability perhaps reflects the vagility of the larval stage and the tropical nature of this species to flourish in alternating wet/dry habitats. Immature Cx. erraticus may be found in a variety of grassy aquatic habitats, including ponds and lakes as well as situations where water levels fluctuate such as the edges of streams (Horsfall 1972). Larval development is favored by water temperatures ranging from 26 to 29°C (Breeland et al. 1961) that occur along the margins of shallow ponds and pools. In North Carolina, the abundance of larval and adult Cx. erraticus decreased with rising lake levels (Robertson et al. 1993), a situation similar to that seen at the Iuka wetland. This species also may occur in large numbers in semipermanent rice fields with floodwater mosquitoes such as Psorophora spp. Unlike Cs. melanura, Cx. erraticus overwinters as inseminated females and seasonal abundance is not dependent on excessive rainfall in the late summer and fall of the preceding year (Hayes and Hess 1964).
Oc. triseriatus is also a competent vector for EEE virus (Davis 1940, Chamberlain et al. 1954), but field isolations from this mosquito are relatively rare (Morris 1988). In Florida, a single EEE virus isolate was made from Oc. triseriatus during peak transmission where several other species (Culex nigripalpus Theobald, Cs. melanura) were considered to be primary vectors (Wellings et al. 1972). Similar to the situation reported here, the MIR of the Florida population was very high (1:58) due to the low number of mosquitoes collected. Oc. triseriatus will select reptiles (turtles) as hosts in the southeastern United States, even when deer and rodents are available (Irby and Apperson 1988, Szumlas et al. 1996). Some common turtle species in the region such as Clemmys gutatta Schneider (spotted turtle) are susceptible to EEE virus infection and develop a viremia (Smith and Anderson 1980), indicating their possible role as a virus reservoir.
Ps. ferox is a competent vector of EEE virus (Chamberlain et al. 1954), but reports of natural infections with this virus are extremely rare. Its importance in maintenance of EEE virus remains unknown. However, as an aggressive blood feeder that routinely attacks large mammals, it may be important as a bridge vector in the mid-South. Although found infected, neither An. crucians nor An quadrimaculatus s.l. is a competent EEE virus vector (Chamberlain et al. 1954). Nevertheless, as indicated by their MIRs, both species made contact with a viremic host, demonstrating that virus was circulating readily in the vertebrate reservoir. The earliest collection date (9 April) in which this virus was detected occurred in a pool of An. crucians.
SLE virus was detected from pools of Cx. erraticus in one of 5 yr of testing during late August. Unlike EEE virus, SLE virus is more broadly distributed in the United States and is much less likely to be found annually in established foci. Therefore, it is not unusual that the occurrence in the wetland site was temporary. However, SLE virus posed problems to the region in epidemic form in 1976 during which time two isolates were made from Cx. erraticus in Memphis, TN (Mitchell et al. 1980). The MIR during the 1976 epidemic was 0.2 which is similar to the figure of 0.48 reported here. To our knowledge, these are the only reports of SLE virus detected from Cx. erraticus.
West Nile virus was not found in the site during 2000–2002. West Nile equine cases were first reported in Mississippi in October 2001 by the Centers for Disease Control and Prevention, but no WNV-positive mosquito pools were detected that year. However, this virus caused a major epidemic in Mississippi in 2002 when human cases were first reported in late July. Horse cases and positive mosquito pools were also reported as the epidemic gained momentum throughout the state. However, during its spread, the virus did not cause human cases in Tishomingo County, where the study site is located (Mississippi State Health Department–Unpublished Epidemiology Reports).
Blood meals derived from eight different bird species were found in the 29 blooded mosquitoes containing avian derived blood meals. The small number of avian-derived blood meals obtained made it difficult to conduct statistical tests of the relative attractiveness of the different bird species observed in the blood meals. Despite this, some tentative observations may be made. For example, of the 29 meals analyzed, more than one-half (16) were derived from a single species, the great blue heron. In the point count estimates of the avifauna at the site, this species represented <1% of the total birds observed. This finding suggested that the great blue heron was being preferentially targeted by the local mosquito population, a hypothesis supported by the feeding indices calculated using the uncorrected abundance data. However, mosquito attraction involves a variety of factors, many of which are related to the overall size of the potential host. For this reason, the point count data were normalized for the mass of each bird species, and the overall feeding indices were recalculated using the adjusted data. When adjusted for biomass, the pairwise feeding indices for the great blue heron were greatly reduced, suggesting that mosquito preference for this species may be largely a factor of its large size. In contrast, the brown-headed cowbird, blue jay, and northern mockingbird were consistently overrepresented in the blood meals compared with both the unadjusted and biomass adjusted abundance data. In this regard, it is interesting to note that a similar pattern of avian host selectivity has been recently reported in studies conducted in eastern Alabama (Hassan et al. 2003). In that location, the yellow-crowned night-heron, Nyctanassa violacea (L.), was found to be consistently selected by the local vector population. Together, these data suggest that EEE vectors may preferentially target certain avian hosts, a hypothesis that could have important implications for the dynamics of EEE transmission.
Acknowledgments
We appreciate the technical assistance provided by Samantha Strickland, Leslie Viguers, and Bruce Lilley. This research was supported by National Institutes of Health grant R01-(AI)-49724.
References Cited
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR, et al. Host-feeding habits of Culex and other mosquitoes (Diptera: Culicidae) in the borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. J Med Entomol. 2002;39:777–785. doi: 10.1603/0022-2585-39.5.777. [DOI] [PubMed] [Google Scholar]
- Bibby CJ, Burgess ND, Hill DA. Bird census techniques. Academic; New York: 1992. [Google Scholar]
- Breeland SG, Snow WE, Pickard E. Mosquitoes of the Tennessee Valley. Tenn Acad Sci. 1961;36:249–319. [Google Scholar]
- Chamberlain W, Sikes RK, Nelson DB, Sudia WD. Studies on the North American arthropod-borne encephalitides. VI Quantitative determinations of virus-vector relationships. Am J Hyg. 1954;60:278–285. doi: 10.1093/oxfordjournals.aje.a119721. [DOI] [PubMed] [Google Scholar]
- Chiang C, Reeves WC. Statistical estimation of virus infection rates in mosquito vector populations. Am J Hyg. 1962;75:377–391. doi: 10.1093/oxfordjournals.aje.a120259. [DOI] [PubMed] [Google Scholar]
- Cupp EW, Klingler K, Hassan HK, Vigeurs LM, Unnasch TR. Eastern equine encephalomyelitis virus transmission in central Alabama. Am J Trop Med Hyg. 2003;68:495–500. [PMC free article] [PubMed] [Google Scholar]
- Davis WA. A study of birds and mosquitoes as hosts for the virus of eastern equine encephalomyelitis. Am J Hyg. 1940;32:45–59. [Google Scholar]
- Dunning JB. Monograph No. 1. Eldon Publishing Co; Cave Creek, AZ: 1984. Body weights of 686 species of North American Birds. [Google Scholar]
- Edman JD, Evans FD, Williams JA. Development of a diurnal resting box to collect Culiseta melanura (COQ.) Am J Trop Med Hyg. 1968;17:451–456. doi: 10.4269/ajtmh.1968.17.451. [DOI] [PubMed] [Google Scholar]
- Hamel PB, Smith WP, Twedt DJ, Woehr JR, Morris E, Hamilton RB, Cooper RJ. A land manager’s guide to point counts of birds in the Southeast. U.S. Dep. Agric. Southern Research Station; Asheville, NC: 1996. [Google Scholar]
- Hassan HK, Cupp EW, Hill GE, Katholi CR, Klingler K, Unnasch TR. Avian host preference by vectors of eastern equine encephalomyelitis virus. Am J Trop Med Hyg. 2003;69:641–647. [PubMed] [Google Scholar]
- Hayes RO, Hess AD. Climatological conditions associated with outbreaks of eastern encephalitis. Am J Trop Med Hyg. 1964;13:851–858. doi: 10.4269/ajtmh.1964.13.851. [DOI] [PubMed] [Google Scholar]
- Horsfall WR. Their bionomics and relation to disease. Hafner Publishing Co; New York: 1972. Mosquitoes. [Google Scholar]
- Hugh-Jones ME, Samui KL. Epidemiology of eastern equine encephalitis in Louisiana and Mississippi. J Fla Mosq Control Assoc. 1993;64:112–118. [Google Scholar]
- Irby WS, Apperson CS. Hosts of mosquitoes in the coastal plain of North Carolina. J Med Entomol. 1988;25:85–93. doi: 10.1093/jmedent/25.2.85. [DOI] [PubMed] [Google Scholar]
- Katholi CR, Toé L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis. 1995;172:1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- Kay BH, Boreham PFL, Edman JD. Application of the “feeding index” concept to studies of mosquito host-feeding patterns. Mosq News. 1979;39:68–72. [Google Scholar]
- King WV, Bradley GH, McNeel TE. The mosquitoes of the southeastern states. US Dep Agric Misc Publ. 1939;336 [Google Scholar]
- King WV, Bradley GH, McNeel TE. The mosquitoes of the southeastern states (Revision) US Dep Agric Misc Publ. 1942;336 [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, et al. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599–604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD. Mosquitoes of a limited area in southern Mississippi. Am Midl Nat. 1947;37:325–374. [Google Scholar]
- Mitchell CJ, Francy DB, Monath TP. Arthropod vectors. In: Monath TP, editor. St Louis encephalitis. American Public Health Association; Washington, DC: 1980. pp. 313–379. [Google Scholar]
- Morris CD. Eastern equine encephalomyelitis. In: Monath TP, editor. The arboviruses: epidemiology and ecology. CRC; Boca Raton, FL: 1988. pp. 1–20. [Google Scholar]
- Nasci RS. A lightweight battery-powered aspirator for collecting resting mosquitoes in the field. Mosq News. 1981;41:808–811. [Google Scholar]
- Nasci RS, Mitchell CJ. Arbovirus titer variation in field-collected mosquitoes. J Am Mosq Control Assoc. 1996;12:167–171. [PubMed] [Google Scholar]
- Powell KE, Blakey DL. St. Louis encephalitis The 1975 epidemic in Mississippi. J Am Med Assoc. 1977;237:2294–2298. doi: 10.1001/jama.237.21.2294. [DOI] [PubMed] [Google Scholar]
- Ralph CJ, Droege S, Sauer JR. Managing and monitoring birds using point counts: standards and applications. In: Ralph CJ, Sauer JR, Droege S, editors. Monitoring bird populations by point counts. U.S. Department of Agriculture, Forest Service General Technical Report PSW-GTR-149; 1995. pp. 161–168. [Google Scholar]
- Robbins CS, Bystrak D, Geissler PH. The breeding bird survey: its first fifty years, 1965–1979. U.S. Department of the Interior; Washington, DC: 1986. [Google Scholar]
- Robertson LC, Prior S, Apperson CS, Irby WS. Bionomics of Anopheles quadrimaculatus and Culex erraticus (Diptera: Culicidae) in the Falls Lake basin, North Carolina: seasonal changes in abundance and gonotrophic status, and host-feeding patterns. J Med Entomol. 1993;30:689–698. doi: 10.1093/jmedent/30.4.689. [DOI] [PubMed] [Google Scholar]
- Scott TW, Weaver SC. Eastern equine encephalomyelitis virus: epidemiology and evolution of mosquito transmission. Adv Virus Res. 1989;37:277–328. doi: 10.1016/s0065-3527(08)60838-6. [DOI] [PubMed] [Google Scholar]
- Smith AL, Anderson CR. Susceptibility of two turtle species to eastern equine encephalitis virus. J Wildl Dis. 1980;16:615–617. doi: 10.7589/0090-3558-16.4.615. [DOI] [PubMed] [Google Scholar]
- Sudia WD, Chamberlain RW. Collection and processing of medically important arthropods for virus isolation. National Disease Center; Atlanta., GA: 1967. [Google Scholar]
- Szumlas DE, Apperson CS, Powell EE, Hartig P, Francy DB, Karabatsos N. Relative abundance and species composition of mosquito populations (Diptera: Culicidae) in a La Crosse virus-endemic area in western North Carolina. J Med Entomol. 1996;33:598–607. doi: 10.1093/jmedent/33.4.598. [DOI] [PubMed] [Google Scholar]
- Wellings FM, Lewis AL, Pierce LV. Agents encountered during arboviral ecological studies: Tampa Bay area, Florida, 1963 to 1970. Am J Trop Med Hyg. 1972;21:201–221. doi: 10.4269/ajtmh.1972.21.201. [DOI] [PubMed] [Google Scholar]


